Abstract
We determined the effect of intravesical instillation of pentosan polysulfate encapsulated in liposomes for refractory interstitial cystitis patients. This was an open label uncontrolled study. Subjects were recruited from a private urology practice. Inclusion criteria included patients who met NIDDK criteria for Interstitial Cystitis (IC) and who were responding poorly to conventional treatments. Exclusion criteria included evidence of a urinary tract infection, bladder cancer, or other forms of chronic cystitis. Patients received 400 mg of Pentosan Polysulfate (PP) encapsulated into liposomes as an intravesical instillation performed every 2 weeks for 3 months. Baseline and post treatment outcome measures were obtained that included the O’Leary-Sant Interstitial Cystitis Symptom and Problem Questionnaire and the Pelvic Pain and Urgency/Frequency Patient symptom Scale tests. A total of 37 instillations were used and no adverse events occurred. Clinically significant decreases in symptom scores greater than 50% were seen in virtually all outcome measures at 3 month follow up. All subjects reported remarkable subjective improvement in pain symptoms marked by decreased use of narcotics and increased enjoyment of daily activities. No patients developed systemic symptoms or poor tolerance of the instillations. Intravesical Pentosan Polysulfate encapsulated into liposomes can significantly decrease frequency, urgency, pain and improve quality of life for two months after deployment. Additional studies are needed to determine cellular effects of glycosaminoglycan restoration, ideal doses, dosing intervals, safety and cost-effectiveness of this therapy.
Keywords: Interstitial cystitis, pentosan polysulfate, liposome, glycosaminoglycan, urothelium, nanospheres
Introduction
The prevalence of IC in the United States ranges from 4 to 12 million people with women affected more than men 10:1. Average time of onset of symptoms until diagnosis is four years. IC is known to have a profound and devastating effect on the health and quality of victim’s lives. IC has been undertreated and is known for its resistance to conventional therapy and insidious progression. The exact pathophysiology of IC has generally been poorly understood and there may be an autoimmune component to the disease. One common and compelling theory suggests that the defect appears to be related to an insufficient glycosaminoglycan urothelial barrier. When this protective layer degrades, the bladder is exposed to the acidic urine which has a toxic effect on the bladder tissue and afferent nerves become overstimulated promoting inflammation. This promotes a cascade of cycles of pain in the bladder and surrounding organs including the pelvic floor eventually leading to a debilitating constellation of symptoms.
Currently one of the most effective FDA approved pharmacological treatments for IC is oral Pentosan Polysulfate PP (Elmiron®), a weak analog of heparin and one of the glycosaminoglycans. Although the Elmiron®, website states that the exact cause of action is unknown, it is commonly considered that the active agent is absorbed by the GI tract and secreted in the urine where it forms a coating which simulates the natural glycosaminoglycan layer resulting in a protective barrier. Only 6% of the orally ingested product is secreted into the bladder and this is washed out relatively rapidly resulting in the need for dosing every 8 hours. Oral PP has been shown benefit over placebo in trials but can be associated with significant systemic side effects such as GI intolerance and hair loss [1-3]. Nevertheless, favorable safety data for the use of intravesical or oral PP is quite extensive [4]. Many clinicians will use PP as an intravesical instillation with literature and anecdotal support for improved efficacy using this delivery method [5].
Other instillation therapies are commonly used that include “cocktails” that contain various combinations of glycosaminoglycans, anesthetics, bicarbonate, and steroids but results are variable. Many remedies for IC concentrate on mitigating nerve transmission by blocking nerve fibers of pain transmitters through a variety of mechanisms. Other interventions that have successfully decreased pain and frequency due to IC include diet modification, behavioral therapy, physical therapy, and sacral neuromodulation. Surgical treatments can include laser eradication of mucosal lesions and ulcers, spot injections of lesions, and even partial or total bladder resection.
Liposomes are phospholipid concentric bilayer nanospheres mimicking human cell walls. Liposomes can encapsulate other agents to protect them and then act as a bio-adhesive by forming a molecular film on the urothelium. Liposomes can adsorb onto cell surfaces and fuse with cells to allow drug delivery inside cell walls [6-8]. There is evidence that empty liposomes alone can mitigate irritative bladder symptoms in comparison to placebo [9,10]. Liposomes are used commonly in foods, cosmetics, and compounded drugs.
There is increasing evidence that glycosaminoglycan barrier restoration therapy is important for the treatment of IC. To our knowledge there are no reports of a GAG agent mixed with a liposome nanocarrier to optimize urothelial contact and penetration. The apparent therapeutic benefit of PP encapsulated in liposomes suggests that this compound may be an effective bio-adhesive therapy for severe IC.
This report describes the preliminary results of a prospective open label study of PP/liposomes in a cohort of subjects with severe IC.
Materials and methods
Subjects
Eight subjects were recruited from a single urology practice and all received IRB format informed consent. Patients underwent history, physical examination, and urinalysis. Patients with soybean intolerance were excluded.
Procedures
Patients had urine dipstick tests to check for infection before treatment. Patients were placed in the dorsal lithotomy position and received sterile prep. Catheterization and bladder evacuation was performed with a 14 Fr Silicone catheter and water soluble lubricant. A 100 cc viscous solution of 400 mg of PP encapsulated in sub-micron (50-200 nm) liposomes made from phosphatidlycholine was mixed with normal saline and then instilled into the bladder using a catheter tip syringe. The delivery catheter was promptly removed. Patients were instructed to retain the compound at least 30-60 minutes if possible. During this retention time, patients performed positional rotisserie (rotating 90 degrees every 10 minutes) to maximize bladder wall contact. After one hour of observation, patients were discharged home from the clinic.
Results
Outcomes: Baseline measurements of all outcomes were made within 1 week prior to PP instillation. Our primary outcome measures were the symptom and bother index from the O’Leary-Sant Interstitial Cystitis Symptom and Problem Questionnaire, and the symptom and bother score from the Pelvic Pain and Urgency/Frequency Patient Symptom Scale PUF testing. Subjects received verbal questions regarding any side effects or adverse reactions to the instillations.
Eight subjects received a total of 37 intravesical instillations of PP encapsulated into liposomes. No adverse events were recorded in this pilot study. Mean O’Leary-Sant index scores decreased from 26.5 ± 7.2 to 13.8 ± 9.6 and mean PUF scores decreased from 24.9 ± 14.3 to 12.1 ± 9.6, Figure 1 shows the baseline and 3 month O’Leary scores, and Figure 2 shows the baseline and 3 months PUF scores for all eight subjects. Median values with corresponding ranges are shown due to the small numbers in this study.
Figure 1.
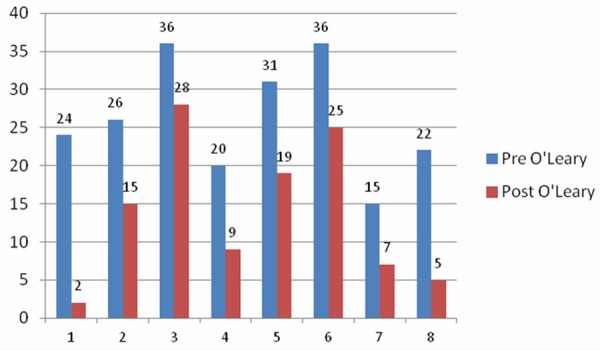
O’Leary-Sant combined symptom index and problem index scores.
Figure 2.
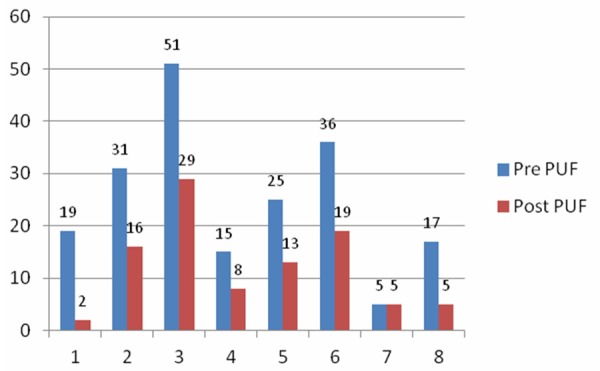
Pelvic Pain and Urgency Frequency combined symptom and bother scores.
Patients who received the PP/Liposome mixture experienced relatively rapid relief of symptoms. Some were able to stop narcotics use. Nearly all subjective outcome measures at 3 months showed a 50% or greater decrease in symptom and bother scores. Patients were followed for greater than 1 year and several of the patients noted durable and sustained relief of symptoms for greater than 15 months.
Discussion
Liposomes are an excellent drug delivery system and are biocompatible and well absorbed into gastrointestinal lining, skin, and urothelium [11]. Liposomes can penetrate tight junctions such as those found in between transitional cells of the urothelium to improve drug dwell time [12]. Other advantages include the ability to encapsulate small hydrophilic molecules like PP protecting them from a high proton environment, and to deliver drugs across cell wall phospholipid bilayers onto cells or even inside cellular components.
Given the small size of this study population (37 treatments), it is difficult to draw conclusive evidence of safety and efficacy, however the PP/liposome compound appears to be effective in patients for whom no other treatment was helpful. Some of the patients had battled IC for 10 and 20 years and all were prior oral and or intravesical PP failures. In view of the dramatic symptom and bother score improvements, the compound certainly requires additional studies.
Outcomes are mitigated by the possibility of placebo effects and Hawthorne effects. It is notable that patients who received empty liposomes also reported some improvement in the literature [13]. However, the fact that all patients were treatment failures with numerous other remedies and were advanced stage IC patients was compelling.
Urologists have instilled intravesical PP for decades with an excellent anecdotal and literature safety record [14]. Liposomes are common in foods and cosmetics and are generally regarded as safe (GRAS) products [15].
Further larger studies are indicated to establish safety of PP in liposomes. The addition of more subjects may reveal as yet unseen adverse effects, decreased efficacy or reduced duration of effects. Long term efficacy is yet to be determined.
PP when delivered in liposomes is expected to be much more effective in delivering the active form of the molecule to the urothelial target [16]. It is possible that other chronic inflammatory urothelial conditions such as chemical, hemorrhagic, and radiation cystitis may also be mitigated by PP/Liposome therapy.
This initial pilot study strongly supports the potential efficacy and safety of intravesical PP encapsulated in small nanosphere liposomes used as a nanocarrier delivery system. Further safety and dosing studies are warranted. Liposomes protect small molecules like PP from a hostile proton environment to increase dwell time and bio-adhesion and potentially improve efficacy over oral and intravesical forms of GAG administration. Multicenter placebo controlled studies will be required to establish the safety, dosing, and long term efficacy.
Disclosure of conflict of interest
None.
References
- 1.Waters MG, Suleskey JF, Finkelstein LJ, Van Overbeke ME, Zizza VJ, Stommel M. Interstitial cystitis: a retrospective analysis of treatment with pentosan polysulfate and follow-up patient survey. J Am Osteopath Assoc. 2000;100(Suppl 3):S13–8. [PubMed] [Google Scholar]
- 2.Hanno PM. Analysis of long-term Elmiron therapy for interstitial cystitis. Urology. 1997;49(Suppl 5A):93–9. [Google Scholar]
- 3.Parsons CL, Mulholland SG. Successful therapy of interstitial cystitis with pentosan polysulfate. J Urol. 1987;138:513–6. doi: 10.1016/s0022-5347(17)43243-5. [DOI] [PubMed] [Google Scholar]
- 4.Anderson VR, Perry CM. Pentosan polysulfate: a review of its use in the relief of bladder pain or discomfort in interstitial cystitis. Drugs. 2006;66:821–35. doi: 10.2165/00003495-200666060-00006. [DOI] [PubMed] [Google Scholar]
- 5.Davis EL, El Khoudary SR, Talbott EO, Davis J, Regan LJ. Safety and efficacy of the use of intravesical and oral pentosan polysulfate sodium for interstitial cystitis: a randomized double-blind clinical trial. J Urol. 2008;179:177–85. doi: 10.1016/j.juro.2007.08.170. [DOI] [PubMed] [Google Scholar]
- 6.Giannantoni A, Di Stasi SM, Chancellor MB, Costantini E, Porena M. New frontiers in intravesical therapies and drug delivery. Eur Urol. 2006;50:1183–93. doi: 10.1016/j.eururo.2006.08.025. discussion 1193. [DOI] [PubMed] [Google Scholar]
- 7.Tyagi P, Wu PC, Chancellor M, Yoshimura N, Huang L. Recent advances in intravesical drug/gene delivery. Mol Pharm. 2006;3:369–79. doi: 10.1021/mp060001j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fraser MO, Chuang YC, Tyagi P, Yokoyama T, Yoshimura N, Huang L, De Groat WC, Chancellor MB. Intravesical liposome administration--a novel treatment for hyperactive bladder in the rat. Urology. 2003;61:656–63. doi: 10.1016/s0090-4295(02)02281-1. [DOI] [PubMed] [Google Scholar]
- 9.Tyagi P, Hsieh VC, Yoshimura N, Kaufman J, Chancellor MB. Instillation of liposomes vs dimethyl sulphoxide or pentosan polysulphate for reducing bladder hyperactivity. BJU Int. 2009;104:1689–92. doi: 10.1111/j.1464-410X.2009.08673.x. [DOI] [PubMed] [Google Scholar]
- 10.Lee WC, Chuang YC, Lee WC, Chiang PH. Safety and dose flexibility clinical evaluation of intravesical liposome in patients with interstitial cystitis or painful bladder syndrome. Kaohsiung J Med Sci. 2011;27:437–40. doi: 10.1016/j.kjms.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 11.GuhaSarkar S, Banerjee R. Intravesical drug delivery: Challenges, current status, opportunities and novel strategies. J Control Release. 2010;148:147–59. doi: 10.1016/j.jconrel.2010.08.031. [DOI] [PubMed] [Google Scholar]
- 12.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4:145–60. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 13.Chuang YC, Lee WC, Lee WC, Chiang PH. Intravesical liposome versus oral pentosan polysulfate for interstitial cystitis/painful bladder syndrome. J Urol. 2009;182:1393–400. doi: 10.1016/j.juro.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 14.Bade JJ, Laseur M, Nieuwenburg A, van der Weele LT, Mensink HJ. A placebo-controlled study of intravesical pentosanpolysulphate for the treatment of interstitial cystitis. Br J Urol. 1997;79:168–71. doi: 10.1046/j.1464-410x.1997.03384.x. [DOI] [PubMed] [Google Scholar]
- 15.Raj S, Jose S, Sumod US, Sabitha M. Nanotechnology in cosmetics: Opportunities and challenges. J Pharm Bioallied Sci. 2012;4:186–93. doi: 10.4103/0975-7406.99016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giannantoni A, Di Stasi SM, Chancellor MB, Costantini E, Porena M. New frontiers in intravesical therapies and drug delivery. Eur Urol. 2006;50:1183–93. doi: 10.1016/j.eururo.2006.08.025. discussion 1193. [DOI] [PubMed] [Google Scholar]


