Abstract
AIMS
Diagnosis of early distal symmetric polyneuropathy (DSP) is challenging. Nerve conduction studies (NCS) are often normal. Skin biopsy for intraepidermal nerve fiber density (IENFD) has better sensitivity, but is invasive. Sudoscan is a novel technology that measures electrochemical skin conductance (ESC, microSimens – uS), which is thought to be proportionate to the number of functional sweat glands. This study evaluated Sudoscan’s diagnostic utility for DSP
METHODS
55 patients with suspected DSP (22 with diabetes, 2 prediabetes, 31 idiopathic) and 42 controls underwent the Utah Early Neuropathy Scale (UENS) and Sudoscan. Each was offered skin biopsy. DSP participants underwent quantitative sudomotor axon reflex testing (QSART) and NCS.
RESULTS
Feet and hands ESC were reduced among DSP participants compared to controls (64 +/− 22 vs. 76 +/− 14 uS p<0.005, and 58 +/− 19 vs. 66 +/− 18 uS p<0.04). There was no difference between diabetic and idiopathic DSP. Receiver operating characteristic curve analysis revealed feet ESC and IENFD had similar areas under the curve (0.761 and 0.752). ESC correlated with Sural amplitude (0.337, p<0.02), UENS (−0.388, p<0.004), and MNSI (−0.398, p<0.005).
CONCLUSIONS
Sudoscan is a promising diagnostic test for diabetic and idiopathic DSP, with diagnostic performance similar to IENFD.
Keywords: peripheral neuropathy, diabetes, sudomotor
Introduction
Distal symmetric polyneuropathy (DSP) is one of the most common and disabling complications of diabetes. Over half of diabetic patients develop DSP(Smith & Singleton, 2012), with an annual health care cost in excess of $10 Billion (in 2003)(Gordois, Scuffham, Shearer, Oglesby, & Tobian, 2003). DSP diagnosis is based on a combination of symptoms, signs and confirmatory diagnostic testing. Current guidelines recommend use of nerve conduction studies (NCS) for diagnostic confirmation(Tesfaye et al., 2010). However, many patients with early DSP, or with preferential loss of small unmyelinated axons have normal NCS. In this setting use of a validated measure of small fiber function is recommended for diagnostic confirmation (when clinically required)(Lauria, Bakkers, et al., 2010a; Tesfaye et al., 2010). The most commonly employed diagnostic tool in this setting is ankle skin biopsy with measurement of intraepidermal nerve fiber density (IENFD)(Peltier et al., 2009; Singleton et al., 2008). IENFD has demonstrated diagnostic utility(Casellini, Parson, Richardson, Nevoret, & Vinik, 2013; Tesfaye et al., 2010), reproducibility(Devigili et al., 2008; Gin, Baudoin, Raffaitin, Rigalleau, & Gonzalez, 2011) and there are published age and sex specific normative data(Lauria, Bakkers, et al., 2010a; Singleton et al., 2008). However, skin biopsy is invasive, expensive and requires a central laboratory. Because sudomotor axons are also involved in small fiber neuropathy, tests that evaluate their function may also have diagnostic utility. Quantitative sudomotor axon reflex testing (QSART) is the most commonly currently available test of sudomotor function(Smith et al., 2005; Tesfaye et al., 2010; Thaisetthawatkul, Fernandes Filho, & Herrmann, 2013), but is technically challenging to perform and demands careful control of testing conditions and patient preparation to obtain adequate reliability(Gibbons, Illigens, Wang, & Freeman, 2009; Singleton et al., 2008).
ESC is a novel noninvasive measurement that depends on the electrochemical reaction of the chloride component of sweat with stainless steel metal(Tesfaye et al., 2010; Thaisetthawatkul et al., 2013) across a spectrum of transcutaneous current. The resulting measurement is proportional to density of cutaneous sweat glands containing functional chloride channels. Several small studies suggest that ESC measured by the Sudoscan device (Impeto Medical, Paris France) may be a sensitive method to detect sudomotor dysfunction (Calvet, Dupin, Winiecki, & Schwarz, 2013; Casellini et al., 2013; Devigili et al., 2008; Peltier et al., 2009). The goal of this study was to evaluate the potential diagnostic utility of ESC as measured by Sudoscan for DSP due to diabetes and idiopathic neuropathy.
Subjects, Materials and Methods
DSP participant recruitment and clinical evaluation
The University of Utah Institutional Review Board approved the study protocol and all participants provided informed consent. Patients with symptoms consistent with DSP were recruited from the Neuromuscular and Peripheral Neuropathy Clinics at the University of Utah. Each potential DSP patient provided a focused history, including a detailed family history, completed standardized symptom questionnaires (the Michigan Neuropathy Screening Instrument -MNSI(Lauria, Bakkers, et al., 2010a; Lauria, Hsieh, et al., 2010b), visual analog pain scale - VAS), and examination scales (the Utah Early Neuropathy Scale - UENS(Singleton et al., 2008) and Michigan Diabetic Neuropathy Score - MDNS(Feldman et al., 1994)). Each underwent a laboratory evaluation for causes for peripheral neuropathy that included, at minimum, evaluation for diabetes (hemoglobin A1c, fasting plasma glucose), serum protein electrophoresis and immunofixation, and vitamin B12 level. Other evaluation such as oral glucose tolerance testing, hepatitis serologies and evaluation for autoimmune disorders was carried out at the discretion of the Neurologist performing the clinical evaluation. The etiology of DSP was recorded for each patient. If a cause could not be determined, DSP was considered idiopathic.
Control participants of similar age were recruited from spouses of participants, patients without neurological illness seeking refractive eye care, and advertisements. A focused neurological and general medical, and family history was obtained to exclude potential control participants with diseases that might predispose them to subclinical peripheral neuropathy (e.g. diabetes, vitamin deficiency, renal disease). Each control participant reported no symmetric foot symptoms and underwent the UENS to exclude subclinical DSP. Each underwent Sudoscan and was offered a distal leg skin biopsy for IENFD assessment.
Nerve conduction studies (NCS) were performed using standard techniques, maintaining limb temperature greater than 32 degrees C (Viking EDX Carefusion Minneapolis MN). Sural sensory amplitude and conduction velocity and Peroneal motor amplitude, distal latency, conduction velocity and minimal F wave latency were measured on each DSP participant. An abnormality of age specific Sural sensory amplitude or Peroneal motor amplitude or conduction velocity was used to judge NCS abnormality.
Skin biopsy for measurement of IENFD was offered to all DSP and control participants. Skin biopsy was performed at the distal leg and processed and stained with PGP 9.5 using established methodology(Lauria, Hsieh, et al., 2010b). IENFD was determined by counting the number of fibers crossing the dermal epidermal junction, measuring epidermal length, and calculating a fiber density (fibers/mm)(Lauria, Bakkers, et al., 2010a).
Quantitative sudomotor axon reflex testing (QSART) was performed at the foot, distal leg, proximal leg and forearm (WR Medical Maplewood, MN) using established methodology(Peltier et al., 2009). Room temperature was maintained at 21-24 degrees C, and skin temperature of 32 degrees C was maintained throughout testing.
Electrochemical skin conductance was measured using the Sudoscan device in all participants using previously described methodology(Casellini et al., 2013). The test is painless and non-invasive. Briefly participants place their hands and feet on stainless steel metal plates. An incremental low voltage direct current (DC) potential (< 4 V) is applied during a two-minute interval. Electrochemical skin conductance (ESC) is defined as the ratio of the resultant current and the constant DC stimulus. At low voltages (<10V) the stratum corneum acts as an insulator, thus the current generated is thought to originate from the sweat gland ion conductance. ESC is automatically calculated by the equipment for skin of palms and soles, and results are expressed in microSiemens (μS)(Feldman et al., 1994; Gin et al., 2011).
Statistical analysis
Two way Students t tests were used to compare groups. Receiver operating characteristic (ROC) curve analysis was used to assess the diagnostic utility of Sudoscan and IENFD. Sensitivity, specificity and predictive values using published normative data were also determined, and Chi Squared statistic used to compare proportions. Because not all patients referred for DSP have the diagnosis, the UENS was used as an agnostic diagnostic standard that did not depend on performance of specific confirmatory electrophysiological evaluation. A score of 4 or greater was required for a diagnosis of peripheral neuropathy for ROC curve purposes(Lauria, Hsieh, et al., 2010b; Singleton et al., 2008). Pearson correlation coefficients were used to evaluate for relationships between variables in the DSP cohort only. Statistical analysis was performed using SPSS version 20 (IBM).
Results
55 patients referred with symptoms consistent with DSP were enrolled; 22 had diabetes, 2 impaired glucose tolerance and 2 had a family history of neuropathy, 1 alcoholic neuropathy and the remaining 28 idiopathic neuropathy. The mean age was 59.1 +/− 10.4 years and 17 were female. 42 Control participants without symptoms or signs of DSP were recruited, with a similar mean age of 55.4 +/− 10.8 (ns); 33 were female (p<0.001). All participants were Caucasian aside from 3 controls (one each Pacific Islander, Asian and Latino) and two DSP participants who were Latino. DSP participants had significantly greater height, weight, body mass index, and blood pressure compared to control participants (table 1). Neuropathy patients with diabetes had higher hemoglobin A1c than those without diabetes (6.3+/− 0.64 vs 5.5 +/− 0.35, p<0.001). However, both groups shared other features of metabolic syndrome(Grundy et al., 2004), and those with non-diabetic neuropathy had higher LDL and total cholesterol (107 +/− 36 vs 77.8 +/− 24, p<0.001, and 186 +/−35 vs 155 +/− 27, p<0.001). BMI, serum triglycerides and blood pressure were similar between the groups. Neuropathy was mild to moderate in severity based on clinical scales (table 1). 18 of 40 control participants and 45 of 55 DSP participants consented to skin biopsy. NCS were abnormal in 59% of participants with possible DSP, and IENFD was abnormal in 46%, consistent with mild neuropathy severity. Compared to controls, the cohort of participants evaluated for DSP symptoms had significantly lower feet ESC (64. +/− 22 uS vs. 75.8 +/− 14 uS, p<0.004)(Figure 1) and lower IENFD at distal leg (3.41 +/− 2.2 vs 5.04 +/− 2.1 fibers/mm, p<0.01) and proximal thigh (5.51 +/− 2.2 vs 6.93 +/− 2.3 fibers/mm, p<0.02)(Table 1). There were no significant differences in either measure between DSP participants with diabetic and idiopathic or non-diabetic neuropathies. Within study groups, there was no significant difference for ESC or IENFD between male and female participants.
Table 1. Clinical features of distal symmetric polyneuropathy (DSP) and control participants.
DSP participants had significantly higher weight, height, body mass index (BMI) and systolic blood pressure compared to control participants. DSP patients had moderately severe neuropathy based on UENS, that was frequently painful. Feet ESC and IENFD at both the distal and proximal sites were significantly reduced among DSP participants who underwent skin biopsy(n=45) compared to controls (n=18). BMI – Body mass index, BP – Blood pressure, MDNS – Michigan Diabetic Neuropathy Score, UENS – Utah Early Neuropathy Score, MNSI – Michigan Neuropathy Screening Instrument, VAS – Visual analog pain scale, QSART – Quantitative sudomotor axon reflex testing, NS – Not significant.
| Measure | DSP (N=55) | Control (N=42) |
P Value | |
|---|---|---|---|---|
|
Clinical
Features |
Age | 59.1 +/− 10.4 | 55.4 +/− 10.8 | NS |
| Height | 69.1 +/− 4.3 | 65.2 +/− 3.3 | 0.001 | |
| Weight | 214.3 +/− 47.8 | 172.2 +/− 42.9 |
0.001 | |
| BMI | 31.7 +/− 7.2 | 28.0 +/− 7.0 | 0.016 | |
| Systolic BP | 128.7 +/− 17.9 | 118.9 +/− 14.8 |
0.014 | |
|
Clinical
Scales |
MDNS | 8.6 +/− 5.6 | ------- | |
| UENS | 12.9 +/− 8.9 | 0.99 +/− 0.16 | 0.001 | |
| MNSI | 5.94 +/− 2.39 | 0.75 +/− 0.84 | 0.001 | |
| VAS (mm) | 37.8 +/− 26 | ------- | ||
|
Nerve
Conduction Studies |
Sural sensory amplitude (uV) | 4.8 +/− 6.9 | ------- | |
| Sural sensory conduction velocity (m/sec) |
45.7 +/− 5.7 | ------- | ||
| Peroneal motor amplitude (mV) | 3.2 +/− 2.5 | ------- | ||
| Peroneal motor conduction velocity (m/sec) |
42.2 +/− 5.1 | ------- | ||
| Peroneal minimal F wave latency (msec) |
54.9 +/− 7.6 | ------- | ||
| QSART | Foot Sweat Volume uL | 0.486 +/− 0.42 | ------- | |
| Distal leg sweat volume uL | 0.500 +/− 0.62 | ------- | ||
| Proximal leg sweat volume uL | 0.688 +/− 0.74 | ------- | ||
| Forearm | 1.29 +/− 1.1 | ------- | ||
| Skin biopsy | Distal leg (fibers/mm) | 3.41 +/− 2/2 | 5.04 +/− 2.1 | 0.01 |
| Proximal thigh (fibers/mm) | 5.51 +/− 2.2 | 6.93 +/− 2.3 | 0.02 | |
| Sudoscan | Feet uS | 64.0 +/− 22 | 75.8 +/− 14 | 0.004 |
| Hands uS | 58.1 +/− 19 | 65.5 +/− 18 | NS | |
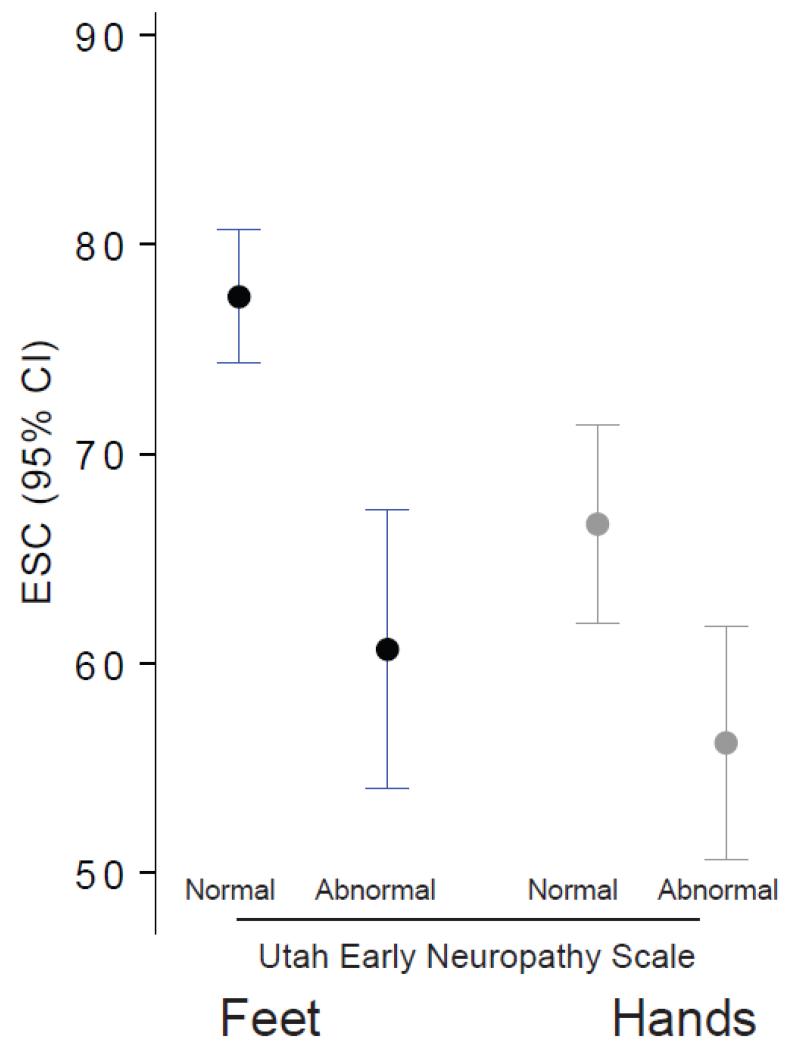
Figure 1. Electrochemical skin conductance (ESC) in the feet and hands was significantly reduced in those with abnormal Utah Early Neuropathy Score (UENS).
Mean ESC (with 95% confidence intervals) in the feet was 60.7 +/− 22.5 uS among those with abnormal UENS and 77.5 +/− 10.5 among those with normal UENS (p<0.001). Hands ESC was 56.4 +/− 18.7 uS versus 66.7 +/− 15.9 uS respectively (p<0.003).
The diagnostic utility of ESC measurement was evaluated using receiver operating characteristic (ROC) curve analysis. The Toronto consensus criteria for diabetic neuropathy define probable DSP based on the combination of symptoms and exam signs(Lauria, Bakkers, et al., 2010a; Tesfaye et al., 2010). The UENS, a validated examination scale for patients with early neuropathy was used to define those with abnormal examination(Peltier et al., 2009; Singleton et al., 2008). The combination of symmetrical, length dependent neuropathic symptoms and abnormal UENS was used to define DSP for the purposes of the ROC curve analysis. This approach permitted comparison of the diagnostic utility of Sudoscan with that of IENFD. IENFD is accepted by the Toronto criteria as a confirmatory test in patients with neuropathy symptoms and signs but normal NCS.
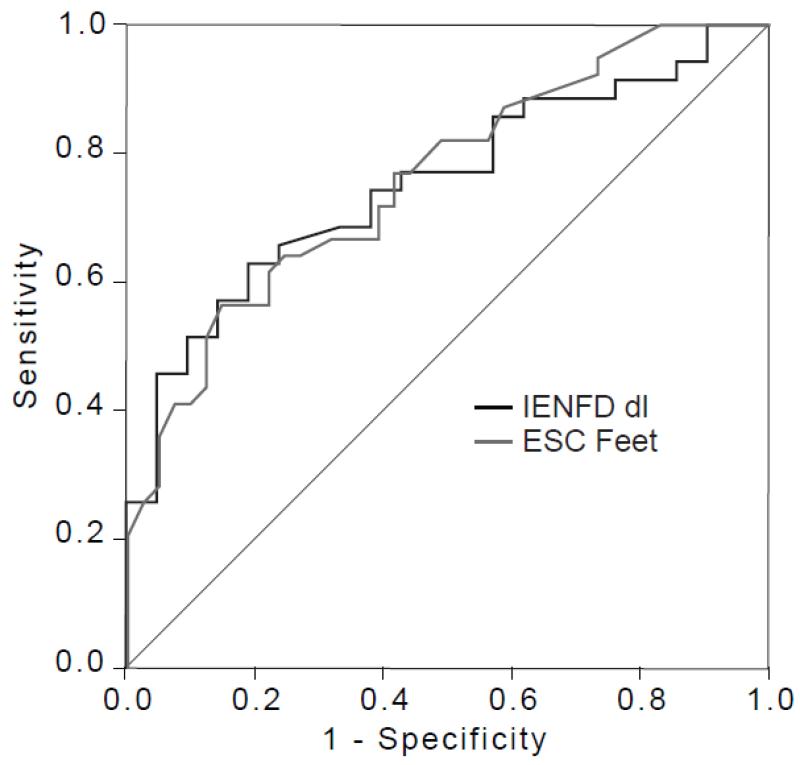
All control participants had a normal UENS. The UENS was abnormal in 45 of the 55 participants with symptoms suggestive of DSP. Sudoscan and IENFD had similar diagnostic efficiency with similar areas under the curve of 0.761, and 0.752 respectively (Figure 2). The lower limit of normal for foot ESC in the Caucasian population has been set by the manufacturer at 70 uS. Using UENS as the gold standard, feet ESC had a diagnostic sensitivity of 77% and a specificity of 67%, with a positive predictive value (PPV) of 59% and negative predictive value of 83%% (NPV) (Chi squared p<0.001). In comparison, IENFD at the distal leg had a lower sensitivity of 63%, similar specificity of 63%, with higher PPV (73%) but lower NPV (52%).
Figure 2. Receiver operating characteristics (ROC) curves for Sudoscan and skin biopsy using abnormal Utah Early Neuropathy Score (UENS) as the gold standard.
Area under the curve (AUC) for Sudoscan (ESC feet), and intraepidermal nerve fiber density (IENFD) were similar (0.761, 0.752 respectively).
One comparison of the utility of diagnostic tests is whether they provide the same diagnostic conclusion (normal/abnormal) for the same patient. The concordance of IENFD, NCS and ESC was examined. Distal leg IENFD and feet ESC were concordant in 58%. In 23% ESC was normal but IENFD was abnormal, and in 19% ESC was abnormal and IENFD normal. NCS and ESC were concordant in 67%. ESC was normal but NCS abnormal in 22% and ESC abnormal but NCS normal in 11%. Among those in the neuropathy group, all tests were 11%, 1 was abnormal in 40%, 2 in 21% and all 3 in 28%. When only those with an abnormal UENS are considered, all were normal in 7.9%, 1 in 37%, 2 in 21% and 34%.
Diagnostic performance was also examined separately in the group referred for possible DSP (not including control participants). Participants were categorized using the Toronto criteria into those with possible neuropathy (symptoms only, 5), probable (symptoms in addition to abnormal UENS, 15), and confirmed (symptoms, abnormal UENS and abnormalities of NCS, QSART or IENFD, 29)(Casellini et al., 2013; Tesfaye et al., 2010). The prevalence of abnormal feet ESC (<70 uS) was 20% among those with possible, 27% with probable, and 68% with definite neuropathy (Chi2 p<0.002). Similar analysis was not performed for IENFD or QSART as they are part of the case definition of neuropathy in the Toronto criteria.
The relationship between DSP measures was examined using Pearson correlation coefficients for participants with DSP (Table 2). ESC in the feet significantly correlated with neuropathy signs (UENS, cc −0.388, p<0.004) and symptoms (MNSI, cc −.398, p<0.005) (Figure 3), as well as Sural sensory amplitude (cc 0.337, p<0.019) and QSART at the foot (cc 0.348, p<0.015). IENFD in the feet correlated with signs (−0.312, p<0.039) and NCS parameters (Sural amplitude cc 0.440, p<0.004, and Peroneal conduction velocity cc 0.342, p<0.009) but not symptoms.
Table 2. Correlation between neuropathy measures.
Pearson correlation coefficients are displayed and significant results are italics with p values in parentheses. Feet ESC was significantly correlated with neuropathy symptoms (MNSI), signs (UENS), Sural sensory amplitude, and QSART sweat volume at the foot. UENS – Utah Early Neuropathy Score, MNSI – Michigan Neuropathy Screening Instrument, PMA- Peroneal Motor Amplitude, PMCV – Peroneal motor conduction velocity, QSART – Quantitative sudomotor axon reflex testing at the foot (ft) and distal leg (dl), IENFD- Intraepidermal nerve fiber density at the distal leg (dl) and proximal thigh (pt).
| Feet ESC | Hands ESC | IENFD (dl) | IENFD (pt) | QSART (ft) | QSART (dl) | |
|---|---|---|---|---|---|---|
| UENS | − .388 (.004) | −.145 | − 312 (.039) | − .424 (.004) | −.164 | −.222 |
| MNSI | − .398 (.005) | − .430 (.002) | .097 | −.168 | −.304 | − .512 (.001) |
| VAS | −.213 | −.256 | −.208 | −.020 | −.239 | −.122 |
| Sural Amp | .337 (.019) | −.017 | .440 (.004) | .229 | −.054 | −.094 |
| PMA | .068 | −.085 | .162 | .293 | .191 | .218 |
| PMCV | .081 | .049 | .342 (.009) | .170 | .155 | .127 |
| QSART (ft) | .348 (.015) | .329 (.023) | .109 | .314 (.043) | ||
| QSART (dl) | .271 | .273 (.055) | −.203 | .054 | ||
| IENFD (dl) | .263 | −.009 | ||||
| IENFD (pt) | .373 (.012) | .246 |
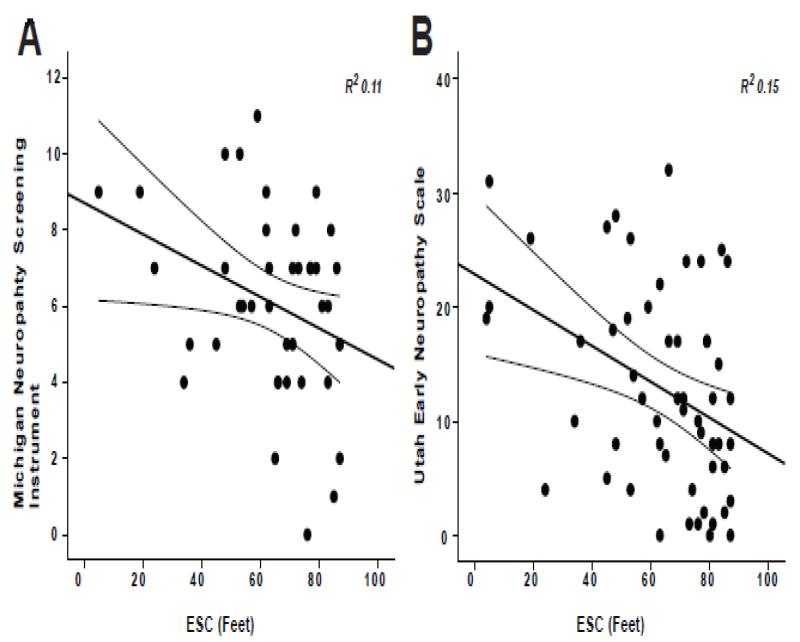
Figure 3. Electrochemical skin conductance (ESC) in the feet correlated with neuropathy symptoms and signs.
(A) The Michigan Neuropathy Screen Instrument and ESC feet had a Pearson correlation coefficient of −.398 (p<0.005) with an R2 of 11%. (B) The UENS and ESC feet had a Pearson correlation coefficient of −.398 (p<0.005) with an R2 of 15%.
Discussion
In this comparison of participants with and without clinically defined neuropathy, feet ESC was significantly different between controls and participants with symptoms of DSP. ESC was similar between idiopathic and diabetic DSP groups, suggesting it reflects sudomotor axon function as opposed to a direct effect of diabetes or hyperglycemia. Idiopathic neuropathy participants had similar BMI to diabetic patients, but had higher serum total and LDL cholesterol, consistent with prior observations linking obesity and its metabolic consequences to neuropathy risk(Rose, Singleton, & Smith, 2006; Smith & Singleton, 2013). ESC was similar in men and women within each cohort. In ROC curve analysis ESC had similar diagnostic utility as skin biopsy with measurement of IENFD, the current gold standard for small fiber neuropathy. These results suggest this form of sudomotor evaluation represents an attractive diagnostic modality for DSP in comparison with IENFD, which is the most commonly employed diagnostic measure of small fiber integrity. IENFD has demonstrated diagnostic utility for DSP(Devigili et al., 2008; Gin et al., 2011) and robust normative data are available(Lauria, Bakkers, et al., 2010a; Singleton et al., 2008). However, skin biopsy is invasive, expensive and unavailable at many centers, necessitating reliance on reference laboratories. While IENFD reproducibility is acceptable when laboratories employ rigorous quality control(Smith et al., 2005; Tesfaye et al., 2010; Thaisetthawatkul et al., 2013), not all referral laboratories use the same methodology or reference age and gender adjusted normative data.
Prior studies of Sudoscan have indicated excellent test retest reliability. ESC as measured by Sudoscan has high reproducibility based on Bland-Altman plots, with a mean difference of 1.7 uS from day to day, among a cohort with a mean feet ESC of 59 +/− 27uS.(Gin et al., 2011) Another study assessed reproducibility of feet ESC using independent measurement by two different Sudoscan units and found a correlation coefficient of 0.93 (p<0.001)(Calvet et al., 2013).
Sweat gland innervation may be directly assessed using skin biopsy stained with PGP 9.5. Sweat gland nerve fiber density is reduced in diabetic subjects and correlates with clinical measures of neuropathy severity(Gibbons et al., 2009; Singleton et al., 2008). Measurement of sweat gland nerve fiber density is appealing because it is performed on the same biopsy using the same processing methodology and stain employed for measurement of IENFD, but it has many of the same limitations, including its invasiveness. Analysis is time consuming and requires a carefully obtained and handled skin biopsy; depending on the depth of the biopsy, sweat glands may not be visualized. The most commonly used test of sudomotor function is QSART, which consists of iontophoresing acetylcholine and measuring the volume of sweat produced by the local axon reflex. QSART has a high diagnostic sensitivity for small fiber neuropathy. Among 101 patients with suspected DSP and normal NCS, QSART had a positive predictive value of 90% and negative predictive value of 80%. By comparison, IENFD had similar PPV of 90% but lower NPV of 68%(Tesfaye et al., 2010; Thaisetthawatkul et al., 2013). For optimum reliability, QSART requires meticulous attention to detail including maintenance of a constant environmental temperature.(Calvet et al., 2013; Casellini et al., 2013; Devigili et al., 2008; Peltier et al., 2009) Maximal QSART reliability is achieved with an overnight fast, and holding medication for 48 hours prior to the test, which is frequently impossible in clinical practice due to risk of medical complications or symptom exacerbations with abrupt medication cessation, particularly in a diabetic population.
Measurement of ESC is an attractive alternative to skin biopsy and QSART. It is easy and rapid to perform and very well tolerated. The procedure requires less than 3 minutes to complete and is not uncomfortable. It is essentially risk free. Our results suggest it has a similar diagnostic utility to IEFND in patients with DSP of mixed etiology. The fact that feet ESC was significantly correlated with both symptoms assessed using the MNSI, and signs based on the UENS supports its clinical relevance. Prior studies show a variable degree of correlation between IENFD and patient symptoms and signs(Lauria, Bakkers, et al., 2010a; Lauria, Hsieh, et al., 2010b). In the current study, IENFD at the distal leg weakly correlated with the UENS but did not correlate with the MNSI. In contrast, QSART correlated with the MNSI but not the UENS. QSART at the foot and feet ESC were correlated, although the magnitude of the relationship was not as high as one might expect. This observation is likely due to the large standard deviation in the QSART data, a reflection of variability in sweat volume produced. Feet ESC was significantly correlated to proximal IENFD (.373, p<.012) but not IENFD at the distal leg. This observation may be related to the floor effect observed with distal leg IENFD, where many patients with neuropathy have very low or absent IENFD. The fact that only ESC measurement correlated with both signs and symptoms suggest it may have equivalent if not superior clinical relevance to the other measures.
The ROC analysis supports comparable diagnostic utility between ESC measurement and IENFD. Given control participants did not have QSART it was impossible to assess its diagnostic utility. Sensitivity and specificity for both IENFD and ESC measurement were lower than published studies(Casellini et al., 2013; Devigili et al., 2008; Singleton et al., 2008). In particular, the diagnostic sensitivity of IENFD was surprising. However studies of IENFD have typically been performed in populations selected for small fiber neuropathy, and those for ESC measurement in populations with established diabetic neuropathy. For instance, Casellini et al demonstrated a sensitivity of 76% with a specificity of 92% for ESC measurement among a cohort of patients with neuropathy secondary to either type 2 or type 1 diabetes(Casellini et al., 2013; Feldman et al., 1994). The participants in the Casellini study had more advanced neuropathy than those in this study: for example those with abnormal ESC had a mean motor score on the Neuropathy Impairment Score Lower Limb (NIS-LL) of over 2, indicating clinically evident weakness, whereas no participant in the current study had any motor findings. NCS were normal in 40% of DSP participants consistent with mild neuropathy with relatively greater small fiber involvement. Inclusion of participants with mild neuropathy including a mixture of small and large fiber involvement likely influenced diagnostic utility. Furthermore, in order to permit comparison of ESC to IENFD, it was impossible to use the Toronto criteria definition as the gold standard since IENFD is part of the case definition, and reliance of NCS for confirmation would not capture those with small fiber predominant neuropathy. It is interesting that NCS, ESC and IENFD were all concordant in approximately one third of participants. This observation emphasizes the fact that each measure assesses a distinct population of peripheral nerve axons (NCS- large myelinated sensory and motor, IENFD- small unmyelinated sensory, and ESC- sudomotor axons). These findings support the need for further exploration of diagnostic utility in patients with DSP of variable severity. The high NPV suggests ESC measurement may have a particular role in excluding neuropathy in patients with isolated suggestive paresthesias or other sensory symptoms, particularly those in whom neurological examination proves normal. Such patients are frequently encountered in clinical practice.
This study has a number of limitations. The control population was not large or diverse enough to derive meaningful cutoff values and had a significantly greater proportion of females than did the DSP symptom cohort. While there was no difference in ESC between sexes, and no correlation with age, it is impossible to exclude age effects (as are seen with IENFD and other measures of nerve function)(Lauria, Bakkers, et al., 2010a) or sex-related differences in ESC. Sex related differences in sweat production have been identified in QSART; women consistently have lower sweat volumes (by a factor of two)(Low et al., 1997; Peltier et al., 2009). Because the majority of controls were female, this difference in sweat production might tend to blunt the observed ESC difference between the study groups and could have underestimated diagnostic performance. Controls and the DSP symptom cohort were significantly different in weight and BMI. This finding is expected given weight and BMI are recognized neuropathy risk factors, and absolute differences in BMI between groups were small and unlikely to account for differences in ESC. Further evaluation is required to demonstrate the responsiveness of ESC to neuropathy progression, and whether its decline is linear. While the available data suggest ESC is reproducible, the potential for medication effects has not been completely explored. A strength of the current study is its performance in a clinical setting. Unlike other published studies, which include a selected population of diabetic participants with established DSP, this study enrolled participants from a clinical population with a spectrum of neuropathy severity more closely reflecting the population evaluated in most practices (i.e. those presenting with new sensory symptoms). The observation of good diagnostic effectiveness in a clinically based population of neuropathy participants supports the potential diagnostic utility of ESC measurement.
While this study suggests ESC measurement has diagnostic utility and correlates with signs and symptoms, the extent to which ESC directly reflects sweat gland density or innervation by autonomic fibers has yet to be conclusively demonstrated. Data from cystic fibrosis patients demonstrate that dESC (the difference between ESC at a stimulus voltage of 3.6V and ESC at a stimulus voltage of 1.6V) is significantly reduced compared to control subjects, with a sensitivity of 93% and a specificity of 100%(Casellini et al., 2013; Hubert, Brunswick, Calvet, Dusser, & Fajac, 2011). These findings support the hypothesis that ESC is related to chloride anion conductance. Demonstration of equivalent ESC in diabetic and idiopathic neuropathies also suggests it reflects axon loss. Future studies incorporating sweat gland nerve fiber density and direct measures of sweat function (e.g. thermoregulatory sweat testing) should be considered.
Neuropathy diagnosis is primarily based on clinical evaluation, and diagnostic criteria for “definite” neuropathy rely on a confirmatory test, most commonly NCS(England et al., 2005; Tesfaye et al., 2010). While these criteria are primarily intended for application in a research setting physicians frequently refer patients for NCS for diagnostic confirmation. While NCS have demonstrated diagnostic value, they are often normal in patients with early or small fiber predominant neuropathy. Electrodiagnostic studies have been identified as one of the largest drivers of health care costs related to neuropathy evaluation(Callaghan et al., 2013). Numbness and sensory complaints are one of the most common symptoms practicing neurologists encounter. The results of this study suggest ESC measurement using Sudoscan holds promise as a well tolerated, rapid, and inexpensive means of excluding neuropathy in this patient population.
Acknowledgments
Study Funding: NIHR01DK064814, ADA08-CR52, Impeto Medical.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors have no conflicts of interest to declare.
All authors had access to the data and a role in writing the manuscript
References
- Callaghan BC, Burke JF, Rodgers A, McCammon R, Langa KM, Feldman EL, Kerber KA. Expenditures in the elderly with peripheral neuropathy: Where should we focus cost-control efforts? Neurology. Clinical Practice. 2013;3(5):421–430. doi: 10.1212/CPJ.0b013e3182a78fb1. doi:10.1212/CPJ.0b013e3182a78fb1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvet JH, Dupin J, Winiecki H, Schwarz PEH. Assessment of small fiber neuropathy through a quick, simple and non invasive method in a German diabetes outpatient clinic. Experimental and Clinical Endocrinology & Diabetes: Official Journal, German Society of Endocrinology [and] German Diabetes Association. 2013;121(2):80–83. doi: 10.1055/s-0032-1323777. doi:10.1055/s-0032-1323777. [DOI] [PubMed] [Google Scholar]
- Casellini CM, Parson HK, Richardson MS, Nevoret ML, Vinik AI. Sudoscan, a noninvasive tool for detecting diabetic small fiber neuropathy and autonomic dysfunction. Diabetes Technology and Therapeutics. 2013;15(11):948–953. doi: 10.1089/dia.2013.0129. doi:10.1089/dia.2013.0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devigili G, Tugnoli V, Penza P, Camozzi F, Lombardi R, Melli G, et al. The diagnostic criteria for small fibre neuropathy: from symptoms to neuropathology. Brain. 2008;131(Pt 7):1912–1925. doi: 10.1093/brain/awn093. doi:10.1093/brain/awn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England JD, Gronseth GS, Franklin G, Miller RG, Asbury AK, Carter GT, et al. Distal symmetric polyneuropathy: a definition for clinical research: report of the American Academy of Neurology, the American Association of Electrodiagnostic Medicine, and the American Academy of Physical Medicine and Rehabilitation. Neurology. 2005;64(2):199–207. doi: 10.1212/01.WNL.0000149522.32823.EA. [DOI] [PubMed] [Google Scholar]
- Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. 1994;17(11):1281–1289. doi: 10.2337/diacare.17.11.1281. [DOI] [PubMed] [Google Scholar]
- Gibbons CH, Illigens BMW, Wang N, Freeman R. Quantification of sweat gland innervation: a clinical-pathologic correlation. Neurology. 2009;72(17):1479–1486. doi: 10.1212/WNL.0b013e3181a2e8b8. doi:10.1212/WNL.0b013e3181a2e8b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gin H, Baudoin R, Raffaitin CH, Rigalleau V, Gonzalez C. Non-invasive and quantitative assessment of sudomotor function for peripheral diabetic neuropathy evaluation. Diabetes Metab. 2011;37(6):527–532. doi: 10.1016/j.diabet.2011.05.003. doi:10.1016/j.diabet.2011.05.003. [DOI] [PubMed] [Google Scholar]
- Gordois A, Scuffham P, Shearer A, Oglesby A, Tobian JA. The health care costs of diabetic peripheral neuropathy in the US. Diabetes Care. 2003;26(6):1790–1795. doi: 10.2337/diacare.26.6.1790. [DOI] [PubMed] [Google Scholar]
- Grundy SM, Brewer HB, Cleeman JI, Smith SC, Lenfant C, American Heart Association, National Heart, Lung, and Blood Institute Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition; Presented at the Circulation; 2004; pp. 433–438. doi:10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- Hubert D, Brunswick P, Calvet J-H, Dusser D, Fajac I. Abnormal electrochemical skin conductance in cystic fibrosis. Journal of Cystic Fibrosis: Official Journal of the European Cystic Fibrosis Society. 2011;10(1):15–20. doi: 10.1016/j.jcf.2010.09.002. doi:10.1016/j.jcf.2010.09.002. [DOI] [PubMed] [Google Scholar]
- Lauria G, Bakkers M, Schmitz C, Lombardi R, Penza P, Devigili G, et al. Intraepidermal nerve fiber density at the distal leg: a worldwide normative reference study. Journal of the Peripheral Nervous System: JPNS. 2010a;15(3):202–207. doi: 10.1111/j.1529-8027.2010.00271.x. doi:10.1111/j.1529-8027.2010.00271.x. [DOI] [PubMed] [Google Scholar]
- Lauria G, Hsieh ST, Johansson O, Kennedy WR, Leger JM, Mellgren SI, et al. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. Eur J Neurol. 2010b;17(7):903–12. e44–9. doi: 10.1111/j.1468-1331.2010.03023.x. doi:10.1111/j.1468-1331.2010.03023.x. [DOI] [PubMed] [Google Scholar]
- Low PA, Denq JC, Opfer-Gehrking TL, Dyck PJ, O’Brien PC, Slezak JM. Effect of age and gender on sudomotor and cardiovagal function and blood pressure response to tilt in normal subjects. Muscle & Nerve. 1997;20(12):1561–1568. doi: 10.1002/(sici)1097-4598(199712)20:12<1561::aid-mus11>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Peltier A, Smith AG, Russell JW, Sheikh K, Bixby B, Howard J, et al. Reliability of quantitative sudomotor axon reflex testing and quantitative sensory testing in neuropathy of impaired glucose regulation. Muscle & Nerve. 2009;39(4):529–535. doi: 10.1002/mus.21210. doi:10.1002/mus.21210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose K, Singleton JR, Smith AG. Increased prevalence of metabolic syndrome in peripheral neuropathy. Neurology. 2006;65(6):103. [Google Scholar]
- Singleton JR, Bixby B, Russell JW, Feldman EL, Peltier A, Goldstein J, et al. The Utah Early Neuropathy Scale: a sensitive clinical scale for early sensory predominant neuropathy. Journal of the Peripheral Nervous System: JPNS. 2008;13(3):218–227. doi: 10.1111/j.1529-8027.2008.00180.x. doi:10.1111/j.1529-8027.2008.00180.x. [DOI] [PubMed] [Google Scholar]
- Smith AG, Singleton JR. Diabetic neuropathy. Continuum (Minneapolis, Minn.) 2012;18(1):60–84. doi: 10.1212/01.CON.0000411568.34085.3e. doi:10.1212/01.CON.0000411568.34085.3e. [DOI] [PubMed] [Google Scholar]
- Smith AG, Singleton JR. Obesity and hyperlipidemia are risk factors for early diabetic neuropathy. Journal of Diabetes and Its Complications. 2013 doi: 10.1016/j.jdiacomp.2013.04.003. doi:10.1016/j.jdiacomp.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AG, Howard JR, Kroll R, Ramachandran P, Hauer P, Singleton JR, McArthur J. The reliability of skin biopsy with measurement of intraepidermal nerve fiber density. J Neurol Sci. 2005;228(1):65–69. doi: 10.1016/j.jns.2004.09.032. [DOI] [PubMed] [Google Scholar]
- Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010;33(10):2285–2293. doi: 10.2337/dc10-1303. doi:10.2337/dc10-1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thaisetthawatkul P, Fernandes Filho JAM, Herrmann DN. Contribution of QSART to the diagnosis of small fiber neuropathy. Muscle & Nerve. 2013;48(6):883–888. doi: 10.1002/mus.23891. doi:10.1002/mus.23891. [DOI] [PubMed] [Google Scholar]