1. Introduction
The potential outcomes framework for causal inference employs a number of assumptions (Neyman, 1923; Rubin, 1974, 1990; Robins, 1986). One of the assumptions that is generally made, either implicitly or explicitly, is an assumption that is sometimes described as the “no-multiple-versions-of-treatment assumption”; the assumption is part of what Rubin defined as the “Stable Unit Treatment Value Assumption” or SUTVA (Rubin, 1980, 1986). The assumption is made so that the potential outcomes for each individual under each possible treatment are well defined and take on a single value. If there are multiple versions of treatment present, as might arise for surgery treatment say if there are different surgeons who perform the surgery, and if these different versions of treatment give rise to different potential outcomes, then this assumption will be violated.
To circumvent the problems created by multiple versions of treatment in such contexts, one might restrict inference to a single version of treatment or, more generally, redefine each version of treatment as a different treatment. For example, one could consider the effect of surgery conducted by each particular surgeon rather that the effect of surgery generally. Such redefinition of the treatment of interest would make the no-multiple-versions-of-treatment assumption more reasonable and we do consider this approach below (see Proposition 1). However, redefining each version of treatment as a different treatment may not always be possible or desirable. One may not have data on which version each patient received. Moreover, if a patient needs to decide whether or not to undergo surgery but has no control over the choice of the surgeon who will actually perform the surgery, the average effect of the surgery treatment generally, rather than the average effect of surgery for each particular surgeon, may be what is most relevant. Such average effects of a particular surgical procedure (averaged over the surgeon who administers it) may also be of interest from a policy perspective. If, for example, in the treatment of cancer patients we are comparing the effects of radiation versus surgery, although different surgeons (i.e. different versions of treatment) may have different effects on survival, from a policy perspective, we could not simply select the most competent surgeon to perform all of the surgeries as the number of surgeries needed would be far too numerous for one surgeon to undertake. The policy question of interest here would be evaluating the overall survival rates of radiation versus surgery, taking into account the fact that not all surgeons are equally skilled.
Motivated by the above considerations, the purpose of this article is to consider causal inference under violations of the no-multiple-versions-of-treatment assumption. We will consider two broad settings: one in which the treatment precedes the version and one in which the version precedes the treatment. In the first setting, treatment is well-defined even before the version has been assigned but an individual’s potential outcomes are not fixed by the treatment alone; the potential outcome may be different under different versions of treatment. For the purposes of identification, we draw a distinction between confounders of the treatment-outcome relationship and the treatment-version relationship. We show that if data is available on version and one is simply interested in causal effects comparing one version to another, then adjustment does not need to be made for treatment-version confounders in the estimation of such causal effects (Proposition 1). We also show that if there is only one version of the control condition, then to estimate the effect of treatment on the treated, adjustment likewise does not need to be made for treatment-version confounders (Proposition 2). However, under multiple versions of treatment, to estimate overall causal effects for a population, adjustment needs to be made both for treatment-version confounders and for treatment-outcome confounders (Proposition 3) and failure to control for treatment-version confounders can result in bias. Propositions 1 and 3 follow also from theory for direct effects and from causal diagrams (e.g. Pearl, 2009; cf. Petersen, 2011) when applied to the multiple versions of treatment context. Further discussion of accommodating multiple versions of treatment using causal diagrams, and of the relative advantages of using the approach like that presented here versus expanded causal diagrams, is given elsewhere (Pearl, 2010; Hernán and VanderWeele, 2011; Petersen, 2011). In this setting in which treatment precedes version, additional identification results are given for several other types of causal effects which compare outcomes under various settings under which the version of treatment is randomly assigned from some specified distribution (Propositions 4–7). Some of these results (Propositions 5 and 6) have analogues in the literature on stochastic interventions in the context of direct effects (Didelez et al., 2006; Geneletti, 2007). In the Appendix, we also consider analogous results when there may be a time-dependent confounder that is affected by treatment assignment but confounds the version-outcome relationship (Propositions 9–11). Finally, we give a result for the perhaps even more common setting in which the version precedes the treatment variable or in which the actual treatment variable used in the analysis is in fact a coarsening of the underlying version of treatment variable. We show that use of the coarsened variable in the analysis yields effect estimates that can be interpreted as the effect of interventions in which the underlying version of treatment is randomly selected from certain distributions naturally arising in the population (Proposition 8). We discuss the relation of these results to those obtained from the treatment then version setting.
Although some of the results follow in a straightforward way from existing theory we believe it is of benefit for applied research to carefully consider, using the results in this paper as guidance, what different versions of treatment might be present and how this may, or may not, affect inferences. In the setting of treatment-then-version, ignoring multiple versions of treatment and potential treatment-version confounders can result in biased estimates of the overall treatment effect. In the version-then-treatment setting, if multiple versions of treatment are ignored, similar problems can arise with regard to bias due to inadequate control for confounding. Moreover, in this case, even if adequate control for confounding is made, the interpretation of the effect estimates are considerably more subtle than they at first may seem.
The remainder of the paper is organized as follows. In section 2 we review the potential outcomes framework and discuss how it can accommodate settings of multiple versions of treatment; we discuss the definition of causal effects under multiple versions of treatment. In section 3, we discuss identification of these effects when first the treatment is assigned and then a particular version of treatment is assigned. We discuss the interpretation of causal effect estimates under multiple versions and we consider when multiple versions of treatment can be ignored. In section 4, we will consider what new questions might be of substantive interest when multiple versions of treatment are present; some of the identification results bear certain resemblances to the analysis of direct and indirect effects (Robins and Greenland, 1992; Pearl, 2001; Didelez et al., 2006), though, as discussed further below, the precise technical details are distinct. In section 5, we discuss cases in which the ordering is version then treatment rather than treatment then version. An illustration is given in section 6. In section 7, we offer some concluding remarks and discuss how the extensions given in this paper to allow for violations of the no-multiple-versions-of-treatment assumption parallels in certain ways extensions described elsewhere for violations of the other major component of SUTVA, the no-interference assumption (Sobel, 2006; Hong and Raudenbush, 2006; Rosenbaum, 2007; Hudgens and Halloran, 2008; VanderWeele and Tchetgen Tchetgen, 2011a b; Halloran and Hudgens, 2012; Tchetgen Tchetgen and VanderWeele, 2012).
2. Potential Outcomes and the No-Multiple-Versions-of-Treatment Assumption
We will use j = 1, …, N to index the individuals in the population. Let Aj and Yj denote respectively the actual treatment received by and the actual outcome for individual j. Under the standard potential outcomes framework (Rubin, 1974, 1990), one might use Yj(a) to denote the potential outcome Y for individual j if treatment A were set, possibly contrary to fact, to the value a. Suppose treatment takes values in some set
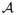 ; often
; often
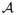 = {0,1} with 0 indicating the control condition and 1 indicating the treatment condition. Articulating the potential outcomes framework in this way requires what Rubin called the “Stable Unit Treatment Value Assumption” or “SUTVA.” Rubin (1980) points out that notation such as Yj(a) effectively presupposes (i) that if individual j is given treatment a then individual j’s outcome under treatment a does not depend on which treatment individual j′ ≠ j received and (ii) that there do not exist multiple versions of treatment a which might give rise to different outcomes depending on which version is administered. The first of these assumptions is sometimes referred to as “no-interference” which Rubin (1980) attributes to Cox (1958); the second assumption is a “no-versions-of-treatment assumption” which Rubin attributes to Neyman (1935). Included also within SUTVA is an assumption which in other literature is sometimes referred to as consistency. The consistency assumption (Robins, 1986) states that Yj(a) = Yj when Aj = a i.e. that the value of Y which would have been observed if A had been set to what it in fact was is equal to the value of Y which was in fact observed. The consistency assumption ties the potential outcomes (or counterfactual data) to the observed data. Under Rubin’s articulation of SUTVA, if there is only one version of treatment, then if Aj = a, the manner in which treatment Aj was in fact set to a is irrelevant, so Yj(a) is well defined and is equal to Yj when Aj = a. Rubin’s SUTVA thus includes a no-multiple-versions-of-treatment assumption and this no-multiple-versions-of-treatment assumption itself includes the consistency assumption.
= {0,1} with 0 indicating the control condition and 1 indicating the treatment condition. Articulating the potential outcomes framework in this way requires what Rubin called the “Stable Unit Treatment Value Assumption” or “SUTVA.” Rubin (1980) points out that notation such as Yj(a) effectively presupposes (i) that if individual j is given treatment a then individual j’s outcome under treatment a does not depend on which treatment individual j′ ≠ j received and (ii) that there do not exist multiple versions of treatment a which might give rise to different outcomes depending on which version is administered. The first of these assumptions is sometimes referred to as “no-interference” which Rubin (1980) attributes to Cox (1958); the second assumption is a “no-versions-of-treatment assumption” which Rubin attributes to Neyman (1935). Included also within SUTVA is an assumption which in other literature is sometimes referred to as consistency. The consistency assumption (Robins, 1986) states that Yj(a) = Yj when Aj = a i.e. that the value of Y which would have been observed if A had been set to what it in fact was is equal to the value of Y which was in fact observed. The consistency assumption ties the potential outcomes (or counterfactual data) to the observed data. Under Rubin’s articulation of SUTVA, if there is only one version of treatment, then if Aj = a, the manner in which treatment Aj was in fact set to a is irrelevant, so Yj(a) is well defined and is equal to Yj when Aj = a. Rubin’s SUTVA thus includes a no-multiple-versions-of-treatment assumption and this no-multiple-versions-of-treatment assumption itself includes the consistency assumption.
The assumption of “no multiple versions of treatment” and SUTVA generally are relevant both to experimental and non-experimental studies. Although SUTVA is often only explicitly noted in non-experimental observational research, the assumption is important in the interpretation of causal effects even in randomized trials.
As noted above, one potential approach for handling multiple versions of treatment would be to redefine the treatment variable A so as to include the version of treatment. This then generates an expanded set of potential outcomes, one for each “treatment level” (defined by the version of treatment) and under this redefined treatment the no-multiple-versions-of-treatment assumption will hold. In a number of settings this may be an adequate solution, though limited sample size for each version may limit the effectiveness of this approach. However, if we do not observe the version, redefining treatment so as to indicate version makes it difficult to identify causal effects since we would then not be observing what was redefined to be the treatment variable. Schafer and Kang (2009), however, have presented work that makes some progress with this approach; their approach does still assume that some indicators related to version are at least available.
Rather than redefining treatment in this way, additional progress can be made by instead introducing separate notation for the treatment itself and for the version of treatment. By taking this approach instead we will sometimes be able to define and identify causal effects even if we do not observe the version of treatment that each individual received. We follow Cole and Frangakis (2009) and VanderWeele (2009) to extend the potential outcomes notation to allow for multiple versions of treatment. Let Yj(a, ka) be the potential outcome for individual j if treatment A is set to value a by means ka where ka takes values in some set
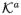 = {1, …, na}. For example, if comparing surgery at a hospital (A = 1) to a control condition (A = 0), the set
= {1, …, na}. For example, if comparing surgery at a hospital (A = 1) to a control condition (A = 0), the set
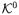 may simply be a singleton if there were only one version of the control condition and
may simply be a singleton if there were only one version of the control condition and
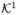 might be the set {1,2,3} indicating surgeon 1, 2 or 3 respectively and the potential outcomes Yj(1,1), Yj(1,2) and Yj(1,3) would indicate how individual j would fare under surgery by surgeon 1, 2 or 3 respectively. For the next two sections we focus on settings in which causal ordering of variables is treatment then version (rather than version then treatment). In the surgery example, an individual is first assigned to surgery then to a surgeon. Note that in this setting in which treatment precedes version, the treatment variable itself is well-defined even before version has been assigned; however, the potential outcome is not fixed by treatment alone. It is not fixed until the version has also been specified. Once treatment has been assigned, but before version has been fixed, one could effectively view the potential outcomes as stochastic counterfactuals (Greenland, 1987; Robins and Greenland, 1989, 2000).
might be the set {1,2,3} indicating surgeon 1, 2 or 3 respectively and the potential outcomes Yj(1,1), Yj(1,2) and Yj(1,3) would indicate how individual j would fare under surgery by surgeon 1, 2 or 3 respectively. For the next two sections we focus on settings in which causal ordering of variables is treatment then version (rather than version then treatment). In the surgery example, an individual is first assigned to surgery then to a surgeon. Note that in this setting in which treatment precedes version, the treatment variable itself is well-defined even before version has been assigned; however, the potential outcome is not fixed by treatment alone. It is not fixed until the version has also been specified. Once treatment has been assigned, but before version has been fixed, one could effectively view the potential outcomes as stochastic counterfactuals (Greenland, 1987; Robins and Greenland, 1989, 2000).
If the two treatments being compared were surgery and radiation, some aspects of treatment variation (e.g. specific hospital, time of treatment initiation) may be common to the treatments being compared but generally not all will be. For each treatment a, we will consider a distinct set of versions
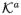 = {1, …, na}. For individuals with Aj = a we let
denote the version of treatment Aj = a actually received by individual j; for individuals with Aj ≠ a we define
so that
. For notational convenience, we define the vector
and recall that
for a ≠ Aj so that Kj thus denotes a vector in which all of the entries are 0 except the entry corresponding to the treatment that individual j actually received and this entry indicates what version of that treatment was in fact received by individual j. Note also that Kj gives no more information than Aj and
together. Note also that there is not variation independence between Aj and Kj; once we know the vector Kj, we know Aj; but Aj does not uniquely determine Kj.
= {1, …, na}. For individuals with Aj = a we let
denote the version of treatment Aj = a actually received by individual j; for individuals with Aj ≠ a we define
so that
. For notational convenience, we define the vector
and recall that
for a ≠ Aj so that Kj thus denotes a vector in which all of the entries are 0 except the entry corresponding to the treatment that individual j actually received and this entry indicates what version of that treatment was in fact received by individual j. Note also that Kj gives no more information than Aj and
together. Note also that there is not variation independence between Aj and Kj; once we know the vector Kj, we know Aj; but Aj does not uniquely determine Kj.
Under this expanded potential outcomes notation the no-multiple-versions-of-treatment assumption can then simply be articulated as that
| (1) |
If (1) holds then the consistency assumption is simply that for all j,
| (2) |
and (1) and (2) together would bring us back to Rubin’s articulation of the no-multiple-versions-of-treatment assumption. VanderWeele (2009) referred to (1) as an assumption of treatment variation irrelevance (which need not necessarily imply consistency assumption (2)). Under multiple versions of treatment, if the version has no relevance to the outcome under consideration then (1) will hold and, for all practical purposes, there are “no multiple versions of treatment,” at least with regard to the outcome Y under consideration. Note, however, that the version of treatment may be irrelevant (i.e. (1) holds) for some outcome but may not be irrelevant for a different outcome.
If the treatment variation irrelevance assumption (1) is violated we may still articulate a consistency assumption as follows. The consistency assumption would then require for all j,
| (3) |
This expanded potential outcomes notation essentially presupposes that, for a subject with Aj = a and
, (i) the potential outcomes Yj(a, k′a) with k′a ≠ ka are well defined, and (ii) the potential outcomes Yj(a*, ka*) with a* ≠ a, ka* ∈
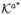 are well defined. In the surgery example, for an individual who in fact received surgery by surgeon 1, we could conceive of what would have happened to this individual had they received surgery from surgeon 2 or surgeon 3, and also what would have happened if surgery had not been given at all; for an individual who did not receive surgery we could conceive of what would have happened to the individual had the individual received surgery from surgeons 1, 2 or 3.
are well defined. In the surgery example, for an individual who in fact received surgery by surgeon 1, we could conceive of what would have happened to this individual had they received surgery from surgeon 2 or surgeon 3, and also what would have happened if surgery had not been given at all; for an individual who did not receive surgery we could conceive of what would have happened to the individual had the individual received surgery from surgeons 1, 2 or 3.
The average causal effect comparing treatment a, version ka with treatment a*, version ka</sup>*</sup> is defined by:
| (4) |
The potential outcomes Y(a, ka) might be conceived of as consisting of joint interventions on both Aj and to respectively set them to levels a and ka (Pearl and Robins, 1995; Pearl, 2001). This likewise is the effect that corresponds to redefining version as a new treatment variable. If the potential outcomes Y(a, ka) or Y(a*, ka*) are only defined for individuals for whom S takes certain levels of some covariate set S then we may instead be interested in the conditional causal effect:
| (5) |
Note that a special case of (4) and (5) would be when the treatment a is the same and only different versions, ka and k′a are being compared.
A well known causal effect is the effect of treatment on the treated, which is usually represented as E{Y(a)|A = a} − E{y(0)|A = a}. In the presence of multiple versions of treatment A = a, the counterfactual outcome Y(a) can be represented as Y(a, Ka). For individuals for whom A = a, Ka is simply the version of treatment that was actually received. Note that Ka is a random variable whose value may be different for different subjects, not a fixed value ka. Suppose now that there is only one version of treatment for the control condition, A = 0, so that K0 = {1} and the only potential outcome for each individual under the control condition is Yj(0) ≡ Yj(0,1). Provided Yj(0) is well-defined for individuals with treatment Aj = a ≠ 0, we could then define the effect of treatment on the treated as:
| (6) |
We will use additional notation to define another familiar causal effect, the overall treatment effect, but here in the setting with multiple versions of treatment. For individuals with Aj = a we defined to be the version of treatment Aj = a actually received by individual j. In some cases, for individuals with Aj = a, we might be willing to conceive of a counterfactual variable , a* ≠ a, corresponding to the version of treatment a* that an individual would have received had they in fact been assigned to treatment a* rather than a. For example, in the surgery context, for an individual j who did not receive surgery (Aj = 0), the variable would denote which surgeon individual j would have been assigned to had the individual in fact undertaken surgery i.e. whether the individual would have been assigned to surgeon 1, 2 or 3. We then assume a consistency assumption for , namely, when Aj = a. For each individual j, we assume there is a fixed version that would have been received had the individual been given treatment a*; as with the case of stochastic counterfactuals (Robins and Greenland, 2000), analogous results to those that follow would hold if these “counterfactual versions of treatment” were assumed stochastic.
The variable , a* ≠ Aj bears some resemblance to the counterfactual value of the mediator in the literature on direct and indirect effects but, unlike in the mediation context, counterfactuals of the form are only defined when a and a* coincide; that is, if a* is the actual (or the counterfactual) treatment the only possible versions of treatment are different versions Ka*of treatment a*. If we are willing to postulate variables , a* ≠ Aj then we can define the overall treatment effect comparing giving everyone treatment a versus treatment a* by:
| (7) |
Assuming the relevant counterfactuals exist we can define, Y(a) ≡ Y(a, Ka(a)) and the expression above is simply E{Y(a)} − E{Y(a*)}. In the following section we will discuss the identification of these various treatment effects. Later we will also consider the definition and identification of some additional causal effect measures.
3. Identification of Causal Effects Under Multiple Versions of Treatment
We now consider identification in the setting of multiple versions of treatment. We partition the set of covariate (potential confounders) into two sets. We let W indicate a set of covariates that may be causes of treatment A or may be, for one or more treatment levels a, causes of which version of treatment Ka is administered but are not causes of Y except through either treatment or the version of treatment; let C denote all other covariates. We partition the covariates in this way because, as we will see below, to identify certain causal effect we do not need data on W i.e. certain effects, but not others, would still be identified even if data on W were unavailable. We can then represent the relations between treatment, version, outcome and covariates as in Figure 1.
Figure 1.
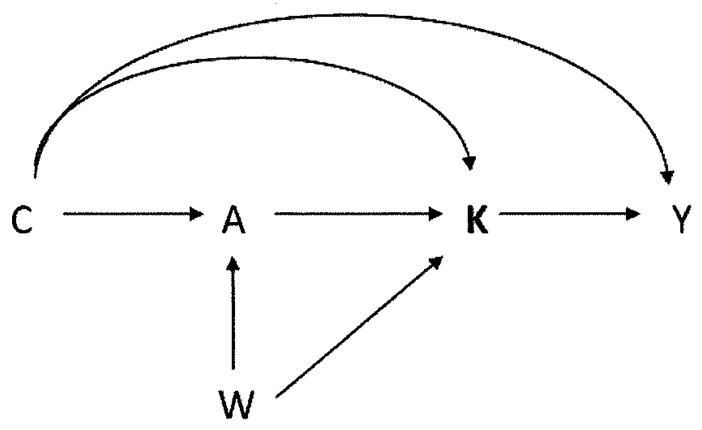
Causal diagram illustrating relationships between treatment A, version K, outcome Y and confounding variables C and W.
Note that because K contains all of the information in A, there is no arrow directed from A to Y. An example of a covariate in W in the context of the surgery example might be the particular health plan that an individual has that might affect both the probability that the individual receives surgery and, if the individual does receive surgery, also which particular surgeon conducts the operation, but would perhaps not affect the outcome except through whether surgery is received and who performs the surgery.
The results below will require that the covariate sets C and W are sufficiently rich so that certain no unmeasured confounding assumptions (also sometimes referred to as “exchangeability” or “ignorability” assumptions) are met. We will use the notation A II B|C to denote that A is independent of B given C. We will first consider the following assumption: whether we have
| (8) |
In other words, we will consider whether, within strata of the covariates C, groups defined by treatment and version are, for all a ∈
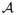 , ka ∈
, ka ∈
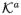 , comparable in their potential outcomes under treatment a, version ka. Note that assumption (8) would hold if treatment A were randomized (or randomized conditional on C) and if, conditional on treatment A = a (or conditional on {A = a, C = c}), version of treatment Ka were also randomized. Intuitively, (8) states that the set C suffices to control for confounding of the joint effect of treatment and version on the outcome. Assumption (8) will hold if Figure 1 represents a causal directed acyclic graph (Pearl, 2009). Note that in Figure 1, C blocks all backdoor paths from the set {A, K} to Y. Note also that since K in fact contains all the information in A, assumption (8) could also be written as Y(a, ka) II K|C; the two are equivalent.
, comparable in their potential outcomes under treatment a, version ka. Note that assumption (8) would hold if treatment A were randomized (or randomized conditional on C) and if, conditional on treatment A = a (or conditional on {A = a, C = c}), version of treatment Ka were also randomized. Intuitively, (8) states that the set C suffices to control for confounding of the joint effect of treatment and version on the outcome. Assumption (8) will hold if Figure 1 represents a causal directed acyclic graph (Pearl, 2009). Note that in Figure 1, C blocks all backdoor paths from the set {A, K} to Y. Note also that since K in fact contains all the information in A, assumption (8) could also be written as Y(a, ka) II K|C; the two are equivalent.
We then have the following identification results. The proofs of all results are given in the appendix; some of the proofs bear resemblance to certain identification results in the literature on direct and indirect effects and we will point out when this is so.
Proposition 1
If Yj(a, ka) and Yj(a*, ka*) are well defined for all individuals j and if the no-unmeasured-confounding assumption (8) holds then
Note for the quantity on the right hand side of the equation in Proposition 1 to itself be estimable from data we would need “positivity” (or “experimental treatment assignment” assumption) to hold that 0 < P(A = a, Ka = ka|C = c) < 1 for all a, ka and c. Similar “positivity” assumptions will be assumed to hold throughout.
Proposition 1 thus allows for the identification of the average causal effect comparing treatment a, version ka with treatment treatment a*, version ka*. We could control for W as well in assumption (8) and Proposition 1, but data on W is not necessary to identify the effect given in Proposition 1; data on W will, however, be needed for the overall treatment effect result given below. The proof of Proposition 1 given in the appendix is for the identification of E{Y(a, ka)}; this proof is completely isomorphic to the proofs often used for the identification of counterfactuals for controlled direct effects (Robins and Greenland, 1992; Pearl, 2001). Note however that the effect itself is somewhat different; even in the special case with a = a* but ka ≠ k′a, the effect E{Y(a, ka)} − E{Y(a, k′a)} corresponds to having the “treatment” variable fixed not the “mediator” variable as in controlled direct effects. If Y(a, ka) and/or Y(a*, ka*) are only defined for those with certain covariate values of S ⊆ C then the result in Proposition 1 can be made conditional on S = s. In the appendix we also discuss settings in which there may be an effect, Q, of treatment A that affects both version K and the outcome Y, a setting sometimes referred to as “time-dependent” confounding. Analogous results hold but identification formulas are different.
As already noted, some of the theory and results given here bear resemblance to results on direct and indirect effects. However a number of the precise technical details differ. First, in the setting considered here in which treatment precedes version, unlike in the context of direct and indirect effects, once the version is known the treatment is also known, whereas, with mediation, the same value of the mediator can arise for different treatment groups. Moreover, in the multiple versions of treatment context there is no direct effect of treatment on the outcome other than through version. Furthermore, within a deterministic potential outcomes framework (Rubin, 1974, 1990), even when studying direct and indirect effects, once treatment is assigned this fixes the potential outcome for an individual, whereas in the multiple versions of treatment context, the potential outcome is not fixed by treatment alone; it is not fixed until the version has also been specified. Finally, in the multiple versions of treatment context, we can distinguish between at least two scenarios, one in which treatment precedes version and one in which version precedes treatment, and it is the former rather than the latter scenario that bears resemblance to results on direct and indirect effects. In this paper, we develop theory and results for both settings. Nevertheless, in several cases, the actual proofs for the results on multiple versions of treatment, like Proposition 1, are formally identical to those for direct effects (Pearl, 2001; Didelez et al., 2006; Geneletti, 2007).
An interesting special case of multiple versions of treatment and the causal effect identified under Proposition 1 is the setting of partial compliance (e.g. Jin and Rubin, 2008). If the treatment A is a randomized dose, the “version” K might be taken as a measure of the extent of compliance. Appeal to Proposition 1 does, however, require that control be made for common causes of the version and the outcome. This will often be a strong assumption and in the compliance setting it may not hold. Proposition 3 below, which identifies overall causal effect (analogous in the non-compliance setting to the intent-to-treat effect) will not require this assumption. However, when the effect of different levels of compliance is of interest, and when it is not possible to control for the confounders of relationship between version/compliance and outcome, then alternative approaches such as that of Jin and Rubin (2008) using principal stratification and a Bayesian analysis may be desirable. As yet another alternative when there are unmeasured version-outcome confounders, it may be possible to adapt sensitivity analysis techniques for controlled direct effects (VanderWeele, 2010) to the multiple versions of treatment setting.
We now consider the identification of the effect of treatment on the treated.
Proposition 2
If there is only one version of treatment for the control condition so that and Yj(0) is well defined for all individuals j and if Y(0) II {A, K0}|C then
Note that Y(0) II {A, K0}|C would hold under assumption (8) above; however, for the application of Proposition 2 we only need the weaker condition Y(0) II {A, K0}|C rather than that (8) hold for all a ∈
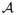 , ka ∈
, ka ∈
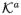 . Once again with Proposition 2 we do not need to control for the A – K confounders, W.
. Once again with Proposition 2 we do not need to control for the A – K confounders, W.
If we are willing to postulate variables , a* ≠ Aj, so that we can define Y(a) = Y(a, Ka(a)) we can also consider the identification of the overall treatment effect. As will be shown in Proposition 3, overall treatment effects are identified if
| (9) |
Assumption (9) requires that, within strata of the covariates {C, W}, groups defined by treatment are comparable in their potential outcomes Y(a) =Y(a, Ka(a)). Assumption (9) would hold if treatment A were randomized (or randomized conditional on {C, W}); assumption (9) holds if Figure 1 is a causal directed acyclic graph (Pearl, 2009).
Proposition 3
If and are well defined for all individuals j and if (9) holds then E{Y(a) − Y(a*)} =
Note for the quantity on the right hand side of the equation in Proposition 3 to itself be estimable from data we would need “positivity” to hold for both {C, W}, not just C i.e. 0 < P(A = a|C = c, W = w) < 1 for all a, c and w.
Proposition 3 states that even under violations of the no-multiple-versions-of-treatment assumption we can use the ordinary identification formula for overall treatment effects but control needs to be made not simply for variables C that might confound the relationship between treatment assignment and outcome but also for variables W that may affect both treatment assignment and version of treatment (even if these variables do not also affect the outcome except through treatment or version of treatment). Control must be made for these variables essentially because for the overall treatment effect we are examining the effect of A on Y and W is a confounder of the relationship between A and Y as it affect both A and also Y through K. Note, however, that if treatment is randomized there will be no common causes of treatment and version. In an observational study, such as that which might be represented in Figure 1, if control is not made for W then there is an unblocked backdoor path from A to Y, namely, A ← W → K → Y. In the context of multiple versions of treatment a sufficient set of confounders for the effect of A on Y would need to include W. In the appendix we give a numerical example showing that without controlling for a common cause of A and K, one can obtain biased estimates of the overall treatment effect. Analytically, it follows from the results of VanderWeele and Arah (2011) that the difference between the estimate adjusted for only C versus that adjusted for C and W is given by
where w′ is an arbitrary reference level of W. The bias thus depends on the effect of W on Y (essentially through K), i.e. E(Y|a, c, w) − E(Y|a, c, w′) and E(Y|a*, c, w)−E(Y|a*, c, w′), and on the conditional distributions of W: P(w|a, c), P(w|a*, c) and P(w|c).
Several observations emerge from the results above. First, Propositions 2 and 3 demonstrate what may be intuitively clear, that it is not necessary to have data on the versions of treatment in order to estimate the effect of treatment on the treated or the overall treatment effect. For the overall treatment effect, adjustment needs to be made not only for common causes of treatment and outcome but also common causes of treatment and version of treatment. For the effect of treatment on the treated, if there are no multiple versions of treatment for the control condition, it is not necessary to have data on common causes of treatment and version. Finally, as shown in Proposition 1, if data are available on the version of treatment then one can identify causal effects comparing a version of one treatment to some other version of the same or a different treatment and to identify such effects one again does not need data on the common causes of treatment and version.
Proposition 3, in particular, has important implications for settings in which the multiple versions of treatment assumption is violated and the violation is ignored. The result implies that the ordinary estimator for average causal effects (i.e. standardizing outcome differences between treatment groups by confounders, ignoring versions) can be interpreted as a contrast between (i) the average outcome that would be expected if everyone had been assigned treatment A = 1 with each individual receiving the version that would have been received had they been assigned A = 1 versus (ii) the average outcome that would be expected if everyone had been assigned treatment A = 0 with each individual receiving the version that would have been received had they been assigned A = 0. The estimate carries this interpretation provided adjustment is made for all confounders of the relationship between treatment A and outcome Y; importantly, however, within the context of multiple versions of treatment, these confounders include common causes of treatment and version. Data on version is not needed, and the no-multiple-versions-of-treatment assumption does not need to hold, but data on common causes of treatment and version are needed to interpret the estimate as a causal effect.
One way to think about these results are that if we conceive of a treatment and version combination, (a, ka), as a “regime”, and a treatment a, along with an unknown rule relating treatment to version as a “policy,” then Proposition 1 states that if the usual conditional independence assumption holds at the level of the regime (assumption 8) we can identify causal effects at the level of the regime. Proposition 3 states that if the usual conditional independence assumption holds at the level of the policy (assumption 9), then for the set of policies in place in the study, we can identify average causal effects at the level of the policy.
4. New Causal Effects and Applications with Multiple Versions of Treatment
In this section we will consider the identification and interpretation of a different type of causal effect which arises by setting the version of treatment to various prespecified distributions. These prespecified distributions may be fixed or may be defined by those of certain treatment groups or by those of individuals with certain pretreatment covariate values. For the results in this section we will rely principally on assumption (8) above but for one result we will consider another “exchangeability” or “no unmeasured confounding” condition, namely whether
| (10) |
In other words, we will consider whether, within strata of the covariates {C, W}, groups defined by treatment are comparable in the versions of treatment they would have received under each possible treatment a. Note that assumption (10) would hold if treatment A were randomized (or randomized conditional on {C, W}. Intuitively, (10) states that within strata of {C, W} the version of treatment which an individual would be assigned if given treatment a is independent of the treatment actually received; note the set {C, W} blocks all backdoor paths from A to K in Figure 1; assumption (10) would thus hold if Figure 1 were a causal directed acyclic graph (Pearl, 2009).
Taubman et al. (2008) considered what the incidence of coronary heart disease would be if everyone exercised at least 30 minutes per day compared to what it actually was, E[Y]. Note that there are clearly multiple versions of treatment for both A = 1 (“exercising at least 30 minutes per day”) and A = 0 (“exercising less than 30 minutes a day”). With slight abuse of notation (by not beginning the indices of each Ka with 1), we might index K1 by {30, 31, 32…} and K0 by {0, 1, 2, …29}. Taubman et al. (2008) considered two hypothetical intervention regimes g that would ensure “exercising at least 30 minutes per day.” Here we consider a simplified version of the Taubman et al. (2008) example, with a point-treatment rather than time-varying treatment, and provide formal identification results for the two hypothetical intervention regimes that Taubman et al. (2008) discussed. This simplification of the Taubman et al. (2008) example could also be analyzed under the partial compliance framework of Jin and Rubin (2008). Under the first intervention regime, those with A = 1 who in fact exercised at least 30 minutes were allowed to retain their actual number of minutes of exercise K1 and those with A = 0 who in fact exercised less than 30 minutes were, under treatment, assigned version of treatment K1 = 30. The counterfactual quantity of interest was thus E[Y(1, G)] where G = K1 if A = 1 and G = 30 otherwise. Taubman et al. described this as a “threshold intervention.” Since, E{Y(1, G)|A = 1} = E{Y(1, K1)|A = 1} = E(Y|A = 1), to identify E{Y(1, G)} it suffices to identify E{Y(1, G)|A = 0} = E{Y(1, 30)|A = 0}. Identification conditions for this quantity are given in the following result.
Proposition 4
If for some a* ≠ a, and some ka, Yj(a, ka) is well defined for all individuals with Aj = a* and if the no-unmeasured-confounding assumption (8) holds then
The second regime g considered by Taubman et al. again lets individuals with A = 1 who in fact exercised at least 30 minutes retain their actual number of minutes of exercise K1; under the second regime those with A = 0 who in fact exercised less than 30 minutes were, under treatment, randomly assigned a version of treatment K1 from the distribution of those with A = 1 who had the same covariates. Taubman et al. described this second regime as a representative regime. If we now let Gj denote a randomly assigned version, K1, of treatment from the distribution of those with A = 1 with covariates Cj then the quantity E{Y(1, G)|A = 0} is needed to identify the counterfactual incidence of coronary heart disease under the “representative regime.” This quantity is identified by the following result.
Proposition 5
For individuals j with Aj = a* ≠ a, let be a random variable with distribution defined by pr(Ka = ka|A = a, C = Cj). If for all j such that Aj = a*, the potential outcome Yj(a, ka) is well defined for all and if the no-unmeasured-confounding assumption (8) holds then
| (11) |
Note that the quantity E{Y(a, Ga)|A = a*} in Proposition 5 does not necessary reflect what would happen if we were to set the version of treatment to the version it would have been if those with A = a* had in fact been given treatment A = a. The formula in (11) will however identify this latter quantity if, as stated formally in the next proposition, for individuals with Aj = a*, is well defined and if the set of covariates for which control is made contains both C and W in Figure 1 so that in addition to identification assumption (8), identification assumption (10) also holds. Whereas Proposition 5 gave a result for randomly setting version of treatment to the distribution of those with A = a, Proposition 6 gives a result for randomly setting version of treatment to the distribution of those with A = a* had they been given treatment A = a.
Proposition 6
If for all individuals j with Aj = a* ≠ a, the potential outcome is well defined then let be a random variable with distribution defined by pr(Ka(a) = ka|A = a*, C = Cj, W = Wj). If for all j such that Aj = a*, the potential outcome Yj(a, ka) is well defined for all and if the no-unmeasured-confounding assumptions (8) and (10) hold then
Under assumptions (8) and (10) of Proposition 6, ordinary estimators of treatment effects will, for each treatment group, estimate the effect of randomly setting the version to one selected from the distribution of versions of those who were in fact in that treatment group; note this is a somewhat stronger interpretation than that provided in Proposition 3. The analytic formulas in Propositions 5 and 6 bear resemblances to those for so-called “natural direct and indirect effects” (Robins and Greenland, 1992; Pearl, 2001) when these effects are identified. Unlike in the literature on natural direct effects, all of the assumptions made here, namely (8)–(10), would be satisfied if both treatment and version were randomized whereas the identification of natural direct and indirect effects requires counterfactual independence assumptions that may not hold even in a doubly randomized trial (Robins, 2003). The effects are thus much closer to those defined in the direct effects literature by stochastic interventions on the mediator (Didelez et al., 2006; Geneletti, 2007) and Propositions 5 and 6 would in fact follow from those results appropriately replacing the mediator by version.
We define and provide an identification result for one further counterfactual quantity. In health disparities research, health outcomes are compared by strata of race or socioeconomic status. In some cases, access to care or receipt of a treatment or procedure may be equal across strata of racial groups but health outcome disparities may still persist. One possibility is that race itself modifies the effect of treatment. Another possibility is that there may in fact be disparities in the version of the treatment being administered. Thus even if outcome disparities are not explained by disparities in the receipt of treatment, they may be explained by disparities in the version of treatment. Let A denote some treatment; for example, for patients with acute respiratory distress syndrome, A = 1 might denote low-volume ventilation and A = 0 traditional ventilation (Acute Respiratory Distress Syndrome Network, 2000). Let K1 denote the version of treatment A = 1 (e.g. K1 might denote the quality of the monitoring for low-volume ventilation) and let Y denote the health outcome (e.g. 180 day survival). Let S ⊆ C denote one or more covariates of interest; here we will let S denote race. We might, for example, then be interested in how much better outcomes would have been for black individuals (S= 1) who received treatment if they had obtained the same quality of treatment as white individuals (S = 0). If for individuals with S = 1, we let Gj denote a randomly assigned version of treatment K1 from the distribution of those with A = 1, S = 0 and with covariates C\S equal to Cj\Sj then this quantity is given by
The counterfactual quantity E{Y(1, G)|A = 1, S = 1} is identified by the following result.
Proposition 7
Let S ⊆ C and let be a random variable with distribution defined by pr(Ka = ka|A = a, S = s′, C\S = Cj\Sj). If for all j such that Aj = a and S = s, the potential outcome Yj(a, ka) is well defined for all and if the no-unmeasured-confounding assumption (8) holds then
Note that the hypothesis that different versions of treatment were given to groups S = 1 and S = 0, not because of discrimination, but because differing versions of treatment, ka and k′a, have differing effects across strata of S could be examined by contrasting E{Y(a, ka)|c, S = 1}− E{Y(a, k′a)|c, S = 1} and E{Y(a, ka)|c, S = 0} − E{Y(a, k′a)|c, S = 0} which would be identified under assumption (8).
5. When Version Precedes Treatment and Consequences of Coarsening the Treatment Variable
All the material thus far considered a setting where treatment is set first and then the version of treatment; in this section we consider the reverse scenario, wherein the version precedes the treatment variable used in the analysis. For example, a researcher may be interested in assessing the effect on five-year all-cause mortality of having low density lipoprotein (LDL) cholesterol of 140 versus 100. In this case, there is no clear unambiguous intervention to fix LDL cholesterol to 140 or 100. Setting LDL cholesterol to a particular level could potentially be accomplished by a dietary intervention or by cholesterol medications or by changes in exercise, but each of these may have different implications for mortality outcomes. The treatment variable itself is poorly defined for the purposes of thinking about causal effects. We might conceive of a broad range of potential interventions to fix LDL cholesterol to 140 or 100 or to some other level. In this case the version (the different ways of setting LDL cholesterol) precedes the treatment variable (the actual level of LDL cholesterol itself).
As another example of when version precedes treatment, we will consider the case of the dichotomization or coarsening of an underlying treatment variable. Often researchers will coarsen, categorize or dichotomize a continuous exposure to simplify an analysis. For example, if the continuous exposure is the number of minutes of exercise, a researcher may form a new dichotomous “treatment” variable defined by exercising at least 30 minutes. One might then speak of different “versions” of the treatment “exercise at least 30 minutes” e.g. exercise 30 minutes, exercise 31 minutes, etc. In the analysis for multiple versions of treatment given above we have presupposed that the causal order of the variables was treatment then version. However, when a continuous exposure has been dichotomized an alternative conceptualization might be that the version precedes treatment.
Suppose then that there is some underlying version of treatment variable K but that the analyst has data on a coarsened variable A where each value of A corresponds to one or more values of K (i.e. the mapping from K to A is a many-to-one map). Let Y be the outcome and Y(k) be the potential outcome for an individual if K had been k. Suppose that in an observational study for a set of covariates L we had Y(k) II K|L i.e. no confounding of the effect of K on Y conditional on covariates L. Suppose also the consistency assumption held such that Y(k) = Y when K = k. An analyst who had used the treatment A might then compute the “causal effect” comparing treatment levels A = a and A = a* by calculating Σl E(Y|A = a, l)pr(l) − Σl E(Y|A = a*, l)pr(l). The following result re-expresses this quantity in terms of interventions on K. We state the result and then consider its interpretation.
Proposition 8
If Y(k) II K|L then
This latter expression can itself be interpreted as a comparison in a randomized trial in which, within strata of covariates L = l, one arm is randomly assigned a “version of treatment” K from the observed distribution of K in the population amongst with A = a and L = l (e.g. if K is minutes exercised, and A = a corresponds to K ≥ 30, then this would be the distribution of minutes of exercise amongst those with L = l who exercise at least 30 minutes) and the other arm is randomly assigned a “version of treatment” K from the observed distribution of K in the population amongst with A = a* and L = l (e.g. the distribution of minutes of exercise amongst those with L = l who exercise less than 30 minutes).
Note that nothing in the analysis above required that K itself be continuous; the variable K might indicate a complex set of potential interventions which are coarsened in treatment variable A by partitioning the support of K. The ordinary estimator for the causal effect adjusting for L, namely, Σl E(Y|A = a, l)pr(l) − Σl E(Y|A = a*, l)pr(l), thus, even in the presence of multiple versions of treatment (and even when the treatment variable A does not unambiguously correspond to a single intervention) has the interpretation of a causal effect comparing two randomized interventions.
We note that in some cases there may be ambiguity as to whether “treatment” precedes “version” as in the previous section or whether “version” precedes “treatment” as in this section. In the exercise example, it is perhaps not unreasonable to argue that the number of minutes of exercise is constituted by a number of decisions (whether to exercise more than 5 minutes, whether to exercise more than 10 minutes, etc.). One of these decisions, namely whether to exercise more than 30 minutes, could be taken as A; once this is determined then there is still the question of which version of A = 1 (how many minutes above 30) or A = 0 (how many minutes below 30) is selected; this was how the issue was conceptualized in the previous section. Interestingly, however, under either conceptualization, one obtains the same empirical formula for the estimate of the overall treatment effect: replacing L with {C, W} in Proposition 8 gives the same empirical formula as in Proposition 3.
In other cases, there is arguably less ambiguity concerning the ordering of version and treatment. If, for example, the exposure A = 1 is experiencing high levels of loneliness and A = 0 experiencing low loneliness, then there are numerous decisions or interventions that may lead to high loneliness and it is difficult to conceive of these as following rather than preceding the high level of loneliness itself. In such cases the approach of this section will be of interest. In the next section we illustrate this approach with an empirical data analysis illustration.
Several important points merit attention, however. First, the ordinary estimate only merits the interpretation of the effect comparing two randomized interventions if we have adequately controlled for confounding for the underlying version of treatment variable K. Second, even if we can potentially interpret the effect of treatment in this manner, an intervention corresponding to the treatment effect we are supposedly estimating will often not be possible to realistically implement in practice. Finally, if we do not know what the underlying version of treatment K is, it may be difficult to assess whether we have indeed controlled for all relevant confounders. We have elsewhere discussed these points in greater detail along with their implications for epidemiologic research (Hernán and VanderWeele, 2011).
6. Illustration
We illustrate some of the prior discussion with an example in which an exposure has been dichotomized. Loneliness (measured on the UCLA-R scale from 20 to 80) has been shown to prospectively predict depressive symptoms (measured on the CES-D scale from 0 to 60) even after control is made for baseline depressive symptoms and other covariates (Cacioppo et al., 2006). Longitudinal data available on loneliness and depressive symptoms in the Chicago Health, Aging, and Social Relations Study of 229 older adults; this data also include as covariates: age, gender, ethnicity, marital status, education, psychiatric conditions and psychiatric medications. Suppose a researcher were to median dichotomize measured at loneliness at follow-up 1 so as to define A = 1 when loneliness is greater than 35. A regression of depressive symptoms at follow-up 2 on dichotomized loneliness at follow-up 1, along with baseline loneliness, baseline depressive symptoms and baseline covariates gives an estimate of 2.44 (95% CI: 0.03,4.85) for dichotomized loneliness at follow-up 1. If we thought that baseline covariates (including baseline loneliness and depressive symptoms) were sufficient to control for confounding of the effect of loneliness at follow-up 1 on depressive symptoms at follow-up 2 then we could interpret this as an estimate of an intervention trial that, conditional on covariates, assigned each individual in one arm to a “version of treatment” of loneliness > 35 randomly drawn from the distribution of “versions of treatment” in the population of those with loneliness > 35 and assigned each individual in the other arm to a “version of treatment” of loneliness ≤ 35 randomly drawn from the distribution of “versions of treatment” in the population of those with loneliness ≤ 35. Note that the estimate only has this interpretation under the strong assumption of no unmeasured confounding, which may not be realistic here.
7. Discussion
In this article we have described how the potential outcomes framework can be extended to allow for multiple versions of treatment, which are present to varying degrees in both observational studies and in randomized clinical trials. In clinical trials, guidelines are often given to reduce the number or relevance of versions but it is generally not possible to eliminate this problem entirely (Hernán and VanderWeele, 2011). Fortunately, as we have seen, even with multiple versions of treatment, it is possible to use the ordinary estimators for causal effects (outcome differences between treatment groups standardized by confounders) to compare the effects of treatments on average under no-unmeasured-confounding assumptions. Ordinary estimators can be interpreted as the causal effects of well-defined interventions that mimic the assignment of versions of treatment in the study population. For such an interpretation in an observational study control must, however, in general be made for common causes of treatment and version; in a randomized trial there will be no such common causes. Although the ordinary estimators have an interpretation as an overall causal effect, multiple versions of treatment still renders ambiguous statements such as “treatment is on average better than control” since these statements will always be with reference to the current policies for assigning versions. Even if treatment is better on average than control under current policies for assigning versions, it is nevertheless possible that certain versions of control, if administered to an entire population, would be better than administering certain versions (or even all versions) of treatment. This could arise if the most effective version of the control were generally infrequently assigned. Analysis of the effects of a specific version of treatment (Proposition 1) or of varying regimes and policies (as considered in section 4) can be useful in assessing this possibility. We have also seen how ignoring multiple versions of treatment can lead to problems with bias and interpretation. In the setting of treatment-then-version, ignoring multiple versions of treatment and potential treatment-version confounding can result in biased estimates of the overall treatment effect. In the version-then-treatment setting, if multiple versions of treatment are ignored, similar problems can arise with regard to bias due to inadequate control for confounding. Moreover, in this case, even if adequate control for confounding is made, the interpretation of the effect estimates are, as noted above, considerably more subtle than is the case with only a single version of treatment.
The contributions in this paper have attempted to extend the potential outcomes framework to allow for multiple versions of treatment. Recent work in causal inference has also attempted to extend the standard potential outcomes notation to accommodate possible interference between units (Sobel, 2006; Hong and Raudenbush, 2006; Rosenbaum, 2007; Hudgens and Halloran, 2008; VanderWeele, 2010; VanderWeele and Tchetgen Tchetgen, 2011a b; Halloran and Hudgens, 2012; Tchetgen Tchetgen and VanderWeele, 2012). We would like to conclude this paper by drawing some parallels between the existing work on interference and our discussion above concerning multiple versions of treatment. First, both the no-interference assumption and the no-multiple-versions-of-treatment assumption are concealed by the notation Yj(a); these two assumptions are often not stated explicitly but are implicitly assumed to hold when using potential outcomes notation such as Yj(a); such notation is in general only justified under the assumptions of no-interference and no-multiple-versions-of-treatment. Second, with both the no-interference assumption and the no-multiple-versions-of-treatment assumption, although the traditional potential outcomes framework presupposes these assumptions, the framework and notation can in fact be extended so as to allow for potential violations; in the case of interference, the potential outcomes notation can be extended so as to allow the potential outcome of one individual to depend on the treatments received by other individuals; in the case of multiple versions of treatment, the notation can be expanded so that an individual may have different potential outcomes for each possible version of treatment. Third, in certain settings, violations of the no-interference assumption or the no-multiple-versions-of-treatment assumption can be ignored; Rosenbaum (2007) showed that the no-interference assumption could be ignored in certain randomized experiments; in our discussion above we have seen that if covariates are available to adjust not just for treatment-outcome confounding but also for “treatment-version confounding” then the multiple versions of treatment can be ignored in the estimation of average causal effects (it is not necessary to have data on which individuals received which version). Fourth, with both the no-interference assumption and the no-multiple-versions-of-treatment assumption, once notation has been introduced to expand the potential outcomes framework in order to accommodate violations, then this new notation can give rise to new questions of theoretical and substantive interest; notation accommodating interference gives rise to questions of the identification and estimation of spillover effects; notation accommodating multiple versions of treatment gives rise to questions about hypothetical interventions on the version of treatment to address policy relevant questions about resource allocation and assignment.
The extension of the potential outcomes framework to address interference and spillover effects has been of use in a variety of substantive contexts (Hong and Raudenbush, 2006; Sobel, 2006; Hudgens and Halloran, 2008; VanderWeele and Tchetgen Tchetgen, 2011a). We hope that the contributions in this article will similarly clarify and extend the possibilities for causal inference when multiple versions of treatment are present.
Appendix
Proofs of Propositions 1–8
Proof of Proposition 1
For any a ∈
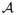 , ka ∈
, ka ∈
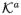 we have that
we have that
Proof of Proposition 2
We have that
Note that if there is only one version of treatment for the control condition, A = 0, then
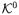 = {1} and the only potential outcome for each individual under the control condition is
. We have that
= {1} and the only potential outcome for each individual under the control condition is
. We have that
where the final equality holds because when A = 0 we have that K0 = 1 since there is only one version of treatment.
Proof of Proposition 3
For any a ∈
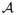 , we have that
, we have that
This completes the proof.
Proof of Proposition 4
We have that
Proof of Proposition 5
We have that
Proof of Proposition 6
We have that
Proof of Proposition 7
We have that
Proof of Proposition 8
If Y(k) II K|L then
where the first equality follows by iterated expectations, the second because K contains all the information in A, the third from consistency and the fourth because Y(k) II K|L.
Example of Treatment-Version Confounding
Let A = 1 denote surgery and A = 0 denote the control (no surgery). Suppose there is only one version of A = 0 (no surgery) but two versions of surgery: surgeon 1 (k1 = 1) and surgeon 2 (k1 = 2). Suppose that there are no confounders C that affect both the outcome Y and either treatment or version but that there is a binary treatment-version confounder W with W = 0 and W = 1 indicating two different health plans. Suppose P(W = 1) = 0.5; P(A = 1|W = 0) = 0.2; P(A = 1|W = 1) = 0.6 so that by Bayes’ Theorem, P(W = 1|A = 1) = 3/4 and P(W = 1|A = 0) = 1/3. Suppose also P(K1 = 1|A = 1, W = w) = (420 + 300W)/1000. Finally, suppose P(Y = 1|A = 0, W = w) = 1/2 and P(Y = 1|A = 1, K1 = k1, W = w) = 1/2 + k1/5. Note that W affects both A and K1 but W has no effect on Y except through A and K1.
Now if the entire population were given surgery (A = 1) then the proportion of the population with K1 = 1 would be {420 + 300 E(W)}/1000 = 0.57 and the proportion with K1 = 2 would thus be 0.43. The proportion with Y = 1 would be 1/2 + E(K1)/5 = 1/2 + (0.57 + 2*0.43)/5 = 0.786. If the entire population were not given surgery (A = 0) the proportion with Y = 1 would be 0.5. The true overall average causal effect of surgery in this population is thus 0.786 − 0.5 = 0.286.
Suppose now that no information is available on version of treatment. Suppose that we did not control for W and simply computed E(Y|A = 1) − E(Y|A = 0). We would obtain:
We would get a biased estimate of the overall average causal effect of surgery. If we did control for W and computed Σw{E(Y|A = 1, w) − E(Y|A = 0, w)}pr(w) we would obtain:
We get a correct estimate of the overall average causal effect of surgery if we control for the treatment-version confounder W but a biased estimate if we do not control for it.
Analogous Results Under Time-Dependent Confounding
Suppose now that there is an effect, Q, of treatment A that affects both version K and the outcome Y as in the Appendix Figure. We will continue to let W denote a set of variables that affects only treatment A and version K.
We replace assumption (8) with
| (A1) |
| (A2) |
Under these two assumptions, the effect of the version of treatment remains identified but data must be available on both version Ka and on the time-dependent confounder Q as stated in the following result which provides the analogue to Proposition 1 under time-dependent confounding. The proof is somewhat analogous to that for “controlled direct effects” in the context of mediation with a time-dependent confounder.
Proposition 9
Under assumptions (A1) and (A2),
Proof
For any a ∈
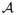 , ka ∈
, ka ∈
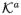 we have that
we have that
Even in the presence of time-dependent confounding as in the Appendix Figure, assumption (9) in the text that Y(a) II A|{C, W} for all a will still hold and the overall causal effect will be identified by the proof of Proposition 3 given above. As before, data on version of treatment is thus not necessary to estimate overall treatment effects. Throughout the paper and in the Appendix we have, however, assumed point treatment. If treatment is time-varying then the version of treatment may serve as a confounder for the effect of subsequent treatment and data would then be needed on the version of treatment for the purposes of confounding control. The development of a formal analytic framework for this setting is left to future research.
In the text, Propositions 5 and 6 considered the effects on those with A = a* of intervening to set A = a with version randomly set to the distribution of those with A = a (Proposition 5) or to the distribution of those with A = a* had they been given treatment A = a. Propositions 10 and 11 give analogous results under time-dependent confounding. Proposition 10 requires assumptions (A1) and (A2). Proposition 11 requires assumptions (A1) and (A2) along with assumption (10) in the text that Ka(a) II A|{C, W} for all a.
Proposition 10
For individuals j with Aj = a* ≠ a, let be a random variable with distribution defined by pr(Ka = ka|A = a, C = Cj). If for all j such that Aj = a*, the potential outcome Yj(a, ka) is well defined for all and assumptions (A1) and (A2) hold then
Proof
We have that
Proposition 11
If for all individuals j with Aj = a* ≠ a, the potential outcome is well defined then let be a random variable with distribution defined by pr(Ka(a) = ka|A = a*, C = Cj, W = Wj). If for all j such that Aj = a*, the potential outcome Yj(a, ka) is well defined for all and if assumptions (A1), (A2) and (10) hold then
Proof
We have that
Appendix Figure.
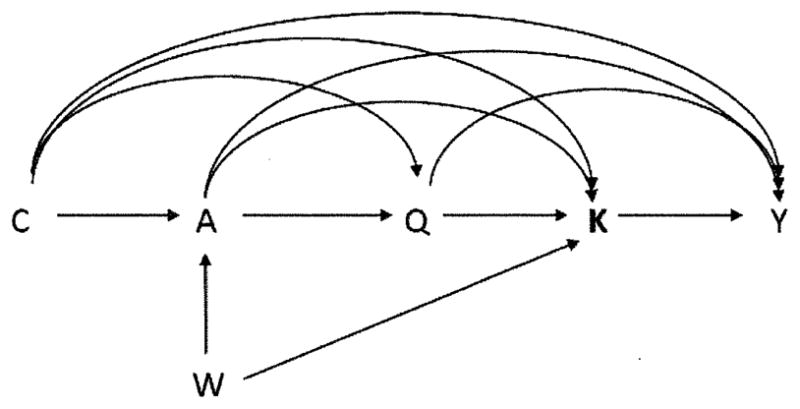
Time-dependent confounding in which an effect, Q, of treatment A, may affect both version K and outcome Y.
Contributor Information
Tyler J. VanderWeele, Email: tvanderw@hsph.harvard.edu.
Miguel A. Hernán, Email: miguel_hernan@post.harvard.edu.
References
- Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. New England Journal of Medicine. 2000;342:1301–8. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- Cacioppo J, Hughes M, Waite L, Hawkley L, Thisted R. Loneliness as a specific risk-factor for depressive symptoms: Cross-sectional and longitudinal analyses. Psychology and Aging. 2006;21:140–151. doi: 10.1037/0882-7974.21.1.140. [DOI] [PubMed] [Google Scholar]
- Cole SR, Frangakis CE. The consistency assumption in causal inference: a definition or an assumption? Epidemiology. 2009;20:3–5. doi: 10.1097/EDE.0b013e31818ef366. [DOI] [PubMed] [Google Scholar]
- Cox DR. Planning of Experiments. New York: John Wiley & Sons; 1958. [Google Scholar]
- Didelez V, Dawid AP, Geneletti S. Direct and indirect effects of sequential treatments. In Proceedings of the Twenty-Second Conference on Uncertainty in Artificial Intelligence.2006. [Google Scholar]
- Geneletti S. Identifying direct and indirect effects in a non-counterfactual framework. Journal of the Royal Statistical Soceity, Series B. 2007;69:199–216. [Google Scholar]
- Greenland S. Interpretation and choice of effect measures in epidemiologic analyses. Am J Epidemiol. 1987;125:761–8. doi: 10.1093/oxfordjournals.aje.a114593. [DOI] [PubMed] [Google Scholar]
- Halloran ME, Hudgens MG. Causal inference for vaccine effects on infectiousness. International Journal of Biostatistics. 2012;8(2):Article 6. doi: 10.2202/1557-4679.1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernán MA, VanderWeele TJ. Compound treatments and transportability of causal inference. Epidemiology. 2011;22:368–377. doi: 10.1097/EDE.0b013e3182109296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong G, Raudenbush SW. Evaluating kindergarten retention policy: A case study of causal inference for multilevel observational data. Journal of the American Statistical Association. 2006;101:901–910. [Google Scholar]
- Hudgens MG, Halloran ME. Towards causal inference with interference. Journal of the American Statistical Association. 2008;103:832–842. doi: 10.1198/016214508000000292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Rubin DB. Principal stratification for causal inference with extended partial compliance. Journal of the American Statistical Association. 2008;103:101–111. [Google Scholar]
- Neyman J. Sur les applications de la thar des probabilities aux experiences Agaricales: Essay des principle. Excerpts reprinted (1990) in English. In: Dabrowska D, Speed T, translators. Statistical Science. Vol. 5. 1923. pp. 463–472. [Google Scholar]
- Neyman J. Statistical problems in agricultural experimentation. Journal of the Royal Statistical Society, II. 1935;2:107–154. [Google Scholar]
- Pearl J. Direct and indirect effects. Proceedings of the Seventeenth Conference on Uncertainty and Artificial Intelligence; San Francisco: Morgan Kaufmann; 2001. pp. 411–420. [Google Scholar]
- Pearl J. Causality: Models, Reasoning, and Inference. Cambridge: Cambridge University Press; 2009. [Google Scholar]
- Pearl J. On the consistency rule in causal inference: an axiom, definition, assumption, or a theorem? Epidemiology. 2010;21:872–875. doi: 10.1097/EDE.0b013e3181f5d3fd. [DOI] [PubMed] [Google Scholar]
- Pearl J, Robins JM. Probabilistic evaluation of sequential plans from causal models with hidden variables. Proceedings of the Eleventh Conference on Uncertainty in Artificial Intelligence; 1995. pp. 444–453. [Google Scholar]
- Petersen ML. Compound treatments, transportability, and the structural causal model: the power and simplicity of causal graphs. Epidemiology. 2011;22:378–381. doi: 10.1097/EDE.0b013e3182126127. [DOI] [PubMed] [Google Scholar]
- Robins JM. A new approach to causal inference in mortality studies with sustained exposure period - application to control of the healthy worker survivor effect. Mathematical Modelling. 1986;7:1393–1512. [Google Scholar]
- Robins JM. Semantics of causal DAG models and the identification of direct and indirect effects. In: Green P, Hjort NL, Richardson S, editors. In Highly Structured Stochastic Systems. Oxford University Press; New York: 2003. pp. 70–81. [Google Scholar]
- Robins JM, Greenland S. The probability of causation under a stochastic model for individual risk. Biometrics. 1989;45:1125–38. [PubMed] [Google Scholar]
- Robins JM, Greenland S. Comment on: “Causal inference without counterfactuals” by A. P. Dawid. Journal of the American Statistical Association. 2000;95:477–82. [Google Scholar]
- Rosenbaum PR. Interference between units in randomized experiments. Journal of the American Statistical Association. 2007;102:191–200. doi: 10.1080/01621459.2012.655954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum PR, Rubin DB. Assessing sensitivity to an unobserved binary covariate in an observational study with binary outcome. Journal of the Royal Statistical Society, Series B. 1983;45:212–218. [Google Scholar]
- Rubin DB. Estimating causal effects of treatments in randomized and nonrandomized studies. Journal of Educational Psychology. 1974;66:688–701. [Google Scholar]
- Rubin DB. Comment on: “Randomization analysis of experimental data in the fisher randomization test” by D. Basu. Journal of the American Statistical Association. 1980;75:591–593. [Google Scholar]
- Rubin DB. Which ifs have causal answers? Comment on: “Statistics and causal inference” by P. Holland. Journal of the American Statistical Association. 1986;81:961–962. [Google Scholar]
- Rubin DB. Formal modes of statistical inference for causal effects. Journal of Statistical Planning and Inference. 1990;25:279–292. [Google Scholar]
- Schafer JL, Kang J. Causal modeling when the treatment is a latent class. Abstract. Joint Statistical Meetings; 2009.2009. [Google Scholar]
- Sobel ME. What do randomized studies of housing mobility demonstrate?: Causal inference in the face of interference. Journal of the American Statistical Association. 2006;101:1398–1407. [Google Scholar]
- Taubman SL, Robins JM, Mittleman MA, Hernán MA. Alternative approaches to estimating the effects of hypothetical interventions. Proceedings of the 2008 Joint Statistical Meetings; Alexandria, VA. 2008. [Google Scholar]
- Tchetgen Tchetgen EJ, VanderWeele TJ. On causal inference in the presence of interference. Statistical Methods in Medical Research – Special Issue on Causal Inference. 2012;21:55–75. doi: 10.1177/0962280210386779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderWeele TJ. Further remarks concerning the consistency assumption. Epidemiology. 2009;20:880–883. doi: 10.1097/EDE.0b013e3181bd5638. [DOI] [PubMed] [Google Scholar]
- VanderWeele TJ. Bias formulas for sensitivity analysis for direct and indirect effects. Epidemiology. 2010;21:540–551. doi: 10.1097/EDE.0b013e3181df191c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderWeele TJ. Direct and indirect effects for neighborhood-based clustered and longitudinal data. Sociological Methods and Research. 2010;38:515–544. doi: 10.1177/0049124110366236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderWeele TJ, Arah OA. Bias formulas for sensitivity analysis of unmeasured confounding for general outcomes, treatments and confounders. Epidemiology. 2011;22:42–52. doi: 10.1097/EDE.0b013e3181f74493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderWeele TJ, Tchetgen Tchetgen EJ. Bounding the infectiousness effect in vaccine trials. Epidemiology. 2011;22:686–693. doi: 10.1097/EDE.0b013e31822708d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanderWeele TJ, Tchetgen Tchetgen EJ. Effect partitioning under interference for two-stage randomized vaccine trials. Statistics and Probability Letters. 2011;81:861–869. doi: 10.1016/j.spl.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]


