Abstract
BACKGROUND:
Motile cilia dysfunction causes primary ciliary dyskinesia (PCD), situs inversus totalis (SI), and a spectrum of laterality defects, yet the prevalence of laterality defects other than SI in PCD has not been prospectively studied.
METHODS:
In this prospective study, participants with suspected PCD were referred to our multisite consortium. We measured nasal nitric oxide (nNO) level, examined cilia with electron microscopy, and analyzed PCD-causing gene mutations. Situs was classified as (1) situs solitus (SS), (2) SI, or (3) situs ambiguus (SA), including heterotaxy. Participants with hallmark electron microscopic defects, biallelic gene mutations, or both were considered to have classic PCD.
RESULTS:
Of 767 participants (median age, 8.1 years, range, 0.1-58 years), classic PCD was defined in 305, including 143 (46.9%), 125 (41.0%), and 37 (12.1%) with SS, SI, and SA, respectively. A spectrum of laterality defects was identified with classic PCD, including 2.6% and 2.3% with SA plus complex or simple cardiac defects, respectively; 4.6% with SA but no cardiac defect; and 2.6% with an isolated possible laterality defect. Participants with SA and classic PCD had a higher prevalence of PCD-associated respiratory symptoms vs SA control participants (year-round wet cough, P < .001; year-round nasal congestion, P = .015; neonatal respiratory distress, P = .009; digital clubbing, P = .021) and lower nNO levels (median, 12 nL/min vs 252 nL/min; P < .001).
CONCLUSIONS:
At least 12.1% of patients with classic PCD have SA and laterality defects ranging from classic heterotaxy to subtle laterality defects. Specific clinical features of PCD and low nNO levels help to identify PCD in patients with laterality defects.
TRIAL REGISTRY:
ClinicalTrials.gov; No.: NCT00323167; URL: www.clinicaltrials.gov
Primary ciliary dyskinesia (PCD) is a rare autosomal recessive disease with an estimated prevalence of one in 16,000.1,2 Patients with PCD display neonatal respiratory distress, chronic sinopulmonary disease, bronchiectasis, recurrent otitis media, and infertility.3 Situs inversus totalis (SI), or mirror-image organ arrangement, occurs in slightly < 50% of patients with PCD; however, a subset of patients with PCD have organ laterality defects that do not meet a classic definition of SI. Instead, findings in this subset of patients correspond to an intermediate classification defined as situs ambiguus (SA) with a spectrum of organ laterality defects. Classification of these defects is complicated because different specialists tend to use different definitions for SA and heterotaxy.4‐8 Some clinicians interchange the terms “situs ambiguus” and “heterotaxy”; however, cardiologists define heterotaxy as a subset of SA with specific congenital heart defects (eg, common atrium, levo-transposition of great arteries). Geneticists have expanded the cardiology definition to include combinations of abdominal defects (eg, asplenia, polysplenia) or vascular defects (eg, interrupted inferior vena cava). Despite this confusion, previous studies of heterotaxy reported a prevalence of one in 10,000, with multimodal forms of inheritance.9,10 We define in this article any laterality defect other than SI as SA and only those with SA combined with complex heart lesions as heterotaxy.
Studies have demonstrated that cilia dysfunction in embryonic nodal plate cells results in laterality defects and congenital heart disease.11,12 In addition, a link between embryonic cilia dysfunction and respiratory cilia dysfunction in mice demonstrates that mutations in Dnai2 and Dnahc5 (homologs to DNAI2 and DNAH5 in humans) lead to heterotaxy and PCD with outer dynein arm (ODA) defects.13,14 Kennedy et al15 demonstrated retrospectively that SA anomalies and heterotaxy are present in at least 6.3% of an international PCD population. Most patients with SA, particularly those with congenital heart disease, have complicated medical courses, including neonatal respiratory distress, cyanosis, and pneumonia. These respiratory manifestations are commonly attributed to a cardiac origin without consideration of possible respiratory cilia dysfunction.16,17 The present prospective study examined the prevalence of laterality defects other than SI in a PCD population and determined which clinical and diagnostic characteristics suggest PCD in a population with complex situs issues.
Materials and Methods
Through our multicenter Genetic Diseases of Mucociliary Clearance Consortium, symptomatic adults and children were referred for previously diagnosed or suspected PCD. Medical records were reviewed and evaluations conducted at one of seven sites in California, Colorado, Maryland, Missouri, North Carolina, Washington, and Ontario, Canada, between May 2006 and September 2012.
Initial situs status was determined by physicians at local consortium sites through review of radiology, surgery, and cardiology reports and radiology images from participant medical records. Additionally, standardized questions to detect situs anomalies were asked of each participant. One consortium physician (A. J. S.) reviewed each potential SA case in a nonblinded fashion, verifying the situs lesions through source documentation before assigning the ultimate situs designation. Participants were grouped into one of the following situs categories: (1) situs solitus (SS); (2) SI; or (3) SA, which includes heterotaxy (Fig 1). Next, we used the congenital heart disease classification schema developed by Botto et al18 to assign participants with SA to one of the following SA subgroups: (1) SA with complex cardiovascular malformation (heterotaxy to most cardiologists), including cardiac isomerism or any cardiovascular defect or groups of defects that do not fit into standard cardiac phenotype classification or associations; (2) SA with simple cardiovascular malformation, including cardiovascular malformations within standard cardiac phenotype classification or associations; (3) SA without cardiac malformation, including vascular, abdominal, or laterality defects but normal cardiac status; and (4) isolated possible laterality defect consisting of one solitary defect, possibly related to issues of laterality, in either cardiac or noncardiac systems. Laterality defects included in each group are detailed in Table 1.
Figure 1 –
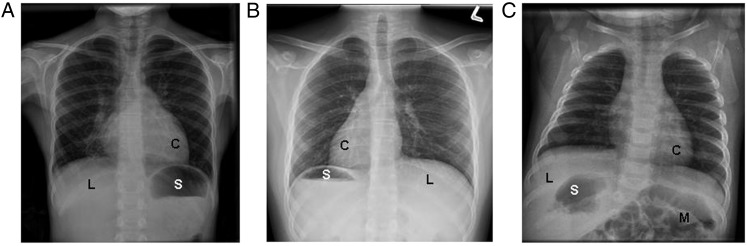
Examples of laterality defects on radiology imaging in various situs groups in the study population. A, A participant with situs solitus, or normal organ arrangement, with left cardiac apex, left-sided stomach bubble, and right-sided liver. B, A patient with situs inversus totalis, or mirror-image organ arrangement, with right-sided cardiac apex, right-sided stomach bubble, and left-sided liver. C, A patient with situs ambiguus with left-sided cardiac apex, right-sided stomach bubble, right-sided liver, and intestinal malrotation who also has right-sided polysplenia visualized on CT scan. C = cardiac apex; L = liver; M = intestinal malrotation; S = stomach.
TABLE 1 ] .
Laterality Defects Included in SA Subgroups
| SA + Complex CVM (Heterotaxy) | SA + Simple CVM | SA Without Cardiac | Isolated Possible Laterality Defect |
| SA plus any of the following cardiac issues: • Cardiac isomerism • Hypoplastic ventricle with other cardiac lesions (except ASD or APVR alone) • l-TGA + LVOTO (PS) • Any defects that do not fit into a standard cardiac phenotype classification or standard associations |
SA plus any of the following standard cardiac phenotypes or associations: • Dextrocardia • Mesocardia • ASD • VSD • AVSD • Common atrium • Tetralogy of Fallot • Pulmonary stenosis or atresia • Overriding aorta • DORV • Ebstein anomaly • Tricuspid atresia • l-TGA • Truncus defect • HLHS + VSD/APVR |
SA including any of the following defects: Vascular • APVR • Right aortic arch • Bilateral or left superior vena cava • Interrupted or duplicated inferior vena cava Abdomen • Asplenia or polysplenia • Intestinal malrotation • Midline liver • Left-sided liver • Right-sided spleen or stomach Pulmonary • Left- or right-side pulmonary isomerism |
Any solitary lesion that may be associated with laterality defects, including cardiac or noncardiac lesions |
Classification criteria used from Botto et al.18 APVR = anomalous pulmonary venous return; ASD = atrial septal defect; AVSD = atrioventricular septal defect; CVM = cardiovascular malformation; DORV = double-outlet right ventricle; HLHS = hypoplastic left heart syndrome; l-TGA = levo-transposition of the great arteries; LVOTO = left ventricular outflow tract obstruction; PS = pulmonary stenosis; SA = situs ambiguus; VSD = ventricular septal defect.
Nasal nitric oxide (nNO) level was measured in participants with either a CLD 88 series (Eco Medics AG), a NIOX Flex (Aerocrine), or a Sievers NOA 280i (General Electric Company) chemiluminescence analyzer, using standard operating procedures. In participants who could cooperate with standard velum closure maneuvers (generally aged > 5 years), values were obtained during a 5-s plateau while exhaling through a resistor.19,20 Reported values are the mean of three maneuvers each in the left- and right-side naris. Otherwise, nNO values were obtained during tidal breathing, using the mean of the five highest tidal peaks from each naris.21 Measured nNO concentration was multiplied by sampling flow to determine nNO production in nanoliters per minute. For velum closure nNO and tidal breathing nNO, values < 77 nL/min20,22,23 and < 40 nL/min,21,24 respectively, were considered low and within PCD range. Velum closure nNO measurements were also collected from 77 healthy pediatric control participants.
Nasal ciliary biopsy specimens were processed for ultrastructural analysis through transmission electron microscopy (Zeiss EM900; Carl Zeiss AG). Photomicrographs were reviewed in a blinded fashion by three independent observers. Each reviewer separately determined whether hallmark disease-causing defects were present in the ODAs, inner dynein arms (IDAs), or central apparatus (CA).3,25,26 Genetic testing for known mutations in PCD-causing genes was performed in cases with hallmark electron microscopic (EM) defects and strong clinical phenotypes. We tested DNAI1 and DNAH5 for patients with ODA defects; DNAAF2, DYX1C1, HEATR2, and LRRC6 for patients with ODA plus IDA defects; CCDC39 and CCDC40 for patients with IDA plus CA and microtubule disorganization defects; DNAH11 for patients with normal ciliary ultrastructure; and all of these genes in patients where ciliary ultrastructure was not available. Both EM and genetic testing were performed in centralized laboratories at the University of North Carolina using standard operating procedures.
Participants with biallelic mutations in PCD-causing genes, hallmark EM defects, or both were given a diagnosis of classic PCD. All remaining participants were labeled as without classic PCD and grouped according to normal or low nNO levels. Those with normal nNO levels above our cutoff formed the SA control group. Final data analyses were limited to participants aged > 1 year in whom nNO measurements were more reliable and adequate clinical symptom history had been established. Continuous data and categorical data were analyzed using Wilcoxon rank sum and χ2 or Fisher exact tests, respectively. Multiple group comparisons were performed using Kruskal-Wallis test. Statistical significance was defined as P < .05 for two-way comparisons and as P ≤ .03 with Bonferroni correction for post hoc comparisons of multiple groups against classic PCD. Institutional review boards at the participating institutions approved this protocol (e-Appendix 1 (247.4KB, pdf) ).
Results
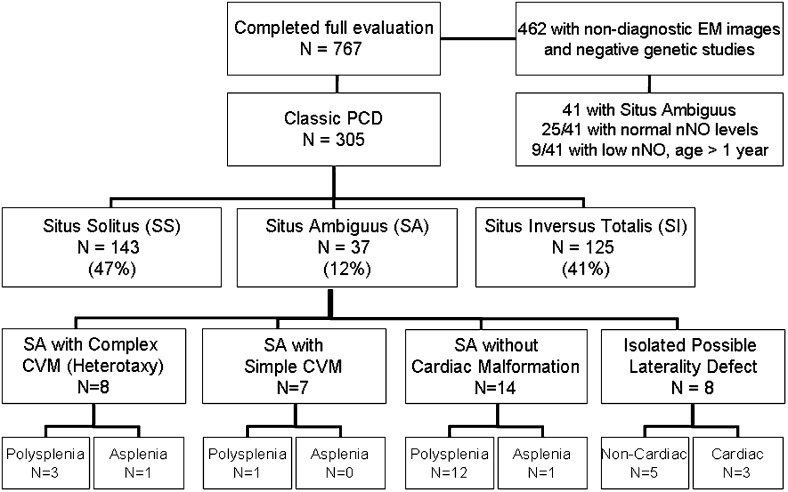
Overall, 305 of 767 participants (39.8%) were given a diagnosis of classic PCD, including 37 (12.1%) with SA and classic PCD. This group with SA and classic PCD included eight with SA plus complex cardiovascular malformation, seven with SA plus simple cardiovascular malformation, 14 with SA without cardiac malformation, and eight with a possible isolated laterality defect (Fig 2, e-Table 1 (247.4KB, pdf) ). SA was identified in 41 of 462 participants without classic PCD. Twenty-five of these participants had normal nNO levels above our cutoff and formed the SA control group. Nine participants with SA without classic PCD had low nNO levels under our cutoff, and seven others were not included in the final data analysis because they were aged < 1 year. The median age of participants with SA and classic PCD was significantly greater than that of participants in the SA control group (Table 2). No specific organ laterality defects were more prevalent in classic PCD; however, dextro-transposition of the great arteries (d-TGA) was more prevalent in SA without classic PCD than in classic PCD. Asplenia and atrioventricular septal defect were more prevalent in SA without classic PCD and nNO levels below our cutoff than in classic PCD (Table 2). Nine participants with SA and classic PCD also had siblings enrolled in the protocol, but none of the siblings had SA.
Figure 2 –
Distribution of participants across laterality defect groups and situs ambiguus subgroups. CVM = cardiovascular malformation, EM = electron microscopic; nNO = nasal nitric oxide; PCD = primary ciliary dyskinesia.
TABLE 2 ] .
Demographics of Participants With SA Aged > 1 Year
| Demographic or Laterality Defect | Classic PCD (n = 35) | Not Classic PCD, nNO Above Cutoffa (SA Control Group) (n = 25) | Not Classic PCD, nNO Below Cutoffa (n = 9) | P Valueb |
| Age, y | 12.4 (1.8-58) | 5.6 (1.2-39) | 8.0 (6.5-26) | .003c |
| Male sex | 34 | 56 | 56 | .20 |
| White race | 80 | 83 | 78 | .92 |
| Chest radiograph for review | 100 | 92 | 100 | .17 |
| CT/MRI scan chest for review | 91 | 72 | 89 | .13 |
| Abdominal CT or ultrasound scan for review | 89 | 76 | 89 | .41 |
| Echocardiogram for review | 80 | 92 | 100 | .18 |
| Any congenital heart disease | 49 | 68 | 78 | .15 |
| Classic heterotaxic heart defect | 20 | 20 | 44 | .31 |
| Atrial situs inversus | 26 | 32 | 22 | .80 |
| Common atrium | 0 | 4 | 0 | .41 |
| Dextrocardia | 46 | 50 | 33 | .31 |
| AVSD | 9 | 8 | 44 | .01d |
| ASD | 20 | 32 | 22 | .56 |
| VSD | 26 | 40 | 0 | .07 |
| Single ventricle | 9 | 12 | 11 | .91 |
| Double-outlet right ventricle | 14 | 4 | 22 | .26 |
| Pulmonary stenosis or atresia | 11 | 32 | 33 | .11 |
| Dextro-transposition of great arteries | 3 | 24 | 11 | .04e |
| l-TGA | 11 | 8 | 22 | .46 |
| Bilateral superior vena cava | 6 | 21 | 11 | .21 |
| Right aortic arch | 46 | 36 | 56 | .56 |
| Interrupted/duplicated inferior vena cava | 34 | 39 | 22 | .66 |
| APVR | 11 | 12 | 33 | .23 |
| Intestinal malrotation | 24 | 21 | 44 | .36 |
| Dextrogastria | 65 | 52 | 44 | .56 |
| Midline liver | 21 | 21 | 33 | .70 |
| Asplenia | 6 | 11 | 43 | .03f |
| Polysplenia | 55 | 53 | 43 | .85 |
Data are presented as median (range) or %. nNO = nasal nitric oxide; PCD = primary ciliary dyskinesia. See Table 1 legend for expansion of other abbreviation.
Velum closure nNO cutoff = 77 nL/min; tidal breathing nNO cutoff = 40 nL/min.
χ2 test among three categorical groups.
Kruskal-Wallis test for median age three-group comparison. Median age of classic PCD vs SA control group Wilcoxon P = .002; median age of classic PCD vs not classic PCD, nNO below cutoff, P = .12.
AVSD in classic PCD vs SA control group Fisher exact P = .65; in classic PCD vs not classic PCD, nNO below cutoff, P = .02.
Dextro-transposition of the great arteries in classic PCD vs SA control group Fisher exact P = .02; in classic PCD vs not classic PCD, nNO below cutoff, P = .38.
Asplenia in classic PCD vs SA control group Fisher exact P = .49; in classic PCD vs not classic PCD, nNO below cutoff, P = .03.
Nineteen of the 37 participants (51%) with SA and classic PCD had ODA defects alone on electron microscopy; eight (22%) had ODA plus IDA defects; five (13%) had IDA plus CA and microtubule disorganization defects; four (11%) had inadequate or indeterminate (no hallmark PCD-causing defects, but not normal either) samples; one (3%) had normal EM findings; and none had isolated CA, IDA, or radial spoke defects. Distribution of ODA defects, ODA plus IDA defects, and IDA plus CA and microtubule disorganization defects in SA was similar to that in SS and SI. None of the participants who had radial spoke defects had laterality defects. There were no significant correlations between genetic mutation and situs anomalies. Distribution of EM defects within the SA population did not correspond to specific laterality defects (Table 3).
TABLE 3 ] .
Diagnostic Testing of Participants With Classic PCD and SA
| ID | Age, y | nNO, nL/min | Situs | EM Defect | Biallelic Mutationa |
| PCD1 | 0.1b | 8.79c | SA + complex CVM | ODA + IDA | HEATR2 |
| PCD2 | 0.2b | 11.2c | SA + simple CVM | ODA + IDA | … |
| PCD3 | 1.8 | 16.1c | SA + complex CVM | ODA | … |
| PCD4 | 3.0 | 4.6c | SA + complex CVM | Indeterminate | DNAH5 |
| PCD5 | 4.7 | 6.1c | SA + simple CVM | ODA | … |
| PCD6 | 7.8 | 10.9c | SA without cardiac defect | Indeterminate | CCDC39 |
| PCD7 | 5.2 | 4.3 | SA + complex CVM | Inadequate sample | DNAH5 |
| PCD8 | 6.4 | Not done | SA without cardiac defect | ODA | DNAH5 |
| PCD9 | 7.3 | 6.8 | SA + simple CVM | IDA + CA, MTD | CCDC40 |
| PCD10 | 7.4 | 5.7 | SA + complex CVM | ODA | DNAH5 |
| PCD11 | 8.1 | 22.0 | SA without cardiac defect | ODA | … |
| PCD12 | 8.1 | 10.4 | SA + simple CVM | ODA + IDA | DYX1C1 |
| PCD13 | 8.5 | 9.6 | SA + simple CVM | IDA + CA, MTD | CCDC40 |
| PCD14 | 9.3 | 10.0 | SA without cardiac defect | ODA | … |
| PCD15 | 10.7 | 35.7 | SA + simple CVM | ODA | DNAH5 |
| PCD16 | 11.1 | 25.6 | SA without cardiac defect | ODA + IDA | … |
| PCD17 | 11.2 | 9.8 | SA without cardiac defect | IDA + CA, MTD | … |
| PCD18 | 11.4 | 10.2 | Isolated possible laterality defect | ODA + IDA | KTU |
| PCD19 | 11.5 | 13.2 | SA + complex CVM | ODA | DNAI1 |
| PCD20 | 12.4 | 25.5 | SA + complex CVM | Normal | DNAH11 |
| PCD21 | 12.9 | 8.9 | SA + complex CVM | Inadequate sample | DNAH5 |
| PCD22 | 13.4 | 20.7 | Isolated possible laterality defect | ODA + IDA defects | HEATR2 |
| PCD23 | 14.0 | 32.5 | Isolated possible laterality defect | ODA defect | DNAH5 |
| PCD24 | 15.6 | 35.8 | SA without cardiac defect | ODA defect | DNAH5 |
| PCD25 | 16.0 | 11.9 | Isolated possible laterality defect | IDA + CA, MTD | … |
| PCD26 | 16.9 | 16.5 | SA without cardiac defect | ODA | … |
| PCD27 | 18.6 | 26.4 | Isolated possible laterality defect | ODA | DNAH5 |
| PCD28 | 20.5 | 29.9 | SA + simple CVM | ODA | … |
| PCD29 | 22.7 | 5.5 | Isolated possible laterality defect | IDA + CA, MTD | … |
| PCD30 | 24.6 | 35.7 | Isolated possible laterality defect | ODA | DNAH5 |
| PCD31 | 30.7 | 5.8 | SA without cardiac defect | ODA | … |
| PCD32 | 31.7 | 13.1 | SA without cardiac defect | ODA+IDA | LRRC6 |
| PCD33 | 34.7 | 47.6 | SA without cardiac defect | ODA | DNAH5 |
| PCD34 | 35.8 | 43.7 | SA without cardiac defect | ODA | … |
| PCD35 | 45.8 | 12.1 | SA without cardiac defect | ODA | DNAH5 |
| PCD36 | 55.2 | 5.7 | Isolated possible laterality defect | ODA | DNAH5 |
| PCD37 | 58.0 | 7.2 | SA without cardiac defect | ODA + IDA | … |
CA = central apparatus; IDA = inner dynein arm; ODA = outer dynein arm; MTD = microtubule disorganization. See Table 1 and 2 legends for expansion of other abbreviations.
Ellipses denote no genetic mutations isolated. We tested DNAI1 and DNAH5 for patients with ODA defects; DNAAF2, DYX1C1, HEATR2, and LRRC6 for patients with ODA + IDA defects; CCDC39 and CCDC40 for patients with IDA + CA and MTD defects; DNAH11 for patients with normal ciliary ultrastructure; and all of these genes for patients in whom ciliary ultrastructure was not available.
Participants aged < 1 y not included in data analysis.
nNO values obtained with the tidal breathing technique.
Year-round wet cough, year-round nasal congestion, neonatal respiratory distress, and digital clubbing were significantly more prevalent in participants with classic PCD than in SA control participants (Table 4). The prevalence of digital clubbing did not correlate with participant age (median age with clubbing, 14 years; median age with no clubbing, 8.5 years; P = .19). The prevalence of neonatal respiratory distress in classic PCD was increased despite a higher prevalence of congenital heart disease, which is known to cause respiratory distress, in SA control participants. The age of onset for nasal congestion was significantly earlier in classic PCD, but age of onset for other sino-oto-pulmonary symptoms was similar in both groups and mainly within the first year of life (Table 4).
TABLE 4 ] .
Clinical Features of Participants With SA Aged > 1 Year
| Clinical Feature | Classic PCD (n = 35) | Not Classic PCD nNO Above Cutoffa (SA Control Group) (n = 25) | Not Classic PCD nNO Below Cutoffa (n = 9) | P Valueb |
| Respiratory symptom | ||||
| Year-round wet cough | 34 of 35 (97) | 9 of 21 (43) | 7 of 9 (78) | < .001c |
| Year-round nasal congestion | 33 of 35 (94) | 16 of 23 (70) | 6 of 8 (75) | .05d |
| Digital clubbing | 7 of 33 (21) | 0 of 22 (0) | 1 of 8 (13) | .07e |
| NRDf | 24 of 29 (83) | 8 of 19 (42) | 6 of 6 (100) | .001g |
| Heart disease known to cause NRD | 15 of 28 (54) | 13 of 19 (68) | 5 of 7 (71) | .38 |
| Chronic otitis media | 30 of 34 (88) | 18 of 23 (78) | 7 of 9 (78) | .58 |
| Sinusitis | 24 of 34 (71) | 18 of 22 (82) | 6 of 8 (75) | .59 |
| Pneumonia/bronchitis | 27 of 35 (77) | 18 of 22 (82) | 8 of 8 (100) | .32 |
| Age of onset for respiratory symptom | ||||
| Year-round wet cough | 0.08 (0.08-13) | 0.59 (0.08-4) | 0.33 (0.08-3) | .81 |
| Year-round nasal congestion | 0.08 (0.08-11) | 0.42 (0.08-15) | 1 (0.08-2) | .07h |
| First episode otitis media | 0.42 (0.08-5) | 0.29 (0.08-8) | 0.5 (0.25-23) | .98 |
Data are presented as No. of total No. (%) participants with indicated respiratory symptom or median age in y (range). NRD = neonatal respiratory disease. See Table 1 and 2 legends for expansion of other abbreviations.
Velum closure nNO cutoff = 77 nL/min; tidal breathing nNO cutoff = 40 nL/min.
χ2 test among three groups.
Cough in classic PCD vs SA control group Fisher exact P < .001; in classic PCD vs not classic PCD, nNO below cutoff, P = .10.
Nasal congestion in classic PCD vs SA control group Fisher exact P = .015; in classic PCD vs not classic PCD, nNO below cutoff, P = .15.
Clubbing in classic PCD vs SA control group Fisher exact P = .021; in classic PCD vs not classic PCD, nNO below cutoff, P = .50.
Not attributable to preterm birth or meconium aspiration.
NRD in classic PCD vs SA control group Fisher exact P = .009; in classic PCD vs not classic PCD, nNO below cutoff, P = .37.
Age of onset for nasal congestion in classic PCD vs SA control group Wilcoxon P = .026; in classic PCD vs not classic PCD, nNO below cutoff, P = .44.
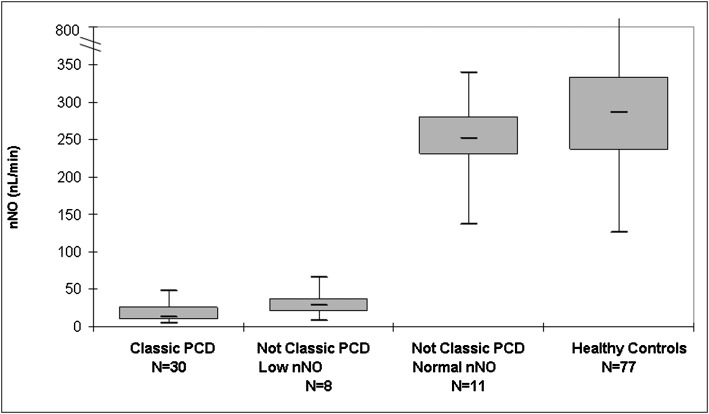
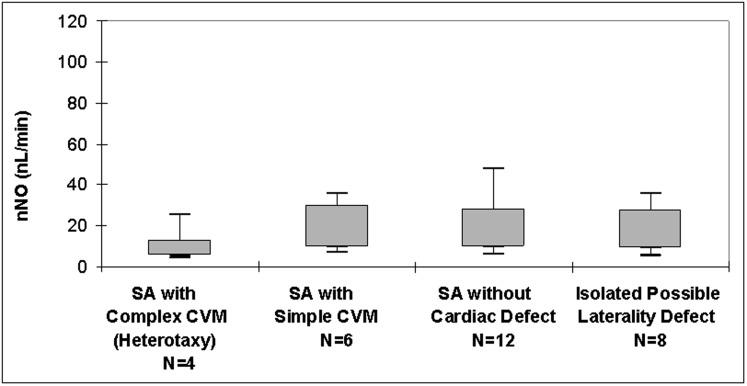
Levels of nNO measured during velum closure were significantly lower in participants with classic PCD (median, 12 nL/min; range, 4-48 nL/min; n = 30) than in all participants without classic PCD (median nNO level above cutoff, 252 nL/min [range, 137-338 nL/min; n = 11]; median nNO level below cutoff, 29 nL/min [range 8-66, n = 8]; P < .001). Levels of nNO during tidal breathing were also significantly lower in participants with classic PCD (median, 8.5 nL/min; range, 5-16 nL/min; n = 4) than in those without classic PCD (median, 128 nL/min; range, 37-276; n = 14; P = .003). For the 77 healthy control participants, median velum closure nNO level was 286 nL/min, which was significantly higher than in participants with classic PCD and SA control participants (range healthy control participants, 125-867 nL/min; Kruskal-Wallis P < .001) (Fig 3). There were no significant differences in velum closure nNO levels among the SA subgroups with classic PCD (Kruskal-Wallis P = .30) (Fig 4).
Figure 3 –
Measurements of nNO in participants by final diagnosis group. Shown are nNO values by velum closure in participants with SA. Box plots show interquartile range, with median denoted by bold line. Whiskers denote minimum to maximum values. See Figure 2 legend for expansion of abbreviations.
Figure 4 –
Measurements of nNO in SA subgroups with classic primary cilia dyskinesia. Shown are nNO values by velum closure in participants with SA according to SA subgroup. Box plots show interquartile range, with median denoted by bold line. Whiskers denote minimum to maximum values. SA = situs ambiguus. See Figure 2 legend for expansion of other abbreviations.
Discussion
SI has been associated with PCD since the first case reports; however, SA with PCD has received attention only recently.15,16,27,28 This study reveals that the prevalence of SA (including heterotaxy) in a cohort of patients with classic PCD is 12.1%, and this increases to 12.8% when excluding radial spoke and CA defects from an overall PCD denominator because these defects are not present in embryonic nodal cilia and, thus, do not present with heterotaxy. PCD is diagnosed in patients with various combinations of laterality defects, including simple and complex heart disease and combinations of noncardiac laterality defects, which do not fit into standard definitions of heterotaxy. Moreover, collections of laterality defects in some patients with SA do not correspond to the phenotypes of left or right isomerism, which often are used to group laterality defects clinically. The participants with confirmed PCD and SA without classic heterotaxic heart defects or isomerism sequence may represent a variant form of heterotaxy that is not fully appreciated.9,29,30 Genetic defects known to cause classic heterotaxy can also cause isolated d-TGA or double-outlet right ventricle, which in isolation are not considered heterotaxy by many cardiologists.31 Furthermore, isolated cardiac septal defects have been linked to ciliary genes.32 Thus, a wide spectrum of laterality defects may be attributable to ciliary dysfunction and associated with PCD.
The SA prevalence within the present PCD population is nearly twice that reported in the Kennedy et al15 study. Although our subclassification schema for SA was different from that of Kennedy et al,15 our definition of PCD and overall definition of laterality and cardiac defects comprising SA were similar. The increased prevalence in the present study may be due to heightened awareness of the link between heterotaxy and PCD, leading to increased referral patterns. Additionally, our use of standardized questions on laterality and cardiovascular anomalies within this prospective protocol may have been more attuned to the discovery of laterality defects than the retrospective protocol of Kennedy et al.15
No specific laterality defects were more prevalent in participants with classic PCD than in SA control participants. d-TGA was significantly more prevalent in SA control participants with normal nNO levels, yet one participant with d-TGA had classic PCD. Asplenia and atrioventricular septal defect, which often are seen together in right isomerism, were more prevalent in those without classic PCD and nNO levels below our cutoff. It is possible that these individuals carry an undiscovered PCD genetic mutation associated with right isomerism and normal findings on electron microscopy. Perhaps with larger SA populations, one could find certain laterality defects associated with classic PCD.
Clinical symptom assessment in this SA population demonstrates that year-round wet cough and year-round nasal congestion were significantly more prevalent in participants with classic PCD than in SA control participants. Although one would expect a large proportion of patients with SA to have neonatal respiratory distress due to an increased burden of congenital heart disease, the prevalence of neonatal respiratory distress in participants with classic PCD was significantly more than in SA control participants. Therefore, in patients with forms of SA sparing the heart or with neonatal respiratory distress out of proportion with their cardiac defect, one should suspect possible PCD. Finally, digital clubbing was significantly more prevalent in participants with classic PCD than in SA control participants, even though clubbing is commonly found in patients with cyanotic congenital heart disease.33
nNO is a useful test for PCD screening, but this has not been broadly studied in patients with SA. We saw significantly lower nNO levels in participants with SA and classic PCD than in SA control participants. All participants with SA and classic PCD had nNO levels below the previously established PCD cutoff of 77 nL/min.20 Levels of nNO did not vary greatly among SA subgroups with classic PCD, which further supports the concept of SA as a spectrum of laterality defects with similar respiratory ciliary dysfunction regardless of the SA defects. A substantial number of participants had some features suggestive of PCD with nNO levels below our cutoff value; however, they did not meet our criteria for classic PCD. With further genetic discovery, some of these may ultimately be defined as PCD. Until a full spectrum of PCD gene mutations is defined, clinicians may encounter patients with some clinical features of PCD and low nNO levels who do not fulfill classic definitions of PCD, as we saw here.
There are several limitations to this study. First, there is no universally accepted definition of heterotaxy or SA. Therefore, we used a classification schema that delineates patients with simple or complex heterotaxic heart defects, who often are followed by cardiologists, from others with noncardiac laterality defects and isolated possible laterality defects, who often are followed outside of cardiology. Some patients with SS or SI in our protocol did not have complete abdominal imaging. Consequently, some abdominal laterality defects may not be identified, and the numbers of participants with SA and classic PCD may be greater than reported here. Additionally, we did not use immunofluorescent ciliary staining, ciliary beat frequency, or functional ciliary analysis in our diagnostic algorithm. Conceivably, these techniques may have detected more cases of PCD, and we may have excluded PCD cases with normal ciliary ultrastructure and genetics but abnormal ciliary function. Second, the SA control group may not have been a pure control group because genetically proven PCD has been reported with normal ciliary ultrastructure34 or rarely with normal nNO levels.35 All SA control participants were referred for chronic sino-oto-pulmonary symptoms, and a healthy SA control group may yield different results. Finally, SA control participants were significantly younger at study enrollment. Because the link between PCD and SA was recently discovered, physicians currently managing patients with SA may be more attuned to the presence of chronic respiratory symptoms in this population. With this knowledge, a higher burden of viral infections causing frequent sinusitis and otitis in young patients with SA may have prompted younger referrals to our consortium. This age disparity may also account for difference in sinopulmonary symptom prevalence because symptom onset may be delayed until an older age. However, this seems unlikely, because most patients with classic PCD exhibit sinopulmonary symptoms at birth and in the first year of life.
Conclusions
Understanding the potential for PCD in patients with laterality defects aside from SI is essential to improve clinical outcomes. Year-round wet cough, year-round nasal congestion, neonatal respiratory distress, and digital clubbing suggest PCD in patients with SA. Any patient with these symptoms and laterality defects apart from SI should have their respiratory cilia studied. Appropriate testing for PCD with SA includes genetic testing, nNO measurement, and EM ciliary examination at an experienced PCD center.
Supplementary Material
Online Supplement
Acknowledgments
Author contributions: A. J. S. had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. A. J. S., S. D. Davis, T. F., S. D. Dell, M. R., K. N. O., S. D. S., C. M., M. A. Z., J. L. C., M. J. H., B. R., M. R. K., and M. W. L. contributed to designing protocol, conducting clinical research visits, clinical investigations, data analysis, and manuscript preparation; W. W. contributed to genetic testing, mutation analysis, and manuscript preparation; M A. Z. contributed to genetic testing and genetic mutation analysis; M. J. H. contributed to creating and monitoring nasal nitric oxide protocols, testing, and data analysis; K. B. contributed to processing and monitoring electron microscopy samples; and B. R. contributed to monitoring cardiac/laterality defect data analysis and manuscript preparation.
Financial/nonfinancial disclosures: The authors have reported to CHEST that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The sponsor had no role in the design of the study, the collection and analysis of the data, or the preparation of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Dr Olivier’s contribution to this manuscript was done as part of his official duties as a National Institutes of Health employee and is a work of the US government.
Other contributions: The authors thank the patients with PCD and their families for participation. The authors also thank Michele Manion and the Primary Ciliary Dyskinesia Foundation, all additional investigators, and the coordinators of the Genetic Disorders of Mucociliary Clearance Consortium who are part of the Rare Diseases Clinical Research Network, including Tanya Glaser, Heather Root, Andrea Henkel, Caroline Smith, Meghan O’Connell (National Institute of Allergy and Infectious Diseases/NIH, Bethesda, MD); Jane Quante, RN (Washington University, St. Louis, MO); Shelley Mann and Carol Kopecky, BS, RRT, CPFT (Children’s Hospital Colorado, Aurora, CO); Sharon McNamara, Liz Cochrane, Molly Elliott, Jennifer Soper, Robert Johnson (Seattle Children’s Hospital, Seattle, WA); Jacquelyn Zirbes, DNP, RN, CNP, CCRC (Stanford University Medical Center, Palo Alto, CA); Donna Wilkes and Melody Miki, RN, BScN (The Hospital for Sick Children, Toronto, ON, Canada); and Susan Minnix, RN, BSN, and Caroline LaFave (University of North Carolina-Chapel Hill, Chapel Hill, NC). The authors further thank Rhonda Pace and Michael Patrone for technical assistance; Elizabeth Godwin for administrative support; Syanne Olson for editorial assistance; Charles R. Esther, MD, PhD, for assistance with statistical analysis; and Kunal Chawla, BS, and Brock Baker, BS, for assistance with nNO collection.
Additional information: The e-Appendix and e-Table can be found in the Supplemental Materials section of the online article.
ABBREVIATIONS
- CA
central apparatus
- d-TGA
dextro-transposition of the great arteries
- EM
electron microscopic
- IDA
inner dynein arm
- nNO
nasal nitric oxide
- ODA
outer dynein arm
- PCD
primary ciliary dyskinesia
- SA
situs ambiguus
- SI
situs inversus totalis
- SS
situs solitus
Footnotes
FOR EDITORIAL COMMENT SEE PAGE 1136
Dr Shapiro was affiliated with the University of North Carolina when this research began.
FUNDING/SUPPORT: Funding for this research was provided to Drs Shapiro, Davis, Ferkol, Dell, Rosenfeld, Olivier, Sagel, Milla, Zariwala, Carson, Hazucha, Knowles, and Leigh and Mss Wolf and Burns by National Institutes of Health (NIH), Office of Rare Diseases Research, National Heart, Lung, and Blood Institute (NHLBI) [Grant 5US54HL096458-06] and to Drs Zariwala and Knowles by NIH, NHLBI [Grant 5R01HL071798] and NIH, National Center for Advancing Translational Science (NCATS) [Grant UL1TR000083]. Funding for Dr Rosenfeld was provided by NIH/NCATS [Grant UL1TR000423]. Funding for Dr Olivier was provided by the Intramural Research Program of the NIH, National Institute of Allergy and Infectious Diseases. Funding for Dr Sagel was by NIH/NCATS Colorado Clinical and Translational Sciences Institute [Grant UL1TR000154]. The Genetic Disorders of Mucociliary Clearance Consortium [U54HL096458] is a part of the NIH Rare Diseases Clinical Research Network supported through collaboration between the NIH Office of Rare Diseases Research at the NCATS and the National Heart, Lung, and Blood Institute.
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Katsuhara K, Kawamoto S, Wakabayashi T, Belsky JL. Situs inversus totalis and Kartagener’s syndrome in a Japanese population. Chest. 1972;61(1):56-61 [DOI] [PubMed] [Google Scholar]
- 2.Torgersen J. Situs inversus, asymmetry, and twinning. Am J Hum Genet. 1950;2(4):361-370 [PMC free article] [PubMed] [Google Scholar]
- 3.Zariwala MA, Knowles MR, Leigh MW. Primary ciliary dyskinesia, In: Pagon RA, Bird TC, Dolan CR, Stephens K, eds. GeneReviews [Internet]. Seattle, WA: University of Washington, Seattle; 1993-2014. National Center for Biotechnology website. http://www.ncbi.nlm.nih.gov/books/NBK1122. Accessed January 1, 2013 [PubMed] [Google Scholar]
- 4.Tynan MJ, Becker AE, Macartney FJ, Jiménez MQ, Shinebourne EA, Anderson RH. Nomenclature and classification of congenital heart disease. Br Heart J. 1979;41(5):544-553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Praagh R. Terminology of congenital heart disease. Glossary and commentary. Circulation. 1977;56(2):139-143 [DOI] [PubMed] [Google Scholar]
- 6.Evans WN. Thoracoabdominal situs: a practical approach accompanied by a short history of descriptive terms. Pediatr Cardiol. 2010;31(7):1049-1051 [DOI] [PubMed] [Google Scholar]
- 7.Jacobs JP, Anderson RH, Weinberg PM, et al. The nomenclature, definition and classification of cardiac structures in the setting of heterotaxy. Cardiol Young. 2007;17(suppl 2):1-28 [DOI] [PubMed] [Google Scholar]
- 8.Aylsworth AS. Clinical aspects of defects in the determination of laterality. Am J Med Genet. 2001;101(4):345-355 [PubMed] [Google Scholar]
- 9.Zhu L, Belmont JW, Ware SM. Genetics of human heterotaxias. Eur J Hum Genet. 2006;14(1):17-25 [DOI] [PubMed] [Google Scholar]
- 10.Ware SM, Peng J, Zhu L, et al. Identification and functional analysis of ZIC3 mutations in heterotaxy and related congenital heart defects. Am J Hum Genet. 2004;74(1):93-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanaka Y, Okada Y, Hirokawa N. FGF-induced vesicular release of Sonic hedgehog and retinoic acid in leftward nodal flow is critical for left-right determination. Nature. 2005;435(7039):172-177 [DOI] [PubMed] [Google Scholar]
- 12.Fliegauf M, Benzing T, Omran H. When cilia go bad: cilia defects and ciliopathies. Nat Rev Mol Cell Biol. 2007;8(11):880-893 [DOI] [PubMed] [Google Scholar]
- 13.Nagao Y, Cheng J, Kamura K, et al. Dynein axonemal intermediate chain 2 is required for formation of the left-right body axis and kidney in medaka. Dev Biol. 2010;347(1):53-61 [DOI] [PubMed] [Google Scholar]
- 14.Tan SY, Rosenthal J, Zhao XQ, et al. Heterotaxy and complex structural heart defects in a mutant mouse model of primary ciliary dyskinesia. J Clin Invest. 2007;117(12):3742-3752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kennedy MP, Omran H, Leigh MW, et al. Congenital heart disease and other heterotaxic defects in a large cohort of patients with primary ciliary dyskinesia. Circulation. 2007;115(22):2814-2821 [DOI] [PubMed] [Google Scholar]
- 16.Bush A. Congenital heart disease in primary ciliary dyskinesia. Pediatr Cardiol. 1998;19(2):191. [DOI] [PubMed] [Google Scholar]
- 17.Brueckner M. Heterotaxia, congenital heart disease, and primary ciliary dyskinesia. Circulation. 2007;115(22):2793-2795 [DOI] [PubMed] [Google Scholar]
- 18.Botto LD, Lin AE, Riehle-Colarusso T, Malik S, Correa A; National Birth Defects Prevention Study. Seeking causes: classifying and evaluating congenital heart defects in etiologic studies. Birth Defects Res A Clin Mol Teratol. 2007;79(10):714-727 [DOI] [PubMed] [Google Scholar]
- 19.American Thoracic Society; European Respiratory Society. ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med. 2005;171(8):912-930 [DOI] [PubMed] [Google Scholar]
- 20.Leigh MW, Hazucha MJ, Chawla KK, et al. Standardizing nasal nitric oxide measurement as a test for primary ciliary dyskinesia.Ann Am Thorac Soc. 2013;10(6):574-581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mateos-Corral D, Coombs R, Grasemann H, Ratjen F, Dell SD. Diagnostic value of nasal nitric oxide measured with non-velum closure techniques for children with primary ciliary dyskinesia. J Pediatr. 2011;159(3):420-424 [DOI] [PubMed] [Google Scholar]
- 22.Noone PG, Leigh MW, Sannuti A, et al. Primary ciliary dyskinesia: diagnostic and phenotypic features. Am J Respir Crit Care Med. 2004;169(4):459-467 [DOI] [PubMed] [Google Scholar]
- 23.Karadag B, James AJ, Gültekin E, Wilson NM, Bush A. Nasal and lower airway level of nitric oxide in children with primary ciliary dyskinesia. Eur Respir J. 1999;13(6):1402-1405 [DOI] [PubMed] [Google Scholar]
- 24.Chawla KK, Shapiro A, Hazucha MJ, et al. Nasal nitric oxide during tidal breathing in children under 6 years of age [abstract]. Am J Respir Crit Care Med. 2009;179(Meeting Abstracts):A3673 [Google Scholar]
- 25.Barbato A, Frischer T, Kuehni CE, et al. Primary ciliary dyskinesia: a consensus statement on diagnostic and treatment approaches in children. Eur Respir J. 2009;34(6):1264-1276 [DOI] [PubMed] [Google Scholar]
- 26.Bush A, Chodhari R, Collins N, et al. Primary ciliary dyskinesia: current state of the art. Arch Dis Child. 2007;92(12):1136-1140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bush A, Hogg C. Primary ciliary dyskinesia: recent advances in epidemiology, diagnosis, management and relationship with the expanding spectrum of ciliopathy. Expert Rev Respir Med. 2012;6(6):663-682 [DOI] [PubMed] [Google Scholar]
- 28.Nakhleh N, Francis R, Giese RA, et al. High prevalence of respiratory ciliary dysfunction in congenital heart disease patients with heterotaxy. Circulation. 2012;125(18):2232-2242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuehl KS, Loffredo C. Risk factors for heart disease associated with abnormal sidedness. Teratology. 2002;66(5):242-248 [DOI] [PubMed] [Google Scholar]
- 30.Sutherland MJ, Ware SM. Disorders of left-right asymmetry: heterotaxy and situs inversus. Am J Med Genet C Semin Med Genet. 2009;151C(4):307-317 [DOI] [PubMed] [Google Scholar]
- 31.D’Alessandro LC, Latney BC, Paluru PC, Goldmuntz E. The phenotypic spectrum of ZIC3 mutations includes isolated d-transposition of the great arteries and double outlet right ventricle. Am J Med Genet A. 2013;161A(4):792-802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gerhardt C, Lier JM, Kuschel S, Rüther U. The ciliary protein Ftm is required for ventricular wall and septal development. PLoS One. 2013;8(2):e57545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martínez-Lavín M, Bobadilla M, Casanova J, Attié F, Martínez M. Hypertrophic osteoarthropathy in cyanotic congenital heart disease: its prevalence and relationship to bypass of the lung. Arthritis Rheum. 1982;25(10):1186-1193 [DOI] [PubMed] [Google Scholar]
- 34.Schwabe GC, Hoffmann K, Loges NT, et al. Primary ciliary dyskinesia associated with normal axoneme ultrastructure is caused by DNAH11 mutations. Hum Mutat. 2008;29(2):289-298 [DOI] [PubMed] [Google Scholar]
- 35.Marthin JK, Nielsen KG. Choice of nasal nitric oxide technique as first-line test for primary ciliary dyskinesia. Eur Respir J. 2011;37(3):559-565 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement