Abstract
BACKGROUND:
New-onset atrial fibrillation (AF) is associated with adverse outcomes during a sepsis hospitalization; however, long-term outcomes following hospitalization with sepsis-associated new-onset AF are unclear.
METHODS:
We used a Medicare 5% sample to identify patients who survived hospitalization with sepsis between 1999 and 2010. AF status was defined as no AF, prior AF, or new-onset AF based on AF claims during and prior to a sepsis hospitalization. We used competing risk models to determine 5-year risks of AF occurrence, heart failure, ischemic stroke, and mortality after the sepsis hospitalization, according to AF status during the sepsis admission.
RESULTS:
We identified 138,722 sepsis survivors, of whom 95,536 (69%) had no AF during sepsis, 33,646 (24%) had prior AF, and 9,540 (7%) had new-onset AF during sepsis. AF occurrence following sepsis hospitalization was more common among patients with new-onset AF during sepsis (54.9%) than in patients with no AF during sepsis (15.5%). Compared with patients with no AF during sepsis, those with new-onset AF during sepsis had greater 5-year risks of hospitalization for heart failure (11.2% vs 8.2%; multivariable-adjusted hazard ratio [HR], 1.25; 95% CI, 1.16-1.34), ischemic stroke (5.3% vs 4.7%; HR, 1.22; 95% CI, 1.10-1.36), and death (74.8% vs 72.1%; HR, 1.04; 95% CI,1.01-1.07).
CONCLUSIONS:
Most sepsis survivors with new-onset AF during sepsis have AF occur after discharge from the sepsis hospitalization and have increased long-term risks of heart failure, ischemic stroke, and death. Our findings may have implications for posthospitalization surveillance of patients with new-onset AF during a sepsis hospitalization.
Sepsis is associated with the development of new-onset atrial fibrillation (AF).1 Approximately 7% of Medicare beneficiaries experience new-onset AF during a hospitalization with sepsis, representing about 50,000 cases of new-onset AF during sepsis among older patients annually.2 The occurrence of new-onset AF during sepsis is associated with a substantial increase in the short-term risk of ischemic stroke and hospital mortality.1,3
In light of rising incidence and falling case-fatality rates,4 understanding long-term outcomes among sepsis survivors is increasingly important. However, long-term outcomes associated with new-onset AF among sepsis survivors are unclear. Prior studies suggest that new-onset AF during hospitalizations for critical illness may be of short duration. For example, a single-center study of surgical patients found that 86% of new-onset AF during septic shock resolved prior to hospital discharge,3 and a prospective, multicenter study of arrhythmias during critical illness showed that the median duration of new-onset AF during critical illness was 180 min.5 The concept that new-onset AF during a potentially “reversible cause” such as sepsis is not associated with adverse long-term risk is also implied in the clinical practice guidelines for AF, which state that “successful treatment of the underlying condition often eliminates AF.”6
Contrary to prevailing opinion, we hypothesized that the prognostic implications of new-onset AF during sepsis are not eliminated after the resolution of sepsis. Rather, we posited that new-onset AF during sepsis represents a marker of long-term risk of recurrent AF and AF-associated complications such as heart failure, ischemic stroke, and death.6 In the current study, we explored the long-term risks associated with the development of new-onset AF among a cohort of Medicare beneficiaries who had survived a hospitalization with sepsis.
Materials and Methods
We used claims data from a nationally representative 5% sample of Medicare beneficiaries. Using the inpatient, outpatient, and professional services’ analytic files and the corresponding denominator files from the Centers for Medicare and Medicaid Services, we identified a cohort discharged alive from a hospitalization with sepsis between January 1, 1999, and December 31, 2010. We used previously published and validated methods to identify sepsis hospitalization (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM]) diagnosis codes for septicemia 038.x, severe sepsis 995.92, or septic shock 785.52 (16%-56% sensitive; 98%-100% specific; positive predictive value [PPV], > 95%).1,7,8 Because no gold standard exists to identify sepsis in administrative data, we performed a sensitivity analysis using an alternate algorithm9 to identify cases of severe sepsis, using ICD-9-CM codes for infection and organ dysfunction (50% sensitive; 96% specific; PPV, 71%).7 For patients with multiple sepsis hospitalizations, we selected the earliest as the index hospitalization. We restricted the analysis to beneficiaries who were 67 years old or older (to classify history of antecedent AF) and living in the United States, who had had continuous fee-for-service Medicare for at least 2 years prior to discharge from the sepsis hospitalization.
AF Definitions
We classified each patient’s AF status during the index sepsis hospitalization as no AF, prior AF, and new-onset AF (e-Table 1 (408.3KB, pdf) ). Patients were classified as having prior AF if they had received a diagnosis of AF or atrial flutter (ICD-9-CM 427.3x, 95% sensitivity, 99% specificity)10 on one inpatient claim or two outpatient or professional claims in the 2 years prior to the sepsis hospitalization. As described previously, we required two outpatient claims to improve the specificity of the AF classification by attenuating the impact of “rule-out” diagnoses.11,12 We defined beneficiaries as having new-onset AF if they had an inpatient AF claim concomitant with the index sepsis hospitalization and were not identified as having prior AF. All other beneficiaries were classified as no AF for the index sepsis hospitalization.
Patient Characteristics
We used the categories black, white, and other to identify self-reported race/ethnicity.13 Comorbid conditions were identified based on previously published algorithms (e-Table 2 (408.3KB, pdf) )14,15 through a search of all claims in the 1-year period preceding the index sepsis hospitalization. Using prior comorbidity claims, we calculated the congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, previous stroke/transient ischemic attack, vascular disease, age 65 to 74 years, sex category (CHA2DS2-VASc) ischemic stroke risk scores for each patient.16 Sepsis factors, such as the site of infection and the type of acute organ failures associated with the sepsis hospitalization, were ascertained from the sepsis hospitalization claim (e-Table 2 (408.3KB, pdf) ).
Long-term Outcomes After Sepsis
We investigated AF, ischemic stroke, heart failure, and mortality following sepsis hospitalization. Ischemic stroke hospitalizations were identified by an inpatient ischemic stroke claim in any position (ICD-9-CM 433.x1, 434.x1, 436.x; 86% sensitivity and 95% specificity; PPV, 90%; k = 0.82).17 We also performed a sensitivity analysis identifying ischemic stroke hospitalization from ischemic stroke claims limited to the principal diagnostic position. We identified the proportion of patients with new-onset AF during sepsis who developed an incident ischemic stroke following the sepsis hospitalization, but who had had no other AF diagnosis prior to the ischemic stroke hospitalization. Acute heart failure hospitalizations were ascertained by a principal inpatient diagnosis of heart failure (ICD-9-CM 428.x, PPV, > 90%)18 occurring after the index sepsis hospitalization. Death was identified from the death date of the Medicare denominator file.
Statistical Analysis
We summarized patient baseline characteristics using means with SDs for continuous variables and frequencies with percentages for categorical variables. We tested for differences among the three AF groups using Kruskal-Wallis tests for continuous variables and χ2 tests for categorical variables.
We treated death as a competing risk and estimated AF rates following sepsis hospitalization using the cumulative incidence function, assessing differences among AF groups using Gray tests. Data for patients who did not die and who did not have postdischarge AF identified were censored at the earliest of 5 years following the index hospitalization discharge, enrollment in a Medicare managed care program, or on December 31, 2011, which was the date of the latest data available for this study.
Other clinical event rates were calculated similarly. Mortality was estimated using Kaplan-Meier methods, whereas heart failure and ischemic stroke rates were estimated using the cumulative incidence function with mortality as a competing risk.
To estimate the association between AF status and postdischarge clinical events, we used proportional hazards regression models. Adjusted hazard ratios were estimated accounting for patient factors alone (demographics, comorbid conditions, and year of hospitalization) and accounting for patient factors in addition to sepsis factors (type of infection and types of organ failure). We tested the proportionality assumption for the indicators associated with each AF group. We used C statistics to summarize the ability of the CHA2DS2-Vasc score to discriminate ischemic stroke risk in sepsis survivors, a cohort in whom such scores have not been validated previously. Analyses were performed using SAS, version 9.3 (SAS Institute, Inc). Study procedures were approved by the Duke University Health System Institutional Review Board (Amd4_Pro00020866).
Results
We identified 138,722 survivors of a sepsis hospitalization between 1999 and 2010 from a Medicare 5% sample. Medicare beneficiaries who survived sepsis were, on average, 80 years old, 42.5% were men, and 83.6% were white. Table 1 demonstrates the characteristics of patients with sepsis stratified by AF status.
TABLE 1 ] .
Baseline Characteristics of Patients Hospitalized With Severe Sepsis by AF Status
| Variable | No AF (n = 95,536) | New-Onset AF (n = 9,540) | Prior AF (n = 33,646) | P Valuea |
| Patient factors | ||||
| Demographics | ||||
| Age, y | 79.5 ± 7.7 | 80.7 ± 7.6 | 81.3 ± 7.3 | < .001 |
| Male sex | 39,327 (41.2) | 4,246 (44.5) | 15,425 (45.8) | < .001 |
| Race | < .001 | |||
| White | 77,643 (81.3) | 8,395 (88.0) | 29,957 (89.0) | |
| Black | 12,499 (13.1) | 761 (8.0) | 2,524 (7.5) | |
| Other | 5,394 (5.6) | 384 (4.0) | 1,165 (3.5) | |
| Comorbid conditions | ||||
| Cancer | 35,300 (36.9) | 3,358 (35.2) | 12,976 (38.6) | < .001 |
| Chronic lung disease | 57,279 (60.0) | 5,902 (61.9) | 24,489 (72.8) | < .001 |
| Dementia | 29,286 (30.7) | 2,316 (24.3) | 10,239 (30.4) | < .001 |
| Diabetes mellitus | 51,445 (53.8) | 4,747 (49.8) | 20,291 (60.3) | < .001 |
| Heart failure | 52,213 (54.7) | 6,398 (67.1) | 28,343 (84.2) | < .001 |
| Hypertension | 85,216 (89.2) | 8,407 (88.1) | 31,982 (95.1) | < .001 |
| Ischemic heart disease | 62,757 (65.7) | 6,704 (70.3) | 28,975 (86.1) | < .001 |
| Peripheral vascular disease | 52,274 (54.7) | 5,021 (52.6) | 22,396 (66.6) | < .001 |
| Prior stroke/TIA | 46,395 (48.6) | 4,304 (45.1) | 19,961 (59.3) | < .001 |
| Renal disease | 33,673 (35.2) | 3,184 (33.4) | 15,405 (45.8) | < .001 |
| Valvular heart disease | 42,705 (44.7) | 5,223 (54.7) | 23,840 (70.9) | < .001 |
| CHA2DS2-VASc score | 6.0 ± 1.9 | 6.1 ± 1.8 | 6.8 ± 1.6 | < .001 |
| Sepsis factors | ||||
| Type of acute organ failure | ||||
| Circulatory | 13,695 (14.3) | 1,625 (17.0) | 5,243 (15.6) | < .001 |
| Hematologic | 5,116 (5.4) | 694 (7.3) | 1,916 (5.7) | < .001 |
| Hepatic | 1,147 (1.2) | 102 (1.1) | 368 (1.1) | .19 |
| Metabolic | 5,654 (5.9) | 618 (6.5) | 1,697 (5.0) | < .001 |
| Neurologic | 5,650 (5.9) | 419 (4.4) | 1,916 (5.7) | < .001 |
| Renal | 25,943 (27.2) | 2,773 (29.1) | 10,077 (30.0) | < .001 |
| Respiratory | 15,159 (15.9) | 2,377 (24.9) | 5,927 (17.6) | < .001 |
| No. organ failures documented | 0.8 ± 1.0 | 0.9 ± 1.0 | 0.8 ± 1.0 | < .001 |
| Type of infection | ||||
| GI infection | 8,045 (8.4) | 885 (9.3) | 2,300 (6.8) | < .001 |
| Pneumonia | 24,216 (25.3) | 2,966 (31.1) | 9,391 (27.9) | < .001 |
| Primary bacteremia or fungemia | 25,900 (27.1) | 2,780 (29.1) | 9,363 (27.8) | < .001 |
| Skin or soft tissue infection | 5,614 (5.9) | 509 (5.3) | 2,387 (7.1) | < .001 |
| Urinary tract infection | 44,751 (46.8) | 3,699 (38.8) | 14,936 (44.4) | < .001 |
| Other infection source | 115 (0.1) | 30 (0.3) | 44 (0.1) | < .001 |
Data are presented as No (%) or mean ± SD. AF = atrial fibrillation; CHA2DS2-VASc = congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, previous stroke/transient ischemic attack, vascular disease, age 65 to 74 years, sex category; TIA = transient ischemic attack.
P value is for comparison among all three atrial fibrillation groups.
Table 2 demonstrates the occurrence of AF after the index sepsis hospitalization stratified by AF status during the sepsis hospitalization. Death presented a strong competing risk: 44.0% of patients died within 1 year of discharge from the index sepsis hospitalization. Within 1 year of the sepsis hospitalization, 44.2% of patients with new-onset AF during sepsis were given another AF diagnosis, as compared with 57.2% of patients with prior AF and 7.7% of patients with no AF (prior to or during the index sepsis hospitalization).
TABLE 2 ] .
Postdischarge Identification of AF by AF Status During Sepsis
| Time From Sepsis Hospitalization | Rate of AF After Sepsis Hospitalization | |||
| No AFa (n = 95,536) | New-Onset AFa (n = 9,540) | Prior AFa (n = 33,646) | P Valueb | |
| 1 y | 7,315 (7.7) | 4,193 (44.2) | 19,147 (57.2) | < .001 |
| 2 y | 9,760 (10.5) | 4,651 (49.3) | 20,304 (60.9) | < .001 |
| 3 y | 11,315 (12.6) | 4,874 (52.0) | 20,695 (62.3) | < .001 |
| 4 y | 12,394 (14.3) | 4,987 (53.6) | 20,877 (63.1) | < .001 |
| 5 y | 13,080 (15.5) | 5,074 (54.9) | 20,967 (63.5) | < .001 |
Data are presented as No. of events (rate [%]). Rates were calculated using the cumulative incidence function, accounting for the competing risk of mortality. See Table 1 legend for expansion of abbreviation.
AF status during index sepsis hospitalization.
P value is for comparison among all three atrial fibrillation groups.
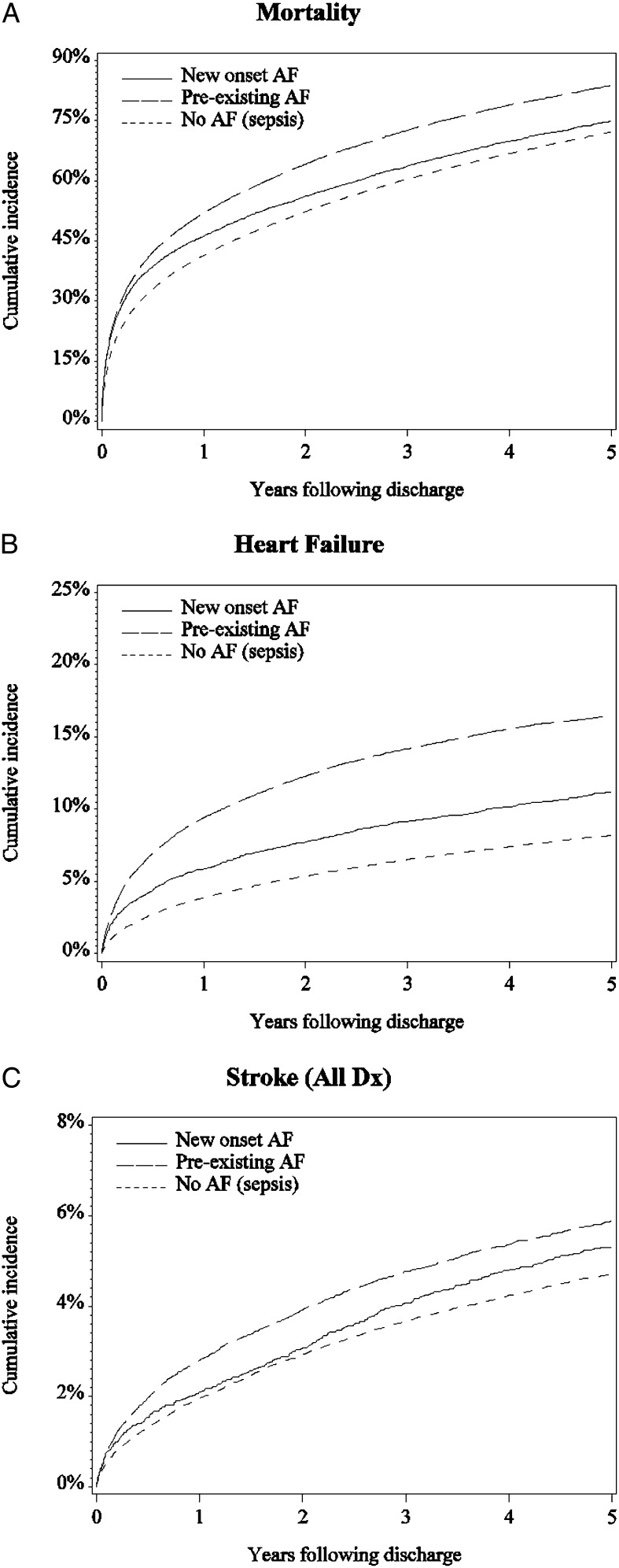
Incidences of mortality, heart failure, and ischemic stroke associated with AF status during sepsis are shown in Table 3. The results of multivariable-adjusted models are shown in Table 4. When compared with patients without AF, patients with new-onset AF during sepsis had a greater risk of postsepsis hospitalization mortality (5-year unadjusted risk, 74.8% vs 72.1%; multivariable-adjusted hazard ratio (HR), 1.04 95% CI, 1.01-1.07), heart failure hospitalization (11.2% vs 8.2%; HR, 1.25; 95% CI, 1.16-1.34), and ischemic stroke hospitalization (5.3% vs 4.7%; HR, 1.22; 95% CI, 1.15-1.47). Cumulative incidence plots and related data for postsepsis hospitalization mortality, heart failure, and ischemic stroke hospitalizations are shown in Figure 1 and Table 5. Hazards associated with one or more of the AF groups were nonproportional for heart failure and mortality (e-Table 3 (408.3KB, pdf) ); relative heart failure and mortality risks associated with new-onset AF (vs no AF) declined over time. More than 99% of our study sample had a CHA2DS2-VASC score ≥ 2. Stratification of long-term ischemic stroke risk by CHA2DS2-VASC score showed similar discrimination for patients with new-onset AF (C statistic, 0.617; prior AF, 0.623; and no AF, 0.624 during the sepsis hospitalization [e-Table 4 (408.3KB, pdf) ]). Among patients with new-AF during sepsis who had an ischemic stroke following the sepsis hospitalization, 47.5% (203 of 427) did not receive another AF diagnosis before the ischemic stroke.
TABLE 3 ] .
Cumulative Incidence of Adverse Outcomes Following Discharge From Sepsis Hospitalization by AF Status During Sepsis
| Outcome | No AF | New-Onset AF | Prior AF |
| Mortality, No. eligible | 95,536 | 9,540 | 33,646 |
| 1 ya | 39,353 (41.5) | 4,383 (46.2) | 17,386 (52.0) |
| 2 y | 48,837 (52.3) | 5,270 (56.2) | 21,127 (64.2) |
| 3 y | 54,899 (60.4) | 5,855 (63.7) | 23,326 (72.7) |
| 4 y | 58,956 (66.8) | 6,293 (69.9) | 24,682 (78.9) |
| 5 y | 61,793 (72.1) | 6,590 (74.8) | 25,554 (83.8) |
| Heart failure, No. eligible | 95,536 | 9,540 | 33,646 |
| 1 yb | 3,666 (3.9) | 556 (5.9) | 3,142 (9.4) |
| 2 y | 4,980 (5.4) | 722 (7.7) | 4,022 (12.3) |
| 3 y | 5,830 (6.5) | 834 (9.2) | 4,517 (14.2) |
| 4 y | 6,397 (7.4) | 903 (10.2) | 4,811 (15.5) |
| 5 y | 6,804 (8.2) | 966 (11.2) | 4,980 (16.5) |
| Ischemic stroke, No. eligible | 93,025 | 9,185 | 32,509 |
| 1 yb | 1,807 (2.0) | 191 (2.1) | 901 (2.8) |
| 2 y | 2,620 (2.9) | 273 (3.1) | 1,231 (3.9) |
| 3 y | 3,159 (3.7) | 349 (4.1) | 1,444 (4.8) |
| 4 y | 3,515 (4.2) | 398 (4.8) | 1,569 (5.4) |
| 5 y | 3,753 (4.7) | 427 (5.3) | 1,659 (5.9) |
Data are presented as No. of events (rate [%]). P value for comparison among all three AF groups < .001 for each outcome. See Table 1 legend for expansion of abbreviation.
Rates calculated using Kaplan-Meier methods.
Rates calculated using the cumulative incidence function, accounting for the competing risk of mortality.
TABLE 4 ] .
Proportional Hazards Regression Model: Association of AF Status With Outcomes
| Outcome Model | New-Onset AF vs No AF | Prior AF vs No AF | New-Onset AF vs Prior AF | |||
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Mortality | ||||||
| Unadjusted | 1.12 (1.09-1.15) | < .001 | 1.39 (1.37-1.41) | < .001 | 0.81 (0.78-0.83) | < .001 |
| Adjusted, patient factors | 1.09 (1.06-1.12) | < .001 | 1.18 (1.17-1.20) | < .001 | 0.92 (0.90-0.95) | < .001 |
| Adjusted, patient and sepsis factors | 1.04 (1.01-1.07) | .009 | 1.19 (1.17-1.21) | < .001 | 0.87 (0.84-0.89) | < .001 |
| Heart failure | ||||||
| Unadjusted | 1.53 (1.43-1.64) | < .001 | 2.70 (2.60-2.80) | < .001 | 0.57 (0.53-0.61) | < .001 |
| Adjusted, patient factors | 1.28 (1.19-1.37) | < .001 | 1.71 (1.65-1.78) | < .001 | 0.75 (0.69-0.80) | < .001 |
| Adjusted, patient and sepsis factors | 1.25 (1.16-1.34) | < .001 | 1.71 (1.65-1.78) | < .001 | 0.73 (0.68-0.78) | < .001 |
| Ischemic stroke | ||||||
| Unadjusted | 1.21 (1.09-1.34) | < .001 | 1.58 (1.49-1.68) | < .001 | 0.76 (0.68-0.85) | < .001 |
| Adjusted, patient factors | 1.20 (1.08-1.33) | < .001 | 1.37 (1.28-1.46) | < .001 | 0.88 (0.78-0.98) | .02 |
| Adjusted, patient and sepsis factors | 1.22 (1.10-1.36) | < .001 | 1.37 (1.28-1.46) | < .001 | 0.89 (0.80-1.00) | .05 |
Patient factors include demographics (age, sex, race), medical history (cancer, COPD, dementia, diabetes mellitus, heart failure, hypertension, ischemic heart disease, peripheral vascular disease, prior ischemic stroke/TIA, renal disease, valvular heart disease) and year of hospitalization. Sepsis factors include type of acute organ failure (circulatory, hematologic, hepatic, metabolic, neurologic, renal, respiratory) and type of infection (GI infection, pneumonia, primary bacteremia or fungemia, skin or soft tissue infection, urinary tract infection, other infection source). HR = hazard ratio. See Table 1 legend for expansion of other abbreviations.
Figure 1 –
Cumulative incidence of mortality, heart failure, and ischemic stroke following discharge from sepsis hospitalization, stratified by AF status during sepsis. See Table 5 for additional information on the number of patients eligible for each outcome at each time point. A, Mortality. B, Heart failure. C, Ischemic stroke. AF = atrial fibrillation; Dx = diagnosis positions on billing claim.
TABLE 5 ] .
Number of Patients at Risk Each Year Following Discharge
| Outcome | Number at Risk Each Year Following Discharge | |||||
| At Discharge | Y 1 | Y 2 | Y 3 | Y 4 | Y 5 | |
| Mortality | ||||||
| No AF | 95,536 | 54,528 | 38,367 | 27,306 | 19,158 | 13,622 |
| New-onset AF | 9,540 | 5,004 | 3,645 | 2,682 | 1,967 | 1,426 |
| Prior AF | 33,646 | 15,807 | 10,049 | 6,488 | 4,135 | 2,623 |
| Heart failure | ||||||
| No AF | 95,536 | 52,415 | 36,304 | 25,494 | 17,714 | 12,463 |
| New-onset AF | 9,540 | 4,692 | 3,359 | 2,420 | 1,748 | 1,243 |
| Prior AF | 33,646 | 14,137 | 8,626 | 5,399 | 3,366 | 2,105 |
| Ischemic stroke | ||||||
| No AF | 93,025 | 52,675 | 36,699 | 25,874 | 17,998 | 12,717 |
| New-onset AF | 9,185 | 4,794 | 3,472 | 2,522 | 1,820 | 1,309 |
| Prior AF | 32,509 | 15,108 | 9,463 | 6,033 | 3,807 | 2,392 |
See Table 1 legend for expansion of abbreviations.
Sensitivity analysis using ischemic stroke identified from a principal diagnosis alone was similar to the primary analysis of ischemic stroke in any diagnostic position (HR new-onset AF, 1.30 [95% CI, 1.15-1.47]; HR prior AF, 1.46 [95% CI, 1.35-1.59] vs no AF). Sensitivity analyses using an alternative algorithm to detect severe sepsis in administrative data identified 252,137 severe sepsis survivors with a distribution of demographics, comorbidities, acute organ failures, and infectious sources by AF status similar to the primary definition of sepsis (e-Table 5 (408.3KB, pdf) ). Outcome results were also similar in the sensitivity analysis: patients with new-onset AF during severe sepsis, as compared with no prior AF during severe sepsis, had greater risks of post-sepsis hospitalization AF (e-Table 6 (408.3KB, pdf) ), mortality, heart failure, and ischemic stroke (e-Fig 1 (408.3KB, pdf) , e-Tables 7, 8 (408.3KB, pdf) ).
Discussion
Among a cohort of Medicare beneficiaries who survived a sepsis hospitalization, we observed that new-onset AF during the sepsis hospitalization was associated with a high risk of AF occurrence within 5 years of the sepsis hospitalization. Our findings challenge the current opinion that new-onset AF during sepsis is generally a transient problem that reverses with resolution of sepsis. In addition, new-onset AF during sepsis was associated with increased risks of postdischarge death, heart failure, and ischemic stroke when compared with sepsis survivors with no AF, but lower risks compared with patients with prior AF. Approximately one-half of the patients with new-onset AF during sepsis who later suffered an ischemic stroke did not have another AF diagnosis preceding the ischemic stroke, raising the possibility that new-onset AF during sepsis may represent a lost opportunity to implement thromboembolism prophylaxis in some patients. Thus, new-onset AF during sepsis appears to be a marker of future AF risk and AF-associated complications that may have implications for postdischarge AF surveillance and treatment.
Few studies have evaluated the long-term risks associated with the development of new-onset AF during sepsis. In an analysis of California state inpatient administrative data with an average of 6 months of follow-up of severe sepsis survivors, we found a nonstatistically significant increased ischemic stroke risk in patients with new-onset AF when compared with patients with no AF.1 The current investigation demonstrates that elevated ischemic stroke risks in patients with new-onset AF during sepsis persist after hospital discharge and adds novel evidence that the occurrence of new-AF during sepsis is associated with elevated risks of postdischarge AF and other AF-associated complications such as heart failure and death. Our findings were robust to adjustment for demographics, year of hospitalization, comorbid conditions, sepsis severity, type of infection, and to sensitivity analyses using alternative algorithms to detect severe sepsis and ischemic stroke. We identified that the CHA2DS2-VASc score discriminated postsepsis ischemic stroke risks with an accuracy similar to that in prior reports,16 interestingly, regardless of AF status.
Although Medicare beneficiaries with new-onset AF during sepsis have an especially high risk of poor outcomes, all sepsis survivors had relatively high 5-year morbidity and mortality. Wunsch et al19 reported a 3-year mortality of 39.5% among Medicare beneficiaries who survived a critical illness, substantially lower than the approximately 60% 3-year mortality after a sepsis hospitalization identified in our study. The sepsis survivors identified in our study may have had greater mortality because of their older age and substantially more comorbidities than Medicare beneficiaries who survived intensive care for other indications. Further, sepsis survivors often have significant long-term cognitive and functional decrements that may worsen prognosis compared with beneficiaries hospitalized with other conditions.20 In a prior report using Medicare Health and Retirement Survey data, 81.9% of Medicare beneficiaries died within 5 years of a severe sepsis hospitalization,21 approximately 10% greater than the 5-year mortality risks in our sample. However, because our 5-year mortality estimate included sepsis survivors only, without accounting for the approximately 30% inhospital mortality associated with sepsis, the mortality rates we present are likely consistent with prior estimates among sepsis survivors.
Rates of heart failure hospitalizations following sepsis were also high. Chen et al22 found heart failure hospitalization rates of approximately 2% per year among Medicare beneficiaries, substantially lower than the 5% heart failure hospitalization rates we identified in the year following a sepsis hospitalization. Potential explanations for the high heart failure risks identified among sepsis survivors include a greater prevalence of comorbidities among sepsis survivors or possible complications of sepsis-induced cardiac dysfunction.23
Ischemic stroke estimates among patients with AF after sepsis hospitalizations are consistent with previous reports among Medicare beneficiaries,24 but appear to differ from the ischemic stroke estimates described in prospective cohorts. For example, the ischemic stroke rate of 2.8% in the year following sepsis among patients with prior AF is lower than previous estimates from patients with AF who were not provided anticoagulation therapy (5% to 8% annual ischemic stroke risks),25,26 but greater than that of patients with AF and CHA2DS2-VASc scores ≥ 2 who were provided anticoagulation (1.7% ischemic stroke yearly risk).27 Shroff et al24 showed that ischemic stroke rates among Medicare beneficiaries with AF have declined since 1992 as the use of anticoagulation therapy has increased. Whether differences in ischemic stroke rates between Medicare samples and prospective cohorts with AF are a result of increased use of anticoagulation, lower sensitivity of ICD-9-CM codes, or changing competing risks is currently unclear and warrants further study.
Long-term outcomes linked to other potentially “reversible conditions” associated with new-onset AF, such as postcardiac surgery, may be similar to our findings in sepsis. For example, Almassi et al28 have shown that patients with new-onset AF after cardiac surgery had elevated long-term risks of stroke, heart failure, and death.
Our study has limitations. Algorithms that identify diagnoses from administrative claims data are generally highly specific but insensitive; we likely underestimated the incidence of AF during sepsis and adverse outcomes after sepsis hospitalization. Although we could not confirm diagnoses with results of ancillary testing (eg, imaging confirmation of ischemic stroke), we used ICD-9 coding algorithms that have been validated previously with known performance characteristics. Whether patients not identified by our coding algorithms would have different risks or whether the previously validated ICD-9 algorithms we used to detect diagnoses have different performance characteristics in Medicare data are unclear. Further, we could not identify whether AF occurred during active sepsis or only during a hospitalization with sepsis. We could not exclude the possibility that patients with new-onset AF or no AF had previously undetected AF. Algorithms that may discriminate among paroxysmal, persistent, and permanent AF using administrative data are currently unavailable and, thus, we were unable to determine the type of AF after the sepsis hospitalization. Further studies will be needed to determine the reasons for the nonproportionality of hazards for mortality and heart failure outcomes after sepsis. We also note that our results may not necessarily represent long-term AF risks in patients not enrolled in continuous fee-for-service Medicare, such as younger patients or those enrolled in private insurance. Because the risk of bleeding and the use of antithrombotic medications is unclear in our study sample, and because the possibility of unmeasured confounding precludes confirmation of a causal relationship between new-onset AF and poor long-term outcomes, our results cannot be used to determine whether anticoagulation may provide a benefit to patients with new-onset AF during sepsis.
Conclusions
In conclusion, patients with new-onset AF during sepsis have greater risks of future occurrence of AF, heart failure, and death than do sepsis survivors without prior known AF. Future studies are needed to determine if patients with new-onset AF during sepsis would benefit from increased postdischarge surveillance for AF or anticoagulation.
Supplementary Material
Online Supplement
Acknowledgments
Author contributions: A. J. W. is the guarantor of the paper and takes responsibility for the integrity of the work as a whole, from inception to published article. A. J. W., L. H. C., and E. J. B. contributed to the concept and design of the study, interpretation of the results, writing of the manuscript, and approval of the final manuscript; and B. G. H. contributed to the concept and design of the study, interpretation of the results, statistical analyses, design of the figures, writing of the manuscript, and approval of the final manuscript.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Curtis has received research support from Johnson & Johnson. Drs Walkey and Benjamin and Mr Hammill have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: The funding sources had no role in the study design; the collection, analysis, and interpretation of data; the writing of the report; or the decision to submit the paper for publication.
Additional information: The e-Figure and e-Tables can be found in the Supplemental Materials section of the online article.
ABBREVIATIONS
- AF
atrial fibrillation
- CHA2DS2-VASc
congestive heart failure, hypertension, age ≥ 75 years, diabetes mellitus, previous stroke/transient ischemic attack, vascular disease, age 65 to 74 years, sex category
- HR
hazard ratio
- ICD-9-CM
International Classification of Diseases, Ninth Revision, Clinical Modification
- PPV
positive predictive value
Footnotes
FOR EDITORIAL COMMENT SEE PAGE 1138
FUNDING/SUPPORT: This study was funded by the US National Institutes of Health, National Heart, Lung, and Blood Institute [K01 HL116768 (to Dr Walker) and R01 HL102214, R01 HL092577, R01NS17950 (to Dr Benjamin)].
Reproduction of this article is prohibited without written permission from the American College of Chest Physicians. See online for more details.
References
- 1.Walkey AJ, Wiener RS, Ghobrial JM, Curtis LH, Benjamin EJ. Incident stroke and mortality associated with new-onset atrial fibrillation in patients hospitalized with severe sepsis. JAMA. 2011;306(20):2248-2254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walkey AJ, Greiner MA, Heckbert SR, et al. Atrial fibrillation among Medicare beneficiaries hospitalized with sepsis: incidence and risk factors. Am Heart J. 2013;165(6):949-955.e3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meierhenrich R, Steinhilber E, Eggermann C, et al. Incidence and prognostic impact of new-onset atrial fibrillation in patients with septic shock: a prospective observational study. Crit Care. 2010;14(3):R108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stevenson EK, Rubenstein AR, Radin GT, Wiener RS, Walkey AJ. Two decades of mortality trends among patients with severe sepsis: a comparative meta-analysis. Crit Care Med. 2014;42(3):625-631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Annane D, Sébille V, Duboc D, et al. Incidence and prognosis of sustained arrhythmias in critically ill patients. Am J Respir Crit Care Med. 2008;178(1):20-25 [DOI] [PubMed] [Google Scholar]
- 6.Fuster V, Rydén LE, Cannom DS, et al. ; American College of Cardiology Foundation/American Heart Association Task Force. 2011 ACCF/AHA/HRS focused updates incorporated into the ACC/AHA/ESC 2006 guidelines for the management of patients with atrial fibrillation: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2011;123(10):e269-e367 [DOI] [PubMed] [Google Scholar]
- 7.Iwashyna TJ, Odden A, Rohde J, et al. Identifying patients with severe sepsis using administrative claims: patient-level validation of the Angus Implementation of the International Consensus Conference definition of severe sepsis. Med Care. 2014;52(6):e39-e43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546-1554 [DOI] [PubMed] [Google Scholar]
- 9.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29(7):1303-1310 [DOI] [PubMed] [Google Scholar]
- 10.Glazer NL, Dublin S, Smith NL, et al. Newly detected atrial fibrillation and compliance with antithrombotic guidelines. Arch Intern Med. 2007;167(3):246-252 [DOI] [PubMed] [Google Scholar]
- 11.Klabunde CN, Potosky AL, Legler JM, Warren JL. Development of a comorbidity index using physician claims data. J Clin Epidemiol. 2000;53(12):1258-1267 [DOI] [PubMed] [Google Scholar]
- 12.Piccini JP, Hammill BG, Sinner MF, et al. Incidence and prevalence of atrial fibrillation and associated mortality among Medicare beneficiaries, 1993-2007. Circ Cardiovasc Qual Outcomes. 2012;5(1):85-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arday SL, Arday DR, Monroe S, Zhang J. HCFA’s racial and ethnic data: current accuracy and recent improvements. Health Care Financ Rev. 2000;21(4):107-116 [PMC free article] [PubMed] [Google Scholar]
- 14.Birman-Deych E, Waterman AD, Yan Y, Nilasena DS, Radford MJ, Gage BF. Accuracy of ICD-9-CM codes for identifying cardiovascular and stroke risk factors. Med Care. 2005;43(5):480-485 [DOI] [PubMed] [Google Scholar]
- 15.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130-1139 [DOI] [PubMed] [Google Scholar]
- 16.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on Atrial Fibrillation. Chest. 2010;137(2):263-272 [DOI] [PubMed] [Google Scholar]
- 17.Tirschwell DL, Longstreth WT, Jr. Validating administrative data in stroke research. Stroke. 2002;33(10):2465-2470 [DOI] [PubMed] [Google Scholar]
- 18.Saczynski JS, Andrade SE, Harrold LR, et al. A systematic review of validated methods for identifying heart failure using administrative data. Pharmacoepidemiol Drug Saf. 2012;21(suppl 1):129-140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wunsch H, Guerra C, Barnato AE, Angus DC, Li G, Linde-Zwirble WT. Three-year outcomes for Medicare beneficiaries who survive intensive care. JAMA. 2010;303(9):849-856 [DOI] [PubMed] [Google Scholar]
- 20.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787-1794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iwashyna TJ, Cooke CR, Wunsch H, Kahn JM. Population burden of long-term survivorship after severe sepsis in older Americans. J Am Geriatr Soc. 2012;60(6):1070-1077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen J, Normand SL, Wang Y, Krumholz HM. National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries, 1998-2008. JAMA. 2011;306(15):1669-1678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Merx MW, Weber C. Sepsis and the heart. Circulation. 2007;116(7):793-802 [DOI] [PubMed] [Google Scholar]
- 24.Shroff GR, Solid CA, Herzog CA. Temporal trends in ischemic stroke and anticoagulation therapy among Medicare patients with atrial fibrillation: a 15-year perspective (1992-2007). JAMA Intern Med. 2013;173(2):159-160 [DOI] [PubMed] [Google Scholar]
- 25.Risk factors for stroke and efficacy of antithrombotic therapy in atrial fibrillation. Analysis of pooled data from five randomized controlled trials. Arch Intern Med. 1994;154(13):1449-1457 [PubMed] [Google Scholar]
- 26.Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation: a major contributor to stroke in the elderly. The Framingham Study. Arch Intern Med. 1987;147(9):1561-1564 [PubMed] [Google Scholar]
- 27.Lip GY, Frison L, Halperin JL, Lane DA. Identifying patients at high risk for stroke despite anticoagulation: a comparison of contemporary stroke risk stratification schemes in an anticoagulated atrial fibrillation cohort. Stroke. 2010;41(12):2731-2738 [DOI] [PubMed] [Google Scholar]
- 28.Almassi GH, Schowalter T, Nicolosi AC, et al. Atrial fibrillation after cardiac surgery: a major morbid event? Ann Surg. 1997;226(4):501-511 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement