Abstract
BACKGROUND:
Deficient nitric oxide-soluble guanylate cyclase-cyclic guanosine monophosphate signaling results from endothelial dysfunction and may underlie impaired cardiac relaxation in patients with heart failure with preserved left ventricular ejection fraction (HFpEF) and pulmonary hypertension (PH). The acute hemodynamic effects of riociguat, a novel soluble guanylate cyclase stimulator, were characterized in patients with PH and HFpEF.
METHODS:
Clinically stable patients receiving standard HF therapy with a left ventricular ejection fraction > 50%, mean pulmonary artery pressure (mPAP) ≥ 25 mm Hg, and pulmonary arterial wedge pressure (PAWP) > 15 mm Hg at rest were randomized to single oral doses of placebo or riociguat (0.5, 1, or 2 mg). The primary efficacy variable was the peak decrease in mPAP from baseline up to 6 h. Secondary outcomes included hemodynamic and echocardiographic parameters, safety, and pharmacokinetics.
RESULTS:
There was no significant change in peak decrease in mPAP with riociguat 2 mg (n = 10) vs placebo (n = 11, P = .6). However, riociguat 2 mg significantly increased stroke volume (+9 mL [95% CI, 0.4-17]; P = .04) and decreased systolic BP (−12 mm Hg [95% CI, −22 to −1]; P = .03) and right ventricular end-diastolic area (−5.6 cm2 [95% CI, −11 to −0.3]; P = .04), without significantly changing heart rate, PAWP, transpulmonary pressure gradient, or pulmonary vascular resistance. Riociguat was well tolerated.
CONCLUSIONS:
In patients with HFpEF and PH, riociguat was well tolerated, had no significant effect on mPAP, and improved exploratory hemodynamic and echocardiographic parameters.
TRIAL REGISTRY:
ClinicalTrials.gov; No.: NCT01172756; URL: www.clinicaltrials.gov
Approximately 30% to 50% of patients with heart failure (HF) have a preserved left ventricular ejection fraction (HFpEF), a condition associated with dismal prognosis1 and for which there are still no proven therapies to improve outcomes.2 Several pathophysiologic mechanisms have been demonstrated in HFpEF, including left ventricular (LV) diastolic dysfunction,3 arterial stiffening, and abnormalities of LV-arterial coupling,4,5 which may compromise LV energetic efficiency. With longstanding pulmonary venous congestion, the majority of patients with HFpEF develop increased pulmonary arterial pressures and pulmonary hypertension (PH). PH has been shown to be a major determinant of mortality in this population and represents a potential novel therapeutic target in HFpEF.6,7
HF- and PH-related diseases are characterized by endothelial dysfunction.8‐12 Preclinical and small clinical studies suggest that deficient nitric oxide-soluble guanylate cyclase-cyclic guanosine monophosphate (NO-sGC-cGMP) signaling is involved in impaired cardiac relaxation/distensibility.13 Furthermore, low cyclic guanosine monophosphate (cGMP) levels in myocardial tissue may underlie abnormal cardiomyocyte function.14 Thus, targeting the NO-sGC-cGMP signaling pathway may be a promising approach for the treatment of HFpEF with PH.
Riociguat is a novel soluble guanylate cyclase (sGC) stimulator15 with a dual mode of action, sensitizing sGC to endogenous nitric oxide (NO) and directly stimulating sGC independent of NO.16 Riociguat induces vasodilation and has antifibrotic, antiproliferative, and antiinflammatory effects.15‐18 In clinical studies, riociguat has proven efficacy in pulmonary arterial hypertension and chronic thromboembolic PH.19,20 In a randomized, placebo-controlled phase 2b study in patients with HF and PH due to systolic LV dysfunction (Left Ventricular Systolic Dysfunction Associated With Pulmonary Hypertension Riociguat Trial [LEPHT]), riociguat was well tolerated and improved cardiac index, pulmonary (PVR) and systemic vascular resistance (SVR), as well as quality of life, without significantly changing mean pulmonary artery pressure (mPAP, primary end point) or systolic BP.21 In the current study (Acute Hemodynamic Effects of Riociguat in Patients With Pulmonary Hypertension Associated With Diastolic Heart Failure [DILATE-1]), we aimed to characterize the hemodynamic effects, safety, and pharmacokinetics of single oral doses of riociguat in patients with HFpEF and PH.
Materials and Methods
Study Design
DILATE-1 (clinicaltrials.gov: NCT01172756) was a double-blind, randomized, placebo-controlled, parallel-group phase 2a study conducted in five centers across Austria, the Czech Republic, and Germany. Patients received oral placebo or riociguat 0.5 mg, 1 mg, or 2 mg in three subsequent ascending dose cohorts.
The following local ethics committees approved the research protocol (EudraCT number: 2010-018436-41): Ethics Committee of the Medical University of Vienna and the General Hospital of Vienna-AKH, Ethics Committee of the A.Ö. Hospital Elisabethinen, Ethics Committee for the State of Salzburg, Ethics Committee of the University of Prague, and Ethics Committee of the Medical Faculty of the University of Cologne. Written informed consent was obtained from patients in accordance with the Declaration of Helsinki.
Patient Population
Eligible patients were those aged ≥ 18 years with symptomatic HFpEF and PH despite optimized therapy with a stable dose of standard medication for the control of symptoms and risk factors for > 30 days (≥ 7 days for diuretic therapy). Detailed inclusion and exclusion criteria are provided in e-Appendix 1 (307.4KB, pdf) .
Study Procedures
Right-sided heart catheterization was performed by insertion of a balloon-tipped pulmonary artery thermodilution catheter via the right internal jugular vein. Hemodynamic and echocardiographic parameters were recorded at regular intervals up to 6 h after intake of study drug. Blood samples for pharmacokinetics and exploratory biomarkers were taken at regular intervals up to 24 h after intake of study drug. Safety was assessed via unblinded data review by an independent data-monitoring committee. Additional safety follow-ups occurred on days 14 and 30 after study drug administration.
Outcome Measures
The primary efficacy variable was the peak decrease from baseline in mPAP, defined as the largest mPAP change from baseline up to 6 h after study drug administration. Secondary variables included additional hemodynamic and echocardiographic parameters, biomarker levels, safety variables, and pharmacokinetics. For the secondary hemodynamic and echocardiographic variables, the mean change from baseline of all evaluations up to 6 h after study drug administration was calculated. Safety was assessed by adverse events (AEs), vital signs, and laboratory evaluations, as defined in e-Appendix 1 (307.4KB, pdf) .
Post hoc exploratory estimation of myocardial oxygen consumption (MVO2) was carried out as defined in e-Appendix 1 (307.4KB, pdf) . All patients who completed hemodynamic assessment were eligible for pharmacokinetic analysis. Details of pharmacokinetic analysis are provided in e-Appendix 1 (307.4KB, pdf) .
Statistical Analysis
Statistical analyses were performed using Statistical Analysis System (SAS Institute Inc). The analysis of efficacy was performed in patients valid for per-protocol analysis. The primary variable was assessed using a two-group, two-sided t test on the riociguat 2 mg group vs placebo at the .05 significance level.
Exploratory analysis of the secondary efficacy and post hoc variables was via analysis of variance, with treatment and time after study drug administration as main effects and a treatment by time interaction. This was followed by sequential pairwise comparisons between the three riociguat doses and placebo based on least squares means. AEs were analyzed descriptively in the safety population (all randomized patients). All values displayed are means ± SD unless stated otherwise.
Results
Demographic Data
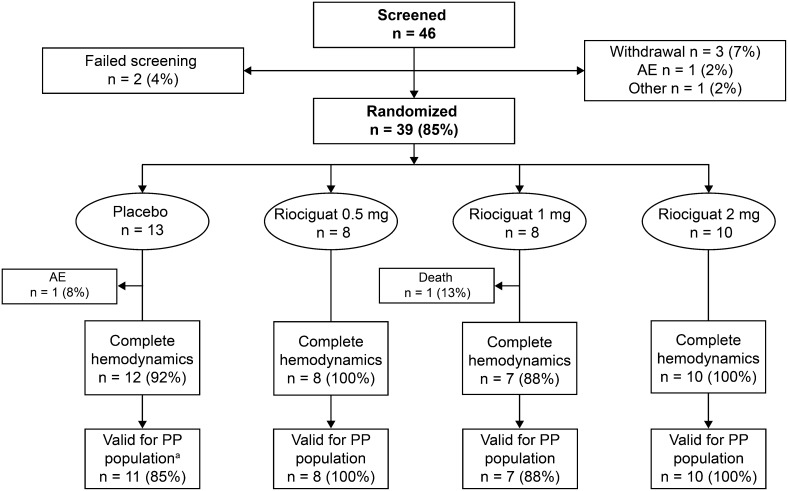
Of the 46 patients screened, 39 were eligible for study participation and 36 completed all study examinations (Fig 1). Demographic and clinical characteristics are shown in Table 1. A high proportion of patients had comorbidities typical of HFpEF, including history of atrial fibrillation (AF) (69%), AF at baseline (44%), diabetes mellitus (44%), coronary artery disease (17%), and COPD (19%). The majority of patients received antihypertensive and diuretic therapy during the study: 47% angiotensin-converting enzyme inhibitors, 36% angiotensin II receptor antagonists, 44% aldosterone antagonists, 81% β-blockers, and 67% loop diuretics (Table 2).
Figure 1 –
Patient disposition. an = 1 invalid for the PP population because of noncompliance with the protocol (laboratory parameters/ECG assessed before informed consent provided). All patients who completed the hemodynamic assessments underwent a valid pharmacokinetic analysis. One patient in the placebo group withdrew because of an AE of pain in the left shoulder. AE = adverse event; PP = per protocol.
TABLE 1 ] .
Demographic and Baseline Characteristics
| Characteristic | Placebo (n = 11) | Riociguat | Total (N = 36) | ||
| 0.5 mg (n = 8) | 1 mg (n = 7) | 2 mg (n = 10) | |||
| Male | 45 | 13 | 43 | 50 | 39 |
| White | 100 | 100 | 100 | 100 | 100 |
| Mean age, y | 75.1 (65.0-86.0) | 68.3 (48.0-80.0) | 65.3 (52.0-79.0) | 72.8 (59.0-83.0) | 71.0 (48.0-86.0) |
| BMI, kg/m2 | 30.2 (21.8-36.0) | 33.5 (22.9-44.9) | 31.0 (21.6-40.8) | 29.3 (23.5-33.4) | 30.8 (21.6-44.9) |
| AF at baseline | 55 | 50 | 43 | 30 | 44 |
| History of AF | 64 | 63 | 71 | 80 | 69 |
| Diabetes mellitus | 45 | 50 | 43 | 40 | 44 |
| Coronary artery disease | 9 | 25 | 14 | 20 | 17 |
| COPD | 36 | 13 | 0 | 20 | 19 |
| Serum creatinine,a mg/dL | 1.15 (0.64-1.96) | 1.15 (0.80-1.50) | 1.06 (0.71-1.80) | 1.20 (0.84-1.62) | … |
| Median NT-proBNP, pg/mL | 1,747.0 (407.0-4,879.0) | 1,097.0 (240.2-4,626.0) | 698.0 (253.5-1,761.0) | 1,067.4 (130.6-7,886.0) | … |
Data presented are mean (range) or percentage of patients in the per-protocol population. Coronary artery disease was not considered relevant at inclusion. AF = atrial fibrillation; NT-proBNP = N-terminal prohormone of brain natriuretic peptide.
Safety population: placebo, n = 13; 0.5 mg, n = 8; 1 mg, n = 8; 2 mg, n = 10.
TABLE 2 ] .
Concomitant Cardiovascular Therapies Administered During the Study
| Drug | Placebo (n = 11) | Riociguat | Total (N = 36) | ||
| 0.5 mg (n = 8) | 1 mg (n = 7) | 2 mg (n = 10) | |||
| Angiotensin-converting enzyme inhibitors | 3 (27) | 2 (25) | 6 (86) | 6 (60) | 17 (47) |
| Angiotensin II receptor antagonists | 5 (45) | 5 (63) | 1 (14) | 2 (20) | 13 (36) |
| Aldosterone antagonists | 7 (64) | 2 (25) | 2 (29) | 5 (50) | 16 (44) |
| β-Blocking agentsa | 10 (91) | 5 (63) | 6 (86) | 8 (80) | 29 (81) |
| Calcium channel blockers | 6 (55) | 3 (38) | 2 (29) | 5 (50) | 16 (44) |
| Amiodarone | 0 | 1 (13) | 0 | 3 (30) | 4 (11) |
| Loop or high-ceiling diuretics | 7 (64) | 3 (38) | 6 (86) | 8 (80) | 24 (67) |
| Thiazides or low-ceiling diuretics | 2 (18) | 3 (38) | 3 (43) | 5 (50) | 13 (36) |
| Digitalis glycosides | 3 (27) | 3 (38) | 2 (29) | 0 | 8 (22) |
| Oral anticoagulants | 7 (64) | 6 (75) | 5 (71) | 5 (50) | 23 (64) |
Data presented are No. (%) of patients in the per-protocol population.
Includes one patient receiving carvedilol in the placebo group.
Hemodynamics
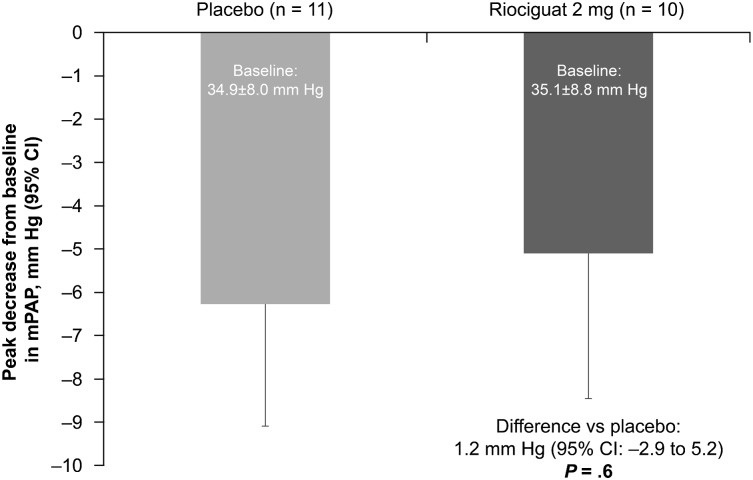
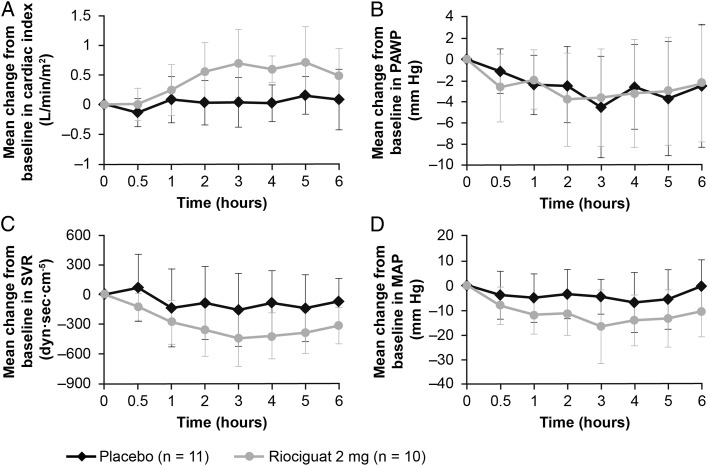
Hemodynamic parameters at baseline are provided in Table 3. There was no significant change in the primary variable—peak decrease in mPAP from baseline up to 6 h after study drug administration—in the riociguat 2 mg group vs placebo group (P = .6) (Figs 2, 3, e-Table 1 (307.4KB, pdf) ). However, stroke volume (SV) (P = .04) and cardiac index (P = .001) were significantly increased in the riociguat 2 mg group compared with the placebo group, in the absence of increases in pulmonary arterial wedge pressure (PAWP) (P = .9) and significant changes in heart rate (HR) (P = .5) (Fig 3, Table 3). In parallel, SVR (P < .05), mean arterial pressure (MAP) (P = .04), and systolic BP (P = .03) significantly decreased in the riociguat 2 mg group vs placebo (Fig 3, Table 3) without significant changes in transpulmonary pressure gradient (TPG) and PVR. Within-group changes in secondary hemodynamic parameters are provided in e-Table 2 (307.4KB, pdf) . All hemodynamic changes occurred without an increase in the estimated MVO2 (mean decrease: −0.1 mL oxygen [O2]/min/100 g) (e-Table 3 (307.4KB, pdf) ).22
TABLE 3 ] .
Baseline Values (± SD) and Changes (Least Squares Mean) From Baseline in Secondary Hemodynamic Parameters
| Parameter | Placebo (n = 11) | Riociguat | Treatment Difference (95% CI)a | ||||||
| 0.5 mg (n = 8) | 1 mg (n = 7) | 2 mg (n = 10) | |||||||
| Baseline | Change | Baseline | Change | Baseline | Change | Baseline | Change | ||
| Cardiac index, L/min/m2 | 2.2 ± 0.8 | 0.04 | 2.7 ± 0.5 | 0.1 | 2.6 ± 0.9 | 0.3 | 2.5 ± 0.5 | 0.5 | 0.4 (0.2 to 0.7) |
| P = .001 | |||||||||
| Cardiac output, L/min | 4.2 ± 1.9 | 0.08 | 5.0 ± 0.7 | 0.3 | 5.2 ± 1.9 | 0.6 | 4.9 ± 1.5 | 1.0 | 0.9 (0.3 to 1.4) |
| P = .002 | |||||||||
| Diastolic BP, mm Hg | 62.3 ± 11.9 | −2.4 | 57.4 ± 9.7 | −2.4 | 61.0 ± 10.2 | −5.8 | 58.7 ± 11.2 | −8.7 | −6.3 (−11.8 to −0.7) |
| P = .03 | |||||||||
| Systolic BP, mm Hg | 129.5 ± 20.2 | −2.1 | 143.3 ± 19.3 | −2.8 | 144.7 ± 22.8 | −16.3 | 141.6 ± 24.9 | −13.7 | −11.7 (−22.4 to −0.9) |
| P = .03 | |||||||||
| Heart rate, bpm | 66.1 ± 14.1 | 2.3 | 63.4 ± 12.2 | 0.9 | 73.0 ± 28.5 | −0.2 | 67.5 ± 7.6 | 4.6 | 2.3 (−4.4 to 9.0) |
| P = .5 | |||||||||
| Stroke volume, mL | 66.6 ± 34.0 | 0.6 | 81.5 ± 18.0 | 5.2 | 77.8 ± 36.7 | 4.0 | 75.1 ± 27.1 | 9.4 | 8.8 (0.4 to 17.3) |
| P = .04 | |||||||||
| Stroke volume index, mL/m2 | 34.2 ± 14.3 | 0.4 | 42.9 ± 7.9 | 2.6 | 39.1 ± 17.1 | 2.2 | 38.4 ± 10.4 | 4.6 | 4.2 (−0.1 to 8.4) |
| P = .05 | |||||||||
| Systemic vascular resistance, dyn/s/cm5 | 1,583 ± 689 | −90 | 1,246 ± 392 | −113 | 1,352 ± 795 | −366 | 1,294 ± 481 | −336 | −247 (−490 to −4) |
| P < .05 | |||||||||
| Systemic vascular resistance index, dyn/s/cm5/m2 | 2,938 ± 1127 | −170 | 2,356 ± 797 | −229 | 2,581 ± 1,379 | −691 | 2,398 ± 633 | −624 | −455 (−905 to −4) |
| P < .05 | |||||||||
| Pulmonary vascular resistance, dyn/s/cm5 | 303.5 ± 168.8 | −4.6 | 217.1 ± 60.5 | −5.7 | 223.7 ± 169.5 | −28.2 | 227.7 ± 105.7 | −20.8 | −16.2 (−78.2 to 45.9) |
| P = .6 | |||||||||
| Pulmonary vascular resistance index, dyn/s/cm5/m2 | 554.5 ± 265.6 | −6.4 | 417.5 ± 140.6 | −13.1 | 435.9 ± 317.4 | −53.2 | 425.0 ± 178.0 | −39.4 | −33.0 (−147.2 to 81.3) |
| P = .6 | |||||||||
| Pulmonary arterial wedge pressure, mm Hg | 21.1 ± 6.4 | −2.8 | 18.6 ± 2.1 | −1.7 | 19.4 ± 2.1 | 0.1 | 21.8 ± 4.6 | −2.9 | −0.2 (−3.0 to 2.6) |
| P = .9 | |||||||||
| Right atrial pressure, mm Hg | 11.6 ± 3.0 | −0.9 | 9.5 ± 3.1 | −0.7 | 12.7 ± 4.6 | 0.5 | 11.7 ± 4.4 | −1.7 | −0.8 (−3.9 to 2.2) |
| P = .6 | |||||||||
| Mean arterial pressure, mm Hg | 83.9 ± 15.9 | −4.3 | 85.0 ± 11.9 | −4.1 | 86.0 ± 13.1 | −9.3 | 85.6 ± 14.8 | −12.3 | −8.0 (−15.5 to −0.6) |
| P = .04 | |||||||||
| Mixed venous oxygen saturation, % | 60.4 ± 7.8 | 1.2 | 66.6 ± 7.3 | −1.1 | 67.8 ± 5.4b | 3.1 | 64.3 ± 5.3c | 2.5 | 1.3 (−3.2 to 5.8) |
| P = .6 | |||||||||
| Transpulmonary pressure gradient, mm Hg | 13.8 ± 5.6 | −0.1 | 13.4 ± 3.1 | 0.4 | 11.7 ± 4.9 | 0.9 | 13.3 ± 6.3 | 0.7 | 0.8 (−2.2 to 3.9) |
| P = .6 | |||||||||
The mean changes from baseline of all evaluations up to 6 h after study drug administration are shown. bpm = beats/min.
Riociguat 2 mg vs placebo.
n = 6.
n = 9.
Figure 2 –
Peak decrease in mPAP from baseline up to 6 h after administration of study drug in the riociguat 2 mg group vs placebo group (primary end point). The difference between treatment groups was analyzed by a two-group, two-sided t test. The treatment difference (95% CI) and P value are also shown. mPAP = mean pulmonary artery pressure.
Figure 3 –
Mean change (± SD) from baseline in selected hemodynamic parameters in the 6 h following administration of study drug. A, Cardiac index. B, PAWP. C, SVR. D, MAP. MAP = mean arterial pressure; PAWP = pulmonary arterial wedge pressure; SVR = systemic vascular resistance.
Although the prospective randomized design minimized baseline imbalances, some differences were evident owing to the small sample size. Following adjustment for baseline differences between groups, changes in SV, SV index, SVR, cardiac output (CO), cardiac index, diastolic BP, and MAP remained significantly different from placebo in the riociguat 2 mg group (e-Table 4 (307.4KB, pdf) ), whereas change in systolic BP did not significantly differ from the placebo group in this analysis.
Echocardiography
At baseline, mean LV ejection fraction was 62.1% ± 6.9%, and LV end-diastolic volume index was 47.1 ± 15.4 mL/m2. Left atrial (LA) area was mildly enlarged (25.4 ± 6.1 cm2; range, 11.1-42.3 cm2) at baseline; eight of 11 patients in the placebo group and nine of 10 in the riociguat 2 mg group had enlarged left atria (> 20 cm2).23 Compared with placebo, there was a significant decrease in right ventricular end-diastolic (RVED) area (−5.6 cm2 [95% CI, −10.9 to −0.3]; P = .04) from 23.8 ± 11.5 cm2 at baseline (97.5 percentile of normal for men > 24.7 cm2 and women 20.7 cm2)24 with riociguat 2 mg and a decrease in LA area (−4.0 cm2 [95% CI, −8.1 to 0.1]; P = .06) (e-Table 5 (307.4KB, pdf) ).
Exploratory Biomarkers and Pharmacokinetics
Plasma N-terminal prohormone of brain natriuretic peptide, asymmetric dimethylarginine, ST2, and galectin-3 revealed no differences in changes from baseline upon administration of riociguat compared with placebo (e-Table 6 (307.4KB, pdf) ). Following single-dose administration of riociguat, plasma concentrations dose-dependently increased across the three active treatment groups (e-Fig 1 (307.4KB, pdf) ). Peak plasma concentrations of riociguat were reached after a median of 1.9 to 2.5 h, and half-life was 13.1 to 14.3 h (e-Table 7 (307.4KB, pdf) ).
Safety and Tolerability
Details of AEs during the study are provided in Table 4; the majority of AEs were of mild or moderate intensity. Drug-related serious AEs were reported by two placebo group patients (15%) (two cases of decreased CO) and three patients (30%) taking riociguat 2 mg (one case of decreased CO and three cases of decreased MAP).
TABLE 4 ] .
Incidence of Adverse Events (Safety Population)
| Adverse Event | Placebo (n = 13) | Riociguat | ||
| 0.5 mg (n = 8) | 1 mg (n = 8) | 2 mg (n = 10) | ||
| Any adverse event | 4 (31) | 3 (38) | 3 (38) | 5 (50) |
| Atrial flutter | 0 | 0 | 0 | 1 (10) |
| Fall | 0 | 0 | 1 (13) | 0 |
| Traumatic hematoma | 1 (8) | 0 | 0 | 0 |
| Serum potassium decreased | 0 | 1 (13) | 0 | 0 |
| Cardiac output decreased | 2 (15) | 0 | 0 | 1 (10) |
| Mean arterial pressure decreased | 1 (8) | 0 | 1 (13) | 3 (30) |
| Muscle hemorrhage | 0 | 0 | 1 (13) | 0 |
| Musculoskeletal pain | 1 (8) | 0 | 0 | 0 |
| Dizziness | 1 (8) | 0 | 0 | 0 |
| Acute renal failure | 0 | 0 | 0 | 1 (10) |
| Dyspnea | 0 | 1 (13) | 0 | 0 |
| Hemothoraxa | 0 | 0 | 1 (13) | 0 |
| Pulmonary hemorrhage | 0 | 0 | 1 (13) | 0 |
| Pulmonary edema | 0 | 1 (13) | 0 | 0 |
| Drug eruptionb | 0 | 0 | 1 (13) | 0 |
| Skin neoplasm excision | 0 | 1 (13) | 0 | 0 |
Data are presented as No. (%).
The events hemothorax and pulmonary hemorrhage occurred in one patient and relate to the insertion of the right-sided heart catheter. The procedure was required by study protocol. Both events were consequently assessed as unrelated to riociguat.
Investigator term “exanthema because of Urosin allergy.”
Regarding AEs of special interest, a fall in invasively measured systemic systolic arterial BP < 80 mm Hg or in invasively measured MAP < 60 mm Hg was reported by one patient (8%) taking placebo, one patient (13%) taking riociguat 1 mg, and three patients (30%) taking riociguat 2 mg. Two patients (15%) taking placebo and one patient (10%) taking riociguat 2 mg experienced a decrease in CO ≥ 20% of baseline. One patient (13%) taking riociguat 0.5 mg developed pulmonary edema, which was considered unrelated to study drug by the investigator. One patient (10%) in the riociguat 2 mg group experienced syncope 6 days after study drug; the AE was reported during the 30-day follow-up period and was associated with additional intake of sildenafil. In the safety population, changes from baseline in arterial O2 saturation between 30 min and 6 h after administration of riociguat were −0.2 to +0.7% in the placebo group, −0.2 to −1.7% in the riociguat 0.5 mg group, −0.4 to +0.8% in the 1 mg group, and −1.0 to −2.1% in the 2 mg group.
There was one death (13%) in the riociguat 1 mg group. The patient died because of pulmonary hemorrhage, which was a complication of the protocol-required right-sided heart catheterization.
There were no clinically relevant changes in vital signs, ECG, or any laboratory variables. A single patient (10%) in the riociguat 2 mg group with a history of paroxysmal AF had AF 24 h after drug intake.
Discussion
The acute hemodynamic and echocardiographic effects of single doses of riociguat were investigated in patients with HFpEF and PH. Despite no significant change in the primary end point—peak decrease in mPAP—favorable hemodynamic effects were observed in the riociguat 2 mg group compared with placebo. Riociguat significantly increased SV and cardiac index, and significantly decreased systolic BP, SVR, and RVED area, without changing HR, PAWP, TPG, or PVR. Riociguat was well tolerated in combination with stable HF therapy.
Riociguat did not have the hypothesized effect—reduction in mPAP—in the current study in patients with HFpEF and PH, which was representative of an elderly, predominantly female HFpEF population with elevated PAWP and common comorbidities. As pulmonary selective vasodilators have been reported to worsen left-sided heart filling pressures and induce pulmonary edema in patients with PH due to HFpEF,25,26 the significant increase in flow without an increase in PAWP might be reflective of the systemic effects of riociguat. Although the contribution of afterload-independent direct myocardial effects to this hemodynamic response was not addressed in the current study, the potential of direct sGC stimulators in HFpEF might go beyond hemodynamic effects and additionally, or even predominantly, rely on nonhemodynamic effects of sGC-derived cGMP signaling in a variety of tissues, including cardiac myocytes.27
The increase in SV was accompanied by a reduction in the size of the right ventricle. Experimental evidence suggests an essential role for sGC activity in load-independent myocardial relaxation.28 An alternative or additional explanation could be a decrease in mitral regurgitant volume as a consequence of reduced LV afterload. A positive inotropic effect of riociguat is less likely, given the unaltered ventricular filling pressures. Furthermore, preclinical data suggest that sGC stimulation does not increase cyclic adenosine monophosphate production despite significant cGMP increases and may blunt the inotropic response to adrenergic stimulation.29,30
Previous attempts to target the NO-sGC-cGMP pathway in HFpEF have shown conflicting results. Despite being widely used as adjunctive therapies in HF, NO donors and organic nitrates are limited by the development of tolerance, oxidative stress, augmentation of endothelial dysfunction, and venoselectivity.31 The hemodynamic response presented here is in agreement with the increase in cardiac index with 16 weeks’ riociguat in the LEPHT.21 By contrast, patients with HFpEF were fourfold more likely than those with HF with reduced ejection fraction to experience a reduction in SV with acute IV nitroprusside, suggesting greater vulnerability of patients with HFpEF to preload reduction.32 As venodilator effects were suggested to play an important role in reducing LV filling pressures with nitroprusside,32 the significant increase in SV with riociguat could potentially be explained by a different regional vasoactivity, which may increase cGMP in the heart and arteries with less of the tolerability-limiting preload reduction of NO donors. Despite 30% of patients experiencing a decrease in invasively measured systemic systolic arterial BP < 80 mm Hg or invasively measured systemic mean arterial BP < 60 mm Hg following single doses of riociguat 2 mg, many patients did not have significant signs and symptoms of hypotension. Nevertheless, as the extent of systemic BP reduction with ad hoc dosing of riociguat 2 mg may be more appropriate in patients with higher baseline BP, riociguat should, like angiotensin-converting enzyme inhibitors, be started at low doses and up-titrated in future longer-term studies. Dosing should be handled similar to the LEPHT in patients with HF and reduced ejection fraction.21
In single-center clinical studies in HFpEF, sildenafil improved pulmonary artery pressure and right-sided heart function.33 However, sildenafil failed to improve peak O2 consumption in the Phosphodiesterase-5 Inhibition to Improve Clinical Status and Exercise Capacity in Diastolic Heart Failure (RELAX) study and showed no significant benefit vs placebo in a range of secondary end points in patients with HFpEF.34 Moreover, subgroup analysis showed no improvement in peak O2 consumption in patients with higher pulmonary artery systolic pressure (median pulmonary artery systolic pressure was 41 mm Hg [range, 32-51 mm Hg] in the treatment group). In contrast to the significant reduction of SVR observed in the present study, and with chronic riociguat therapy in the LEPHT,21 SVR was unchanged after 6 months of treatment with sildenafil.34 The RELAX investigators speculated that the ability of phosphodiesterase-5 inhibitors to enhance cGMP might be limited by insufficient endogenous stimulation of sGC secondary to reduced NO bioavailability in HFpEF. The results of the present study are, therefore, promising regarding the efficacy of direct, NO-independent sGC stimulation in patients with HFpEF.35
The main limitation of this single-dose study was the relatively small number of patients in each treatment group, which prevented analysis of subgroups of particular interest (eg, high vs low TPG, AF vs sinus rhythm). The number of patients with diastolic pressure gradient (DPG) ≥ 7 mm Hg was five of 36, thus precluding a valid statistical evaluation of this subgroup. Assessment of riociguat in patients with combined precapillary and postcapillary PH (PAWP > 15 mm Hg, DPG ≥ 7 mm Hg), as opposed to isolated postcapillary PH (PAWP > 15 mm Hg, DPG < 7 mm Hg),36 could be considered for future studies to explore treatment benefits in this population.
Baseline differences between groups included higher rates of AF at baseline in the riociguat 2 mg group compared with placebo and higher LV end-diastolic volume, SV, LA area, and right ventricular area in riociguat groups than in placebo. Thus, an influence of the extent of remodeling and structural changes on the observed results cannot be excluded.
The presence of patients with AF in the DILATE study may have affected the accuracy of hemodynamic measurements. However, this limitation was minimized by the study protocol recommending that investigators use an average of five measurements during thermodilution in patients with AF vs three for those in sinus rhythm.
Hemodynamic tracings and echocardiography were conducted and read on site in accordance with the standards at each institution; only key echocardiographic parameters were measured to allow for adherence to the protocol-defined acquisition intervals (as short as 30 min), without processing of results for central assessment by core laboratories. The observed changes in flow were only assessed by a single method. There was no significant change in mixed venous oxygen saturation, suggesting that the actual change in CO with riociguat was lower than that observed using thermodilution, although direct assessment of oxygen consumption was not performed. Furthermore, there were no study-specific standardization procedures regarding end-expiratory capture and analysis of PAWP at mid-A; local standards defined their acquisition.
Finally, the hemodynamic and echocardiographic effects of single doses of riociguat in this exploratory phase 2b study cannot be extrapolated to short-term (weeks) or long-term (months to years) treatment responses. Chronic, large-scale, placebo-controlled studies are required, which should include blinded central reading of hemodynamic and echocardiographic measurements.
Conclusions
In conclusion, single doses of riociguat were well tolerated and showed favorable hemodynamic and echocardiographic effects in patients with HFpEF and PH. Riociguat 2 mg significantly increased SV and cardiac index, and decreased systolic BP, SVR, and RVED area, without altering HR, TPG, or PVR. The ventricular filling required to establish an increased SV was not accompanied by increased PAWP, raising the hypothesis that in addition to its systemic vasodilatory effect, riociguat could improve diastolic function. Future clinical studies should include the titration of oral sGC stimulators over a longer period of time in patients with HFpEF.
Supplementary Material
Online Supplement
Acknowledgments
Author contributions: D. B. had full access to the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. D. B. contributed to the study conception and design, acquisition and interpretation of data, drafting and critical review of the article, and approval of the final version to be published; I. P., R. S.-M., P. J., S. R., C. T., A. B., C. S. P. L., and I. M. L. contributed to the acquisition and interpretation of data, critical review of the article, and approval of the final version to be published; R. F. and S. U. contributed to the analysis and interpretation of data, critical review of the article, and approval of the final version to be published; M. O. K. contributed to the study conception and design, interpretation of data, critical review of the article, and approval of the final version to be published; and L. R. contributed to the study conception and design, analysis and interpretation of data, critical review of the article, and approval of the final version to be published.
Financial/nonfinancial disclosures: The authors have reported to CHEST the following conflicts of interest: Dr Bonderman has received speaker fees from Actelion Pharmaceuticals Ltd, AOP Orphan Pharmaceuticals, Bayer HealthCare Pharmaceuticals, Pfizer Inc, and United Therapeutics Corp; has received research support from Bayer HealthCare Pharmaceuticals; and has served on a board or advisory committee for Actelion Pharmaceuticals Ltd and Bayer HealthCare Pharmaceuticals. Dr Pretsch has received speaker fees from Orion Corp and Boehringer Mannheim GmbH and has served on a board or advisory committee for Orion Corp, Eli Lilly and Co, and Boehringer Mannheim GmbH. Dr Jansa has received speaker fees from and has served as a consultant for Actelion Pharmaceuticals Ltd, AOP Orphan Pharmaceuticals, Bayer HealthCare Pharmaceuticals, and GlaxoSmithKline and has served on a board or advisory committee for Bayer HealthCare Pharmaceuticals and United Therapeutics Corp. Dr Rosenkranz has received fees for lectures and/or consultancy from Actavis, Actelion Pharmaceuticals Ltd, Bayer HealthCare Pharmaceuticals, GlaxoSmithKline, Eli Lilly and Co, Novartis Corp, Pfizer Inc, and United Therapeutics Corp and fees for clinical trials/research support from Actavis, Actelion Pharmaceuticals Ltd, Bayer HealthCare Pharmaceuticals, GlaxoSmithKline, Novartis Corp, Pfizer Inc, and United Therapeutics Corp. Dr Lam has received fees for lectures and/or consultancy from Bayer HealthCare Pharmaceuticals, Novartis Corp, AstraZeneca, and Vifor Pharma and has received research support from Boston Scientific, Medtronic, Inc, and Vifor Pharma. Drs Frey, Ochan Kilama, and Roessig and Ms Unger are full-time employees of Bayer HealthCare Pharmaceuticals. Dr Lang has acted as a consultant for Actelion Pharmaceuticals Ltd, AOP Orphan Pharmaceuticals, AstraZeneca, Bayer HealthCare Pharmaceuticals, GlaxoSmithKline, Medtronic, Inc, Pfizer Inc, Servier, and United Therapeutics Corp; has served on a board or advisory committee for Actelion Pharmaceuticals Ltd, AOP Orphan Pharmaceuticals, Bayer HealthCare Pharmaceuticals, and United Therapeutics Corp; has received speaker fees from Actelion Pharmaceuticals Ltd, AOP Orphan Pharmaceuticals, AstraZeneca, Bayer HealthCare Pharmaceuticals, GlaxoSmithKline, Pfizer Inc, Servier, and United Therapeutics Corp; and has received research support from Actelion Pharmaceuticals Ltd, AOP Orphan Pharmaceuticals, Bayer HealthCare Pharmaceuticals, and United Therapeutics Corp. Drs Steringer-Mascherbauer and Bojic and Ms Tufaro have reported that no potential conflicts of interest exist with any companies/organizations whose products or services may be discussed in this article.
Role of sponsors: Bayer HealthCare Pharmaceuticals contributed to the design of the study, the collection and analysis of the data, and the preparation of the manuscript.
Other contributions: We thank Veselin Mitrovic, MD (Kerckhoff-Klinik, Bad Nauheim, Germany) for his assistance with the calculation of MVO2.
Additional information: The e-Appendix, e-Figure, and e-Tables can be found in the Supplemental Materials section of the online article.
ABBREVIATIONS
- AE
adverse event
- AF
atrial fibrillation
- cGMP
cyclic guanosine monophosphate
- CO
cardiac output
- DILATE
Acute Hemodynamic Effects of Riociguat in Patients With Pulmonary Hypertension Associated With Diastolic Heart Failure
- DPG
diastolic pressure gradient
- HF
heart failure
- HFpEF
heart failure with preserved left ventricular ejection fraction
- HR
heart rate
- LA
left atrial
- LEPHT
Left Ventricular Systolic Dysfunction Associated With Pulmonary Hypertension Riociguat Trial
- LV
left ventricular
- MAP
mean arterial pressure
- mPAP
mean pulmonary artery pressure
- MVO2
myocardial oxygen consumption
- NO
nitric oxide
- NO-sGC-cGMP
nitric oxide-soluble guanylate cyclase-cyclic guanosine monophosphate
- O2
oxygen
- PAWP
pulmonary arterial wedge pressure
- PH
pulmonary hypertension
- PVR
pulmonary vascular resistance
- RVED
right ventricular end-diastolic
- sGC
soluble guanylate cyclase
- SV
stroke volume
- SVR
systemic vascular resistance
- TPG
transpulmonary pressure gradient
Footnotes
FUNDING/SUPPORT: The study was supported by Bayer HealthCare Pharmaceuticals (Berlin, Germany). Editorial assistance was provided by Adelphi Communications Ltd (Bollington, England), supported by Bayer HealthCare Pharmaceuticals.
References
- 1.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355(3):251-259 [DOI] [PubMed] [Google Scholar]
- 2.McMurray JJ, Adamopoulos S, Anker SD, et al. ; ESC Committee for Practice Guidelines. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2012;33(14):1787-1847 [DOI] [PubMed] [Google Scholar]
- 3.Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure—abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004;350(19):1953-1959 [DOI] [PubMed] [Google Scholar]
- 4.Borlaug BA, Olson TP, Lam CS, et al. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2010;56(11):845-854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kawaguchi M, Hay I, Fetics B, Kass DA. Combined ventricular systolic and arterial stiffening in patients with heart failure and preserved ejection fraction: implications for systolic and diastolic reserve limitations. Circulation. 2003;107(5):714-720 [DOI] [PubMed] [Google Scholar]
- 6.Bursi F, McNallan SM, Redfield MM, et al. Pulmonary pressures and death in heart failure: a community study. J Am Coll Cardiol. 2012;59(3):222-231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lam CS, Roger VL, Rodeheffer RJ, Borlaug BA, Enders FT, Redfield MM. Pulmonary hypertension in heart failure with preserved ejection fraction: a community-based study. J Am Coll Cardiol. 2009;53(13):1119-1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akiyama E, Sugiyama S, Matsuzawa Y, et al. Incremental prognostic significance of peripheral endothelial dysfunction in patients with heart failure with normal left ventricular ejection fraction. J Am Coll Cardiol. 2012;60(18):1778-1786 [DOI] [PubMed] [Google Scholar]
- 9.Galiè N, Hoeper MM, Humbert M, et al. ; ESC Committee for Practice Guidelines (CPG). Guidelines for the diagnosis and treatment of pulmonary hypertension: the Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS), endorsed by the International Society of Heart and Lung Transplantation (ISHLT). Eur Heart J. 2009;30(20):2493-2537 [DOI] [PubMed] [Google Scholar]
- 10.Katz SD, Hryniewicz K, Hriljac I, et al. Vascular endothelial dysfunction and mortality risk in patients with chronic heart failure. Circulation. 2005;111(3):310-314 [DOI] [PubMed] [Google Scholar]
- 11.Lam CS, Brutsaert DL. Endothelial dysfunction: a pathophysiologic factor in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2012;60(18):1787-1789 [DOI] [PubMed] [Google Scholar]
- 12.Mathier MA, Rose GA, Fifer MA, et al. Coronary endothelial dysfunction in patients with acute-onset idiopathic dilated cardiomyopathy. J Am Coll Cardiol. 1998;32(1):216-224 [DOI] [PubMed] [Google Scholar]
- 13.Stasch JP, Pacher P, Evgenov OV. Soluble guanylate cyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation. 2011;123(20):2263-2273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Heerebeek L, Hamdani N, Falcão-Pires I, et al. Low myocardial protein kinase G activity in heart failure with preserved ejection fraction. Circulation. 2012;126(7):830-839 [DOI] [PubMed] [Google Scholar]
- 15.Schermuly RT, Janssen W, Weissmann N, Stasch JP, Grimminger F, Ghofrani HA. Riociguat for the treatment of pulmonary hypertension. Expert Opin Investig Drugs. 2011;20(4):567-576 [DOI] [PubMed] [Google Scholar]
- 16.Grimminger F, Weimann G, Frey R, et al. First acute haemodynamic study of soluble guanylate cyclase stimulator riociguat in pulmonary hypertension. Eur Respir J. 2009;33(4):785-792 [DOI] [PubMed] [Google Scholar]
- 17.Sharkovska Y, Kalk P, Lawrenz B, et al. Nitric oxide-independent stimulation of soluble guanylate cyclase reduces organ damage in experimental low-renin and high-renin models. J Hypertens. 2010;28(8):1666-1675 [DOI] [PubMed] [Google Scholar]
- 18.Stasch JP, Hobbs AJ. NO-independent, haem-dependent soluble guanylate cyclase stimulators. Handb Exp Pharmacol. 2009;(191):277-308 [DOI] [PubMed] [Google Scholar]
- 19.Ghofrani HA, Galiè N, Grimminger F, et al. ; PATENT-1 Study Group. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med. 2013;369(4):330-340 [DOI] [PubMed] [Google Scholar]
- 20.Ghofrani HA, D’Armini AM, Grimminger F, et al. ; CHEST-1 Study Group. Riociguat for the treatment of chronic thromboembolic pulmonary hypertension. N Engl J Med. 2013;369(4):319-329 [DOI] [PubMed] [Google Scholar]
- 21.Bonderman D, Ghio S, Felix SB, et al. ; Left Ventricular Systolic Dysfunction Associated With Pulmonary Hypertension Riociguat Trial (LEPHT) Study Group. Riociguat for patients with pulmonary hypertension caused by systolic left ventricular dysfunction: a phase IIb double-blind, randomized, placebo-controlled, dose-ranging hemodynamic study. Circulation. 2013;128(5):502-511 [DOI] [PubMed] [Google Scholar]
- 22.Rooke GA, Feigl EO. Work as a correlate of canine left ventricular oxygen consumption, and the problem of catecholamine oxygen wasting. Circ Res. 1982;50(2):273-286 [DOI] [PubMed] [Google Scholar]
- 23.Lang RM, Bierig M, Devereux RB, et al. ; American Society of Echocardiography’s Nomenclature and Standards Committee; Task Force on Chamber Quantification; American College of Cardiology Echocardiography Committee; American Heart Association; European Association of Echocardiography, European Society of Cardiology. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7(2):79-108 [DOI] [PubMed] [Google Scholar]
- 24.Ogunyankin KO, Liu K, Lloyd-Jones DM, Colangelo LA, Gardin JM. Reference values of right ventricular end-diastolic area defined by ethnicity and gender in a young adult population: the CARDIA study. Echocardiography. 2011;28(2):142-149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boilson BA, Schirger JA, Borlaug BA. Caveat medicus! Pulmonary hypertension in the elderly: a word of caution. Eur J Heart Fail. 2010;12(1):89-93 [DOI] [PubMed] [Google Scholar]
- 26.Yin N, Kaestle SM, Kuppe H, et al. Iloprost inhalation causes lung edema in overperfused heart failure lungs [abstract]. Am J Respir Crit Care Med. 2009;179:A3376 [Google Scholar]
- 27.Paulus WJ, Tschöpe C. A novel paradigm for heart failure with preserved ejection fraction: comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J Am Coll Cardiol. 2013;62(4):263-271 [DOI] [PubMed] [Google Scholar]
- 28.Buys ES, Sips P, Vermeersch P, et al. Gender-specific hypertension and responsiveness to nitric oxide in sGCalpha1 knockout mice. Cardiovasc Res. 2008;79(1):179-186 [DOI] [PubMed] [Google Scholar]
- 29.Cawley SM, Kolodziej S, Ichinose F, Brouckaert P, Buys ES, Bloch KD. sGCalpha1 mediates the negative inotropic effects of NO in cardiac myocytes independent of changes in calcium handling. Am J Physiol Heart Circ Physiol. 2011;301(1):H157-H163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ramos-Espiritu LS, Hess KC, Buck J, Levin LR. The soluble guanylyl cyclase activator YC-1 increases intracellular cGMP and cAMP via independent mechanisms in INS-1E cells. J Pharmacol Exp Ther. 2011;338(3):925-931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Münzel T, Daiber A, Mülsch A. Explaining the phenomenon of nitrate tolerance. Circ Res. 2005;97(7):618-628 [DOI] [PubMed] [Google Scholar]
- 32.Schwartzenberg S, Redfield MM, From AM, Sorajja P, Nishimura RA, Borlaug BA. Effects of vasodilation in heart failure with preserved or reduced ejection fraction implications of distinct pathophysiologies on response to therapy. J Am Coll Cardiol. 2012;59(5):442-451 [DOI] [PubMed] [Google Scholar]
- 33.Guazzi M, Vicenzi M, Arena R, Guazzi MD. Pulmonary hypertension in heart failure with preserved ejection fraction: a target of phosphodiesterase-5 inhibition in a 1-year study. Circulation. 2011;124(2):164-174 [DOI] [PubMed] [Google Scholar]
- 34.Redfield MM, Chen HH, Borlaug BA, et al. ; RELAX Trial. Effect of phosphodiesterase-5 inhibition on exercise capacity and clinical status in heart failure with preserved ejection fraction: a randomized clinical trial. JAMA. 2013;309(12):1268-1277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghofrani HA, Grimminger F. Soluble guanylate cyclase stimulation: an emerging option in pulmonary hypertension therapy. Eur Respir Rev. 2009;18(111):35-41 [DOI] [PubMed] [Google Scholar]
- 36.Gerges C, Gerges M, Lang MB, et al. Diastolic pulmonary vascular pressure gradient: a predictor of prognosis in “out-of-proportion” pulmonary hypertension. Chest. 2013;143(3):758-766 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Supplement