Abstract
Purpose
Homonymous visual field defects (HVFDs) may critically interfere with quality of life. The aim of this study was to assess the impact of HVFDs on a supermarket search task and to investigate the influence of visual search on task performance.
Methods
Ten patients with HVFDs (four with a right-sided [HR] and six with a left-sided defect [HL]), and 10 healthy-sighted, sex-, and age-matched control subjects were asked to collect 20 products placed on two supermarket shelves as quickly as possible. Task performance was rated as “passed” or “failed” with regard to the time per correctly collected item (TC -failed = 4.84 seconds based on the performance of healthy subjects). Eye movements were analyzed regarding the horizontal gaze activity, glance frequency, and glance proportion for different VF areas.
Results
Seven of 10 HVFD patients (three HR, four HL) passed the supermarket search task. Patients who passed needed significantly less time per correctly collected item and looked more frequently toward the VFD area than patients who failed. HL patients who passed the test showed a higher percentage of glances beyond the 60° VF (P < 0.05).
Conclusion
A considerable number of HVFD patients performed successfully and could compensate for the HVFD by shifting the gaze toward the peripheral VF and the VFD area.
Translational Relevance
These findings provide new insights on gaze adaptations in patients with HVFDs during activities of daily living and will enhance the design and development of realistic examination tools for use in the clinical setting to improve daily functioning. (http://www.clinicaltrials.gov, NCT01372319, NCT01372332)
Keywords: Homonymous visual field defects, supermarket search task, compensation, eye movements, visual search
Introduction
Homonymous visual field defects (HVFDs; i.e., binocular VFDs respecting the vertical midline and affecting corresponding areas of the VF in both eyes), may critically interfere with quality of life. Postchiasmal visual pathway lesions are among the most common causes of such defects in the binocular visual field. They occur frequently in stroke, brain tumor, or traumatic brain injuries. HVFDs are found in approximately 8% of stroke patients1 and 25% of traumatic brain injury patients.2,3 There are many types of HVFD, ranging from homonymous paracentral scotomas and homonymous quadrantanopia to (complete) homonymous hemianopia, with loss of an entire hemifield of vision. HVFDs may be congruous (i.e., the VFDs in each eye are identical) or incongruous (i.e., the VFDs differ between eyes). In addition, the degree of macular sparing may vary, and its presence is usually associated with preserved reading ability and higher everyday functioning.
Patients with HVFDs may experience severe impairment in everyday activities, such as reading, mobility, or driving.4,5 Patients complain mainly of difficulties with reading and scanning scenes fast enough to make sense of things as a whole.6 Consequently, they fail to notice relevant obstacles or avoid obstacles on their affected side and may collide with approaching people or cars. Reading is also commonly affected in patients with HVFDs.7,8 Left HVFDs cause difficulties with eye movements required to find the beginning of a new line, resulting in omissions of the first word or syllables of the line. Right HVFDs cause more severe reading difficulties, with loss of the anticipatory parafoveal scanning process, increased number of saccades, and significant reduction of reading speed, which result in a characteristic reading disorder termed “hemianopic dyslexia,” which in some patients is nearly equivalent to spelling.9
Several studies have attempted to assess the impact of HVFDs on quality of life (e.g., by means of questionnaires),10 which provide a subjective assessment of the difficulties encountered by patients with HVFDs, or by performing objective analysis of the factors related to the functional abilities of HVFD patients under laboratory conditions or in simulated environment.11–17 These studies have concluded that successful completion of everyday activities is associated with effective visual search behavior. The most realistic attempt to assess the functional impairment of patients with HVFDs in everyday activities is under real-world conditions (e.g., in the context of driving).18–21 Driving is indeed an important aspect of everyday life with a tremendous impact on personal mobility and independence. However, it has been suggested that compensatory gaze movements are highly specific and intrinsically related to the specific task.22 Hence, gaze patterns during driving may not necessarily coincide with the visual behavior in other everyday activities. However, there are only few studies investigating patients with HVFDs during activities of daily living, mainly because real-world experiments require specialized equipment, are expensive, time-consuming, difficult to standardize, and measure the outcomes.19–21 Furthermore, there is variability of patients' strategies in coping with activities of daily living.
The above studies agree on certain aspects: (1) task performance is variable among patients with HVFDs, (2) HVFDs are not always associated with impaired performance, and (3) HVFDs can be compensated by effective head and eye movements. This means that patients with identical VFDs may display different degrees of impairment in everyday activities due to their individual compensatory capacity by means of gaze movements.
A common scenario in everyday life is shopping. Shopping involves constant visual scanning and search for specific items. Most prior work on visual search during shopping has mainly investigated consumer's psychology.23–26 To the best of our knowledge, no studies investigating the visual search of patients with HVFDs during shopping tasks have been performed up to date. However, assessment of activities of daily living is necessary for a better understanding of the compensatory strategies of patients with HVFD. Assessment of visual exploration during daily activities will be helpful in evaluating global, vision-targeted quality of life, in order to improve the correlation between visual function and its perception, design training strategies for improvement of daily functioning, and develop examination tools for use in the clinical setting.
Therefore, the objective of our study was to assess the visual search performance of patients with HVFDs in a supermarket special offer search task. The patients' performance was compared with that of healthy-sighted control subjects. Furthermore, we investigated the factors affecting task performance and studied features of the visual search strategy. We hypothesized that performance of patients with HVFDs is not primarily associated to the extent of the VFD, but is mainly related to their visual-scanning strategy.
Methods
Participants
Twenty participants were enrolled in this study: 10 patients with HVFDs (we refer to this group as HP, age 52.5 ± 12.8 years), including four patients with a right-sided defect (HR) and six with a left-sided HVFD (HL), and 10 healthy-sighted control subjects (HC, age 51 ± 11.7 years), matched with respect to age and sex (Supplementary Table S1 (166.7KB, docx) ). The study was conducted in August 2011.
All participants were recruited by the Neuro-Ophthalmology service of the University of Tübingen, Germany. To be included in the study, all participants were required to be at least 18-years old, to have the ability to understand and comply with the requirements of the study, and have a Minimental Status Examination Score above 24, which indicates normal cognitive status.27 In addition, function and morphology of the anterior visual pathways should be normal, as evaluated by standard ophthalmological tests (fundus and slit-lamp examinations, ocular alignment, and ocular motility). The age- and sex-matched control subjects should additionally have normal visual fields, normal cup-to-disc ratio (≥0.5) and no history of brain injury or physical impairment. The best corrected monocular distant visual acuity of control subjects should be less than 20/20 for those aged-up to 60 years, less than 20/25 for those aged between 60 and 70 years, and less than 20/33 for those aged more than 70 years. Patients' best corrected monocular distant visual acuity should be at least 20/40. Time since lesion onset for HP should be at least 6 months. Mean time since onset of lesion for HP was 7.05 years (± 4.36 years). Exclusion criteria for HP were multiple sclerosis, Alzheimer disease, Parkinson disease, hemiparesis, and visual hemineglect as determined by horizontal line bisection, copying of figures, and by means of the “Bells test.”
Visual fields were assessed by means of binocular semi-automated 90° kinetic perimetry (SKP) obtained with the OCTOPUS 101 Perimeter (background luminance 10 cd/m2, angular velocity 3°/s; Fa. HAAG-STREIT, Koeniz, Switzerland). We used the binocular VF because it provides more realistic information about the VF a patient uses for performing daily activities.28
The research study was approved by the institutional review board of the University of Tübingen and was performed according to the Declaration of Helsinki. Following verbal and written explanation of the experimental protocol all subjects gave their written consent, with the option of withdrawing from the study at any time.
The study was registered at ClinicalTrials.gov (http://www.clinicaltrials.gov, NCT01372319, NCT01372332).
Supermarket Search Task
The experiment was performed in a drugstore in the city center of Tübingen. Twenty different special-offer products were randomly chosen among other products in two racks (10 products each in the right and in the left rack) along a corridor of 7.5-m length and 1.3-m width (Fig. 1).
Figure 1.
The drug store corridor with all marked products (orange signs) on two shelves.
Furthermore, each of the racks included six shelves at different heights (Fig. 1). The products of interest varied in color, shape, and size and were marked in each shelf using orange signs (Fig. 1). They were distributed homogeneously over height and width of both shelves. More specifically, the products of interest were placed such that the distribution of the items according to the site (left and right) and height was the same. The subjects had to look for the orange signs and collect the above standing product as quickly as possible by walking through the corridor only once. Eye movements were recorded by means of an Ergoneers Dikablis mobile eye tracker, a light-weighted, head-mounted monocular unit, which does not interfere with glasses.
The supermarket search task was repeated four times for each participant to address the individual learning effect. In order to record the eye movements of subjects during the search task, the subjects were wearing eye-tracking equipment during the first run. Due to time constraints and logistic reasons (the experiments could only be performed within a 2-hour interval before the opening of the supermarket), we were unable to record eye movements except for the initial run. During the other three runs of a subject the eye-tracking device was calibrated for the next subject. Therefore, we decided to report exclusively on the initial run and to address the learning aspect in another paper. For each trial, we documented the time needed to complete the task, the number of correctly collected items, and the number of wrongly collected items.
Performance Assessment
Performance of subjects was assessed by means of the following parameters: (1) average number of correctly collected items NC over all runs, (2) average performance time t over all runs, and (3) average time per correctly collected item TC = t/NC.
Passing Criterion
The TC values followed a normal distribution with (mean) μ = 3.18 seconds and (SD) σ = 0.55 seconds in the control group (Shapiro-Wilk test). Based on performance of control subjects, the threshold value of TC for failing the task was defined as TC -failed = μ + 3σ. More specifically, TC-failed = 4.84 seconds (i.e., a subject who needed longer than 4.84 seconds per correctly collected item were rated as failing the task).
In order to identify parameters associated with successful task performance, NC, t, and TC were compared across the following participant groups: HVFD control subjects who passed (HCp), right-sided HVFD patients who passed (HRp), left-sided HVFD patients who passed (HLp), and HVFD patients who failed (HPf) by one-way analysis of the variance. Subsequent post hoc comparisons were performed using the Tukey's HSD test. As multiple tests were carried out, the significance level was adjusted using a Bonferroni correction to an alpha-level of 0.05 for multiple comparisons. All data sets were tested for normality by the Shapiro-Wilk test; for nonnormally distributed data, the Kruskal-Wallis test for multiple comparisons and the Mann-Whitney U test were used. In addition, dependencies between eye movement–related parameters (HGA, PGP, and GF) and performance-related parameters (NC, t, and TC) were also explored using linear regression (Pearson Correlation Coefficient) in the patient group. Matlab R2013b (The MathWorks, Inc., Natick, MA) was used for data analysis.
In order to investigate the effect of the VFD on task performance, the size of the binocular VFD was calculated from measurements obtained by means of binocular semi-automated 90° kinetic perimetry (SKP) as described above (Supplementary Table S1 (166.7KB, docx) ). Only the stimulus III/4e was considered because this is a functionally relevant target that is typically used to define driving fitness and legal blindness in Germany.
Analysis of Eye Movements Data
Eye movements were recorded using the D-Lab software tool (Ergoneers Inc., Manching, Germany) at a frequency of 25 frames/s. The recorded data was analyzed using both D-Lab and self-developed algorithms.29–31 In order to quantify the frequency and duration of saccades toward the area of VFD or toward the peripheral visual field, we superimposed the area of VFD as Area of Interest (AOI) for each participant. These models were transferred into D-Lab to analyze the viewing behavior of participants toward such regions in terms of glance proportion and frequency.32 More specifically, the following gaze-related parameters were assessed.
Horizontal Gaze Activity (HGA)
In order to investigate the horizontal exploration ability of a subject, we assessed the SD of the horizontal pupil position, which was expressed as HGA.
Glance Proportion in Percentage (PGP)
PGP describes the percentage of glance duration in a defined AOI during a given-time interval. We computed the PGP for the area of VFD (PGP-VFD), for the VF area beyond 30° (PGP-30c), and for the VF area beyond 60° (PGP-60c).
Glance Frequency (GF)
GF describes the average number of glances toward a defined AOI during the time unit of 1 second. Similar to PGP, we computed the GF for the area of VFD (GF-VFD), the VF area beyond 30° (GF-30c), and the VF area beyond 60° (GF-60c).
Results
Task Performance
For each subject subgroup, Figure 2 presents (a) the average number of correctly collected items NC, (b) the average time t to complete the task, and (c) the average time per correctly collected item TC. The respective values can be found in Supplementary Table S4 (166.7KB, docx) .
Figure 2.
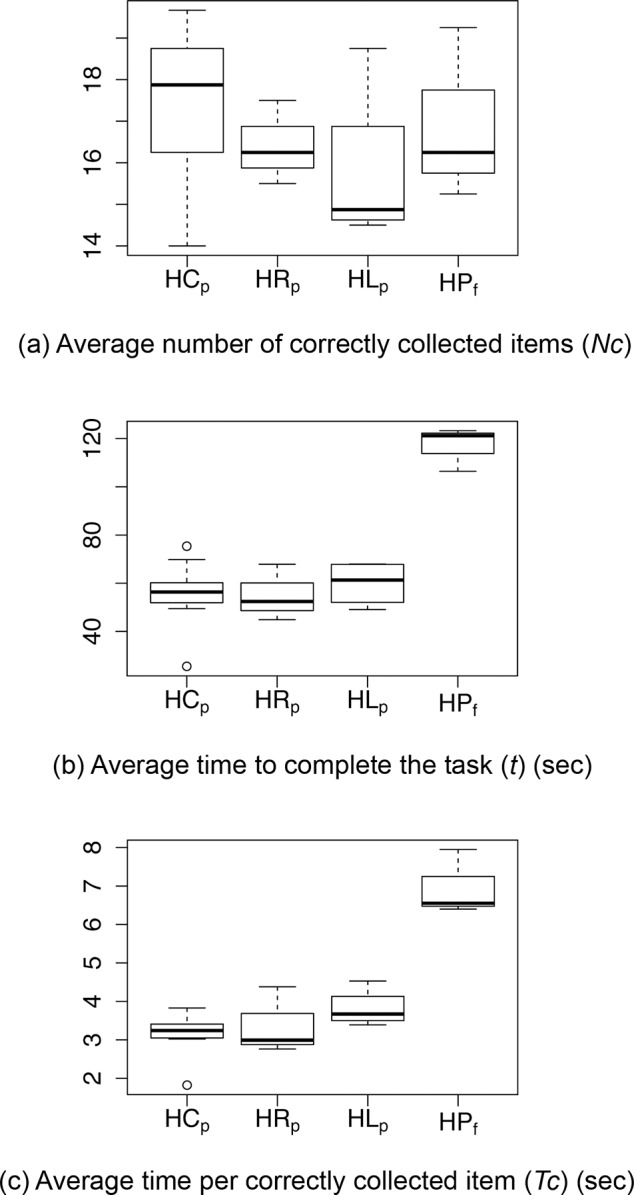
Value range for (a) the average number of correctly collected items over all runs, (b) the average time needed to complete the supermarket search task over all runs, and (c) the average time (over all runs) per correctly collected item. The participant subgroups are marked by HCp, HRp, HLp, and HPf.
Number of Correctly Collected Items (NC)
None of the subjects were able to collect all 20 items successfully Figure 2. However, contrary to our expectation the general task performance regarding NC was good (e.g., the worst performer collected 14 of 20 items). We found no significant differences in the number of correctly collected items between the subject subgroups HCp–HRp–HLp–HPf. However, patients with HL detected significantly less items than their controls (HCL), Table 2.
Table 2.
Performance and Gaze Related Parameters For HVFD Control Subjects Who Passed (HCp), Right-Sided HVFD Patients Who Passed (HRp), Left-Sided HVFD Patients Who Passed (HLp), And HVFD Patients Who Failed (HPf) Statistical Comparisons Were Made Between Groups. *P < 0.05; **P < 0.01; ***P < 0.001. n. s., indicates nonsignificant comparisons
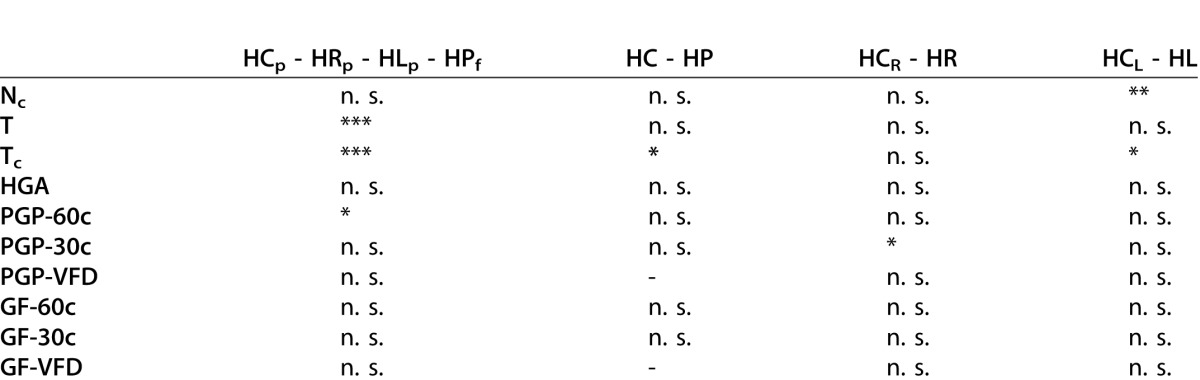
Table 1.
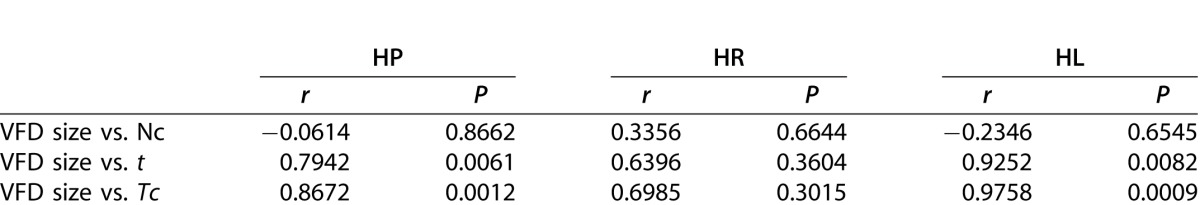
Pearson's Correlation Coefficient (r) Between VFD Size and Parameters Related to Task Performance
The number of wrongly collected items was very small; there were overall only nine wrongly collected items for all participants and all runs (two times one error, one time three errors, one time four errors), therefore no further analyses were performed.
Average Performance Time (t)
Regarding the performance time, we found a highly significant difference between the HVFD subgroups HCp, HRp, HLp, and HPf (P < 0.001, Table 2). While the average performance time t for HPf was 116.98 seconds, HCp, HRp, and HLp performed nearly twice as fast (i.e., 55.8, 55.04, and 59.92 seconds, respectively; Supplementary Table S4 (166.7KB, docx) ). In summary, control subjects and patients who passed the supermarket search task needed considerably less time to complete the trial than patients who failed the task.
Average Time per Correctly Collected Object (TC)
According to the passing threshold TC = 4.84 seconds (see Methods Section) all control subjects completed the supermarket search task successfully. The control group displayed in general significantly shorter t and Tc than the HVFD group. With respect to TC, control subjects (HC) were significantly faster than patients (HP), Table 2. Furthermore, patients with an HL also showed significantly higher Tc values than their controls (HCL).
Seven of 10 HVFD patients passed the supermarket test. More specifically, three of four HR patients and four of six HL patients showed successful task performance.
Since the parameter TC depends on t, we found the same significant differences between subject subgroups as for t. More specifically, subjects who passed the task (HPp) needed significantly shorter time per collected item than subjects who failed the task (P < 0.001, Table 2).
Influence of VFD Size on Task Performance
We computed the correlation between the size of the VFD and the parameters Nc, t, and Tc for all patients, and the subgroups HR and HL. We found a weak, positive correlation between the VFD size and the number of correctly collected items Nc (Table 2). Thus, the VFD size does not seem to influence the number of correctly collected items. In contrast, we found a strong correlation between VFD size and t (and Tc) for HP (all patients) and HL, while there was a moderate but not significant correlation for HR.
Gaze-Related Parameters
Horizontal Gaze Activity (HGA)
Contrary to our expectations, no difference was found in HGA between the participant subgroups (Table 2).
Furthermore, no significant relationship was found between HGA and Nc or the average time per correctly collected object (Tc) in any of the subject subgroups. Thus, it seems that HGA does not influence task performance.
Proportion of Glances in Percent (PGP)
There was no significant difference between the subject subgroups (HCp–HRp–HLp–HPf) regarding the proportion of glances beyond the 30° VF (PGP-30c), Table 2. However, we found a significantly higher PGP-30° in patients with a right-sided defect (HR) compared with their controls (HCR), Table 2. Furthermore, subgroup comparison (HCp–HRp–HLp–HPf) revealed a significant difference in PGP beyond 60° (PGP-60c) between patients with left-sided HVFDs who passed (HLp) and patients with HVFDs who failed (HPf). HLp performed significantly longer glances toward the visual-field periphery beyond 60° than HPf (P < 0.05, Table 2).
Glance Frequency (GF)
There was no significant difference between the subject subgroups in GF beyond 30° (GF-30c) and beyond 60° (GF-60c). In HVFD patients who passed the task, there was a trend for more frequent glances toward the area of the VFD (GF-VFD), when compared with HVFD patients who failed the task. However, this difference (P = 0.06) was not significant (Table 2).
Discussion
In this study, we investigated the performance of patients with homonymous VFDs in comparison with healthy-sighted control subjects during a special-offer supermarket search task. A considerable number of patients managed to pass the test despite the binocular homonymous visual-field loss. Our main finding, namely that some patients with HVFDs are able to fully compensate for their deficit, is in accordance with prior driving studies of patients with HVFDs.18,21 No significant differences were found in the number of correctly collected items between the subject subgroups. This finding suggests that patients with HVFDs are able to perform similarly to a healthy-sighted control group if there are no time restrictions. However, this setting does not fully represent real-world scenarios, which often involve high-risk driving conditions and time pressure. Such scenarios pose a high demand on the individual and the need to scan the scene quickly and efficiently. Therefore, a time-related passing criterion based on the performance of healthy subjects was set. According to this criterion, patient groups needed on average longer time per correctly collected item (Tc) than the respective control groups. However, a subgroup of patients completed the task within the defined time period (HPp), while patients classified as “failed” (HPf) needed significantly longer time to complete the task. Successful task performance seems to be associated with effective visual exploration; however, more data will be needed to confirm this assumption. Our results indicate that an effective compensation strategy in patients with HVFDs employs more glances beyond the 60° VF (i.e., toward the peripheral visual field). In the present supermarket search task, this visual exploration strategy is associated with successful task performance. Related studies have reported that a primary focus on the central VF is associated with better performance, due to functional specialization of the human visual field.16,21,33 However, this finding was either related to visual search of static scenes with the use of a chin rest and a restricted field of view (29° × 22°),16 or to an on-road driving test,21 which is substantially different from the present experiment. During the supermarket search task, the significance of the central part of the visual scene relative to a straight-ahead position is presumably less important than the periphery, due to the eccentric location of the targets on each side of the corridor. In addition, bottom-up information processing, which is based on incoming data from the environment to form a perception, may be less pronounced in the present task than in driving tasks. Our findings are in accordance with a recent study (Tomasi M, et al. IOVS. 2014;ARVO E-Abstract 4131), which investigated the compensatory gaze patterns of hemianopic patients when walking outside. The authors in found that hemianopes looked far peripherally (30°) from their body heading direction and directed their gaze toward the blind side significantly more often than toward the seeing side (Tomasi M, et al. IOVS. 2014;ARVO E-Abstract 4131).
Furthermore, HVFD patients who passed the supermarket search task showed a tendency to look toward the VFD area. Patients with HVFDs shifted their gaze toward the side of the VFD, in order to bring a larger portion of the scene into the unaffected part of the visual field. This bias has been observed in several prior studies with static displays, virtual environments (Tomasi M, et al. IOVS. 2014;ARVO E-Abstract 4131)13,34–37 and driving tasks.15,16,18,21,38 Explicit scanning of the area of VFD seems to be associated with superior task performance, which is not influenced by the nature of the visual search task.
Interestingly, our results are at odds with a naturalistic experiment by Martin et al.,14 who investigated the visual behavior of three longstanding homonymous hemianopes while they assembled wooden models. The authors found little evidence of a compensatory gaze bias for hemianopes, possibly because the targets changed spatial location only through the activity of the participants, so they could rely more on spatial memory and less on visual scanning.14 However, the present task involved a more complex environment with several distractors over a larger area. Hence, compensation was not possible only by relying on visuospatial memory and additional gaze adaptation was required. In a related study by Riley et al.,39 the same hemianopic participants fixated more frequently in the direction of their blind field than their sighted field, when their task was to detect moving targets in a virtual environment.39 Similarly, during a previous simple visual sampling paradigm, hemianopic participants near-normal gaze behavior.13 However, additional gaze compensation was required in order to complete a more complex comparative visual search task.13 These findings confirm the hypothesis that compensatory gaze strategies in patients with HVFDs are task-specific and become more obvious with increasing task complexity.13,15,22
Our finding that patients with HVFDs display on average significantly longer search times per correctly collected object than their controls is also in agreement with prior studies, reporting longer search or response time of patients with HVFDs in laboratory-based visual search tasks.13,36,40–42 Prolonged search duration in hemianopic patients has been attributed to an increased number of fixations and scan-path length in an attempt to scan the areas located within the VFD.13 Some authors have demonstrated a higher number of refixations, possibly due to spatial working memory deficits, a lack of strategic planning leading to chaotic scanpaths, or an impairment of object recognition and visual attention, caused by the underlying brain damage.41
In addition, trial duration was prolonged in failed patients, probably due to ineffective visual exploration. This finding suggests that they may try to compensate by increasing working memory involvement, in order to avoid gaze saccades while memorizing larger chunks of information.13 This strategy appears to require longer processing time, either due to task complexity or due to deficits in higher visual functions. This result is partially consistent with previous findings during comparative visual search: “bad performers” with HVFDs attempted to compensate by both scanning and increased working memory involvement.13 Differences in the experimental design may account for this observation: sitting (in the above study) versus walking participants (in the current experiment). Recent evidence suggests that heading toward a specific direction during a peripheral detection task, places an additional attentional load on walking hemianopes: they seem to use their limited attentional resources toward path-associated visual cues, rather than adopting strategies to maximize target detection.34 On the other hand, our study cannot fully confirm the hypothesis of the study by Iorizzo et al.34 that the adaptive gaze strategies adopted by cortically blind individuals are strongest and most effective when seated and stationary, because our group of passed patients achieved a normal performance by gaze compensation while walking. A possible reason is that in the study by Iorizzo et al.34 no “pass-fail” criterion was applied to differentiate between “good” and “bad” performers, and the experiment involved detection of moving stimuli within a narrower field of view (48° × 36°) under virtual reality conditions.
In summary, visual search patterns of homonymous hemianopes under naturalistic conditions may be related to several different aspects such as the sensory deficit itself, involvement of spatial working memory and higher visual processing, or compensatory eye movements.41 Our study is novel, since eye movements of patients with HVFDs were assessed, for the first time, during a daily activity, such as shopping. Up-to-now there was a lack of data regarding the patterns of visual exploratory movements and their effect on functional compensation of HVFDs, neither in activities of daily living (ADL) nor with regard to quality of life. Hence, our results will have important implications, especially for clinicians and rehabilitation workers, given the aging population and the growing need for focusing on older individuals and their potential vision problems, Insight into the compensatory gaze strategies of patients with HVFDs will first provide useful data about their visual function in everyday life. These results should further enhance the development of an examination tool, assessing the compensatory gaze “potential” of subjects for use in a clinical setting. Finally, in terms of rehabilitation, our findings may enable the development of a training set-up, possibly with feedback elements, for improving compensatory gate strategies in visually impaired subjects.
Limitations of the Study
Although the total number of participants in this study (20 subjects) was large compared with the number of patients in similar studies under naturalistic conditions, the number of patients for subgroup analysis was possibly too small to detect statistical significance. Under consideration of the sample size in the current study, univariate comparisons for exploratory purposes were carried out to avoid possible side effects or implications of a multivariate analysis. Our study was conducted in a naturalistic setting, however, the task was kept rather simple (detect special objects indicated by a bright orange flag) in comparison to a real-life trip to the supermarket where patients have to detect desired objects that are not highlighted by reading the labels. Since, the impact of HVFL on ADLs could have been underestimated in the present task further studies need to be conducted. Another important issue is the role of head movements during natural visual search. Previous studies have suggested that patients with binocular VFD compensate by head movements, especially when the task requires exploration of a wide horizontal field of view (Tomasi M, et al. IOVS. 2014;ARVO E-Abstract 4131).21 In the present task no differences were found in HGA, which expresses the horizontal SD of the pupil position and is an indirect measure of eye movement (saccadic) amplitudes. This observation indirectly suggests the use of head movements in order to scan peripheral locations of the field of view. We have performed video recording of the scene by means of two additional cameras. However, head movement evaluation was not possible due to the highly dynamic nature of the task and the free movement of the participants in all directions. Additionally, trial duration analysis should take into account the motor component of the present task. Although there is no reason to assume any motor differences between groups and we have excluded patients with obvious motor deficits, the trial duration inevitably included the visual search period plus the motor response time (collection of item and heading toward the end of the corridor). Although the majority of the patients had suffered an occipital stroke, our sample also included patients with trauma and brain surgery, leading to lesions in variable brain locations. Hence, it cannot be excluded that some cognitive abilities, including navigation, spatial memory, visual attention, object recognition, action planning, decision-making, and execution might have interfered with visual exploration, depending on the site of cerebral lesion. For this reason, detailed neuropsychological tests and brain lesion analysis in future studies with more homogenous patient samples will shed light into the effect of those parameters on visual exploration. Finally, one may argue that some of the participants had some degree of macular sparing, which might affect gaze patterns. However, due to the free navigation of participants under naturalistic conditions and the need to locate targets in the far periphery, immediate visual input from the area of macular sparing is unlikely, as also shown by the gaze bias toward the peripheral visual field.
Conclusion
Homonymous VFDs may critically interfere with quality of life. In a special-offer supermarket search task, we investigated the performance of 10 patients with homonymous VFDs and 10 control subjects. A considerable number of patients completed the task successfully despite the visual impairment and performed indistinguishably from the control subjects. On average, homonymous visual-field loss was associated with longer search time. Analysis of eye-tracking data revealed that efficient visual search strategy may help some patients to compensate for their visual impairment. For the present supermarket search task, effective visual exploration included longer glancing toward the peripheral visual field.
Acknowledgments
The authors thank Müller Ltd. & Co. KG for enabling this study. We would further like to thank Pfizer and MSD for supporting and enabling this study.
*Enkelejda Kasneci and Katrin Sippel contributed equally to this work.
Disclosure: E. Kasneci, None; K. Sippel, None; M. Heister, None; K. Aehling, None; W. Rosenstiel, None; U. Schiefer, None; E. Papageorgiou, None
References
- 1.Gilhotra JS, Mitchell P, Healey PR, Cumming RG, Currie J. Homonymous visual field defects and stroke in an older population. Stroke. 2002;33:2417–2420. doi: 10.1161/01.str.0000037647.10414.d2. [DOI] [PubMed] [Google Scholar]
- 2.Van Stavern GP, Biousse V, Lynn MJ, Simon DJ, Newman NJ. Neuro-ophthalmic manifestations of head trauma. J Neuro Ophthalmol. 2001;21:112–117. doi: 10.1097/00041327-200106000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Bruce BB, Zhang X, Kedar S, Newman NJ, Biousse V. Traumatic homonymous hemianopia. J Neurol Neurosurg Psychiatry. 2006;77:986–988. doi: 10.1136/jnnp.2006.088799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coleman R, Rapport L, Ergh T, Hanks R, Ricker J, Millis SR. Predictors of driving outcome after traumatic brain injury. Arch Physical Med Rehab. 2002;83:1415–1422. doi: 10.1053/apmr.2002.35111. [DOI] [PubMed] [Google Scholar]
- 5.Papageorgiou E, Hardiess G, Schaeffel F, et al. Assessment of vision-related quality of life in patients with homonymous visual field defects. Graefes Arch Clin Exp Ophthalmol. 2007;245:1749–1758. doi: 10.1007/s00417-007-0644-z. [DOI] [PubMed] [Google Scholar]
- 6.Zihl J. Rehabilitation of Visual Disorders After Brain Injury. Hove, East Sussex: Psychology Press;; 2000. [Google Scholar]
- 7.Gall C, Lucklum J, Sabel BA, Franke GH. Vision- and health-related quality of life in patients with visual field loss after postchiasmatic lesions. Invest Opthalmol Vis Sci. 2009;50:2765–2776. doi: 10.1167/iovs.08-2519. [DOI] [PubMed] [Google Scholar]
- 8.Chen CS, Lee AW, Clarke G, et al. Vision-related quality of life in patients with complete homonymous hemianopia post stroke. Top Stroke Rehabil. 2009;16:445–453. doi: 10.1310/tsr1606-445. [DOI] [PubMed] [Google Scholar]
- 9.Gall C, Wagenbreth C, Sgorzaly S, Franke GH, Sabel BA. Parafoveal vision impairments and their influence on reading performance and self-evaluated reading abilities. Graefes Arch Clin Exp Ophthalmol. 2010;248:863–875. doi: 10.1007/s00417-009-1296-y. [DOI] [PubMed] [Google Scholar]
- 10.Owsley C, Ball K, McGwin G, et al. Visual processing impairment and risk of motor vehicle crash among older adults. JAMA. 1998;279:1083–1088. doi: 10.1001/jama.279.14.1083. [DOI] [PubMed] [Google Scholar]
- 11.Bowers A, Mandel A, Goldstein RB, Peli E. Driving with hemianopia, I: detection performance in a driving simulator. Invest Opthalmol Vis Sci. 2009;50:5137–5147. doi: 10.1167/iovs.09-3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bowers AR, Mandel AJ, Goldstein RB, Peli E. Driving with hemianopia, II: lane position and steering in a driving simulator. Invest Opthalmol Vis Sci. 2010;51:6605–6613. doi: 10.1167/iovs.10-5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hardiess G, Papageorgiou E, Schiefer U, Mallot HA. Functional compensation of visual field deficits in hemianopic patients under the influence of different task demands. Vision Res. 2010;50:1158–1172. doi: 10.1016/j.visres.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 14.Martin T, Riley ME, Kelly KN, Hayhoe M, Huxlin KR. Visually-guided behavior of homonymous hemianopes in a naturalistic task. Vision Res. 2007;47:3434–3446. doi: 10.1016/j.visres.2007.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papageorgiou E, Hardiess G, Mallot HA, Schiefer U. Gaze patterns predicting successful collision avoidance in patients with homonymous visual field defects. Vision Res. 2012;65:25–37. doi: 10.1016/j.visres.2012.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Pflugshaupt T, von Wartburg R, Wurtz P, et al. Linking physiology with behaviour: functional specialisation of the visual field is reflected in gaze patterns during visual search. Vision Res. 2009;49:237–248. doi: 10.1016/j.visres.2008.10.021. [DOI] [PubMed] [Google Scholar]
- 17.Warren M. Pilot study on activities of daily living limitations in adults with hemianopsia. Am J Occup Ther. 2009;63:626–633. doi: 10.5014/ajot.63.5.626. [DOI] [PubMed] [Google Scholar]
- 18.Wood J, McGwin G, Elgin J, et al. On-road driving performance by persons with hemianopia and quadrantanopia. Invest Opthalmol Vis Sci. 2009;50:577–585. doi: 10.1167/iovs.08-2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wood J, McGwin G, Elgin J, et al. Hemianopic and quadrantanopic field loss, eye and head movements, and driving. Invest Opthalmol Vis Sci. 2011;52:1220–1225. doi: 10.1167/iovs.10-6296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elgin J, McGwin G, Wood J, et al. Evaluation of on-road driving in people with hemianopia and quadrantanopia. Am J Occup Ther. 2010;64:268–278. doi: 10.5014/ajot.64.2.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kasneci E, Sippel K, Aehling K, et al. Driving with binocular visual field loss? A study on a supervised on-road parcours with simultaneous eye and head tracking. PLoS One. 2014;9:e87470. doi: 10.1371/journal.pone.0087470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schuett S, Kentridge RW, Zihl J, Heywood CA. Adaptation of eye movements to simulated hemianopia in reading and visual exploration: transfer or specificity? Neuropsychologia. 2009;47:1712–1720. doi: 10.1016/j.neuropsychologia.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 23.Degeratu AM, Rangaswamy A, Wu J. Consumer choice behavior in online and traditional supermarkets: the effects of brand name, price, and other search attributes. Internatl J Res Mark. 2000;17:55–78. [Google Scholar]
- 24.Tonkin C, Duchowski AT, Kahue J, Schiffgens P, Rischner F. Proceedings of the 1st International Workshop on Pervasive Eye Tracking & Mobile Eye-Based Interaction. New York, New York: ACM;; 2011. Eye tracking over small and large shopping displays; pp. 49–52. In. [Google Scholar]
- 25.Reutskaja E, Nagel R, Camerer CF, Rangel A. Search dynamics in consumer choice under time pressure: An eye-tracking study. Am Econ Rev. 2011;101:900–926. [Google Scholar]
- 26.Clement J, Kristensen T, Grønhaug K. Understanding consumers' in-store visual perception: the influence of package design features on visual attention. Journal of Retailing and Consumer Services. 2013;20:234–239. [Google Scholar]
- 27.Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatric Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- 28.Esterman B. Functional scoring of the binocular field. Ophthalmology. 1982;89:1226–1234. doi: 10.1016/s0161-6420(82)34647-3. [DOI] [PubMed] [Google Scholar]
- 29.Tafaj E, Kasneci G, Rosenstiel W, Bogdan M. Proceedings of the Symposium on Eye Tracking Research and Applications. New York, New York: ACM;; 2012. Bayesian online clustering of eye movement data; pp. 285–288. In. [Google Scholar]
- 30.Tafaj E, Kübler TC, Kasneci G, Rosenstiel W, Bogdan M. Online Classification of Eye Tracking Data for Automated Analysis of Traffic Hazard Perception, Artificial Neural Networks and Machine Learning. Berlin, Germany: Springer;; 2013. [Google Scholar]
- 31.Kasneci E, Kasneci G, Kübler T, Rosenstiel W. Proceedings of the Symposium on Eye Tracking Research and Application. New York, New York: ACM;; 2014. The applicability of probabilistic methods to the online recognition of fixations and saccades in dynamic scenes; pp. 323–326. In. [Google Scholar]
- 32.Holmqvist K, Nyström M, Andersson R, Dewhurst R, Jarodzka H, Van de Weijer J. Eye Tracking: A Comprehensive Guide to Methods and Measures. Oxford, United Kingdom: Oxford University Press;; 2011. [Google Scholar]
- 33.Tatler BW. The central fixation bias in scene viewing: selecting an optimal viewing position independently of motor biases and image feature distributions. J Vis. 2007;7(14):1–17. doi: 10.1167/7.14.4. [DOI] [PubMed] [Google Scholar]
- 34.Iorizzo DB, Riley ME, Hayhoe M, Huxlin KR. Differential impact of partial cortical blindness on gaze strategies when sitting and walking – sn immersive virtual reality study. Vision Res. 2011;51:1173–1184. doi: 10.1016/j.visres.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tant MLM, Cornelissen FW, Kooijman AC, Brouwer WH. Hemianopic visual field defects elicit hemianopic scanning. Vision Res. 2002;42:1337–1346. doi: 10.1016/s0042-6989(02)00044-5. [DOI] [PubMed] [Google Scholar]
- 36.Pambakian ALM, Mannan SK, Hodgson TL, Kennard C. Saccadic visual search training: a treatment for patients with homonymous hemianopia. J Neurol Neurosurg Psychiatry. 2004;75:1443–1448. doi: 10.1136/jnnp.2003.025957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ishiai S, Furukawa T, Tsukagoshi H. Eye-fixation patterns in homonymous hemianopia and unilateral spatial neglect. Neuropsychologia. 1987;25:675–679. doi: 10.1016/0028-3932(87)90058-3. [DOI] [PubMed] [Google Scholar]
- 38.Hamel J, Kraft A, Ohl S, De Beukelaer S, Audebert HJ, Brandt SA. Driving simulation in the clinic: testing visual exploratory behavior in daily life activities in patients with visual field defects. J Vis Exp. 2012 doi: 10.3791/4427. e4427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Riley M, Kelly K, Martin T, Hayhoe M, Huxlin K. Homonymous hemianopia alters distribution of visual fixations in 3-dimensional virtual environments. J Vis. 2007;7:289. [Google Scholar]
- 40.Cavézian C, Gaudry I, Perez C, et al. Specific impairments in visual processing following lesion side in hemianopic patients. Cortex. 2010;46:1123–1131. doi: 10.1016/j.cortex.2009.08.013. [DOI] [PubMed] [Google Scholar]
- 41.Machner B, Sprenger A, Kömpf D, et al. Visual search disorders beyond pure sensory failure in patients with acute homonymous visual field defects. Neuropsychologia. 2009;47:2704–2711. doi: 10.1016/j.neuropsychologia.2009.05.016. [DOI] [PubMed] [Google Scholar]
- 42.Zihl J. Visual scanning behavior in patients with homonymous hemianopia. Neuropsychologia. 1995;33:287–303. doi: 10.1016/0028-3932(94)00119-a. [DOI] [PubMed] [Google Scholar]



