Abstract
Polycyclic aromatic hydrocarbons (PAHs) are found widely in the ambient air and result from combustion of various fuels and industrial processes. PAHs have been associated with adverse human health effects such as cognitive development, childhood IQ, and respiratory health. The Fresno Asthmatic Children’s Environment Study (FACES) enrolled 315 children ages 6-11 years with asthma in Fresno, CA and followed the cohort from 2000 to 2008. Subjects were evaluated for asthma symptoms in up to three 14-day panels per year. Detailed ambient pollutant concentrations were collected from a central site and outdoor pollutants were measured at 83 homes for at least one 5-day period. Measurements of particle-bound PAHs were used with land use regression models to estimate individual exposures to PAHs with 4-, 5- or 6-member rings (PAH456) and phenanthrene for the cohort (approximately 22 000 individual daily estimates). We used a cross-validation based algorithm for model fitting and a generalized estimated equation approach to account for repeated measures. Multiple lags and moving averages of PAH exposure were associated with increased wheeze for each of the three types of PAH exposure estimates. The odds ratios for asthmatics exposed to PAHs (ng/m3) ranged from 1.01 (95% CI, 1.00-1.02) to 1.10 (95% CI, 1.04-1.17)]. This trend for increased wheeze persisted among all PAHs measured. Phenanthrene was found to have a higher relative impact on wheeze. These data provide further evidence that PAHs contribute to asthma morbidity.
Keywords: Asthma, polycyclic aromatic hydrocarbons, PAH, air pollution
Introduction
Asthma is the most prevalent, chronic health condition among children in the United States. Approximately 9 million children and 7 million adults are currently afflicted by the disease in the US (National Institutes of Health, 2009). Annually, asthma causes over 10.5 million physician visits and is projected to cost the US over 20 billion dollars in health expenditures and lost productivity (National Institutes of Health, 2009). Traffic emissions are associated with increased asthma morbidity (Ma, et al., 2008; Ward and Ayres, 2004) and possibly asthma onset (McConnell, et al., 2010). Air pollution has been linked to a variety of health outcomes, but the strongest evidence for pathways from exposure to disease exists for traffic-related air pollution and cardiovascular disease (Rosenlund, Berglind, Pershagen, Hallqvist, Jonson and Bellander, 2006; Tonne, Melly, Mittleman, Coull, Goldberg and Schwartz, 2007), all cause mortality (Jerrett, et al., 2009a; Jerrett, et al., 2009b), and asthma exacerbations (Kunzli, et al., 2000). Recently, exposure to PAHs, which are a major component of primary traffic emissions (HEI Panel on the Health Effects of Traffic-Related Air Pollution 2010), has been associated with relevant biomarkers (Delfino, et al., 2009) and implicated as a contributor to the onset of asthma (Burton, 2009; McConnell, et al., 2010).
PAHs are a class of chemicals defined by two or more fused aromatic rings that are products of incomplete combustion of fossil fuels, wood, coal, and tobacco. Major sources of ambient PAHs are vehicular emissions, home heating, woodland fires, prescribed burning, and power plants (Holgate, 1999). PAHs are important components of PM2.5 (particulate matter ≤2.5 µm) and PM10 (PM ≤10 µm), both of which have been linked to respiratory health (Krewski, et al., 2009; Pope, 1989). PAHs have received particular attention because of their oxidative potential and related cytotoxicity. Exposure to PAHs has been linked to several adverse outcomes in children including cognitive development (Perera, et al., 2006), childhood IQ (Perera, et al., 2009), and respiratory health (Jedrychowski, et al., 2005).
The evidence for the link between PAHs and asthma requires further investigation (Delfino, 2002). PAHs and other anthropogenic oxidants are hypothesized to affect the risk for asthma by causing bronchial inflammation and bronchoconstriction (Jedrychowski, et al., 2007). There is evidence to suggest that PAHs act through the immunoglobulin E (IgE) (Tsien, DiazSanchez, Ma and Saxon, 1997) pathway to stimulate inflammatory responses (Nel, Diaz-Sanchez, Ng, Hiura and Saxon, 1998) and enhance allergic inflammation (Lubitz, et al., 2010). PAHs have a well-established ability to generate reactive oxygen species that may affect asthma morbidity (Nel, Diaz-Sanchez and Li, 2001). Li and colleagues have provided evidence that PAHs in diesel exhaust can initiate a cascade of oxidative stress that leads to airway inflammation (Li, et al., 2003; Nel, Diaz-Sanchez and Li, 2001). In this model the reactive oxygen species that result from redox cycling of PAH intermediates activates signaling pathways that lead to transcriptional up-regulation of genes that control synthesis of cytokine and chemokines that participate in the airway inflammation associated with asthma (Nel, Diaz-Sanchez and Li, 2001). Increased production of IgE is one consequence of the PAH-induced oxidative stress (Nel, Diaz-Sanchez, Ng, Hiura and Saxon, 1998).
Exposure estimates to ambient levels of air pollutants are typically based on measurements from single fixed site monitors that can capture temporal variability but not often spatial variability (Wilson, Kingham, Pearce and Sturman, 2005). For PAHs, this poses two problems. First, ambient concentrations of PAHs are not routinely measured at EPA monitoring sites. Second, PAHs are highly dependent on local sources that have a high degree of spatial variability within an urban environment (Lehndorff and Schwark, 2004; Levy, Houseman, Spengler, Loh and Ryan, 2001). Exposure assessment for spatially heterogeneous air pollutants is best done with a model of exposure that accounts for both the temporal and spatial distributions. Land use regression (LUR) models can estimate pollution exposure by exploiting the relationship between the measurement site and local environmental variables (Briggs, et al., 2000). This relationship, formalized with regression equations, can incorporate small area variation and be used to assign estimated exposures for all participants in a cohort study.
In this paper we sought to evaluate the association between wheeze in a cohort of children with asthma in Fresno, CA, and two different methods to characterize the daily PAH exposures in Fresno, California: the daily concentration of particle-bound PAH measured continuously at a central site from 2000-2008 and two estimated individual-level daily exposures calculated from temporally-resolved land use regression models, namely phenanthrene, which is one of the most common PAHs, and PAH456, an estimated sum of PAHs with 4, 5 or 6 rings, which is closely correlated with the continuous PAH central site measurements. Our hypothesis was that there is a positive association between PAH exposure and wheeze, and that the LUR PAH exposure methods will yield stronger associations between wheeze and PAH.
Materials and Methods
Study Population
The data for this analysis come from the Fresno Asthmatic Children’s Environment Study (FACES), a longitudinal cohort study of children with current asthma in Fresno, California. The details on recruitment, health evaluations and environmental measurements have been reported previously (Mann, et al., 2010; Margolis, et al., 2009; Tager, Lurmann, Haight, Alcorn, Penfold and Hammond, 2010). Briefly, FACES collected data to explore short-term and long-term effects of ambient air pollution on children with asthma (as measured by spirometry, wheeze, and disease severity). The children were between the ages of 6-11 years at baseline and lived within a 20 km radius of the central monitoring site in Fresno (see below). FACES followed children (n=315) from 2000 to 2008 and assessed exposure over time to a variety of factors including aeroallergens, secondhand tobacco smoke, particulate matter constituents including PAHs and criteria gaseous air pollutants (NO2, SO2, CO, and ozone). In addition to detailed biannual or annual evaluations in the study field office (health questionnaire and spirometry), each study participant performed twice-daily spirometry with an EasyOne portable spirometer (ndd, Zurich, Switzerland) and answered symptom questions that were programmed in the EasyOne (see Appendix Table 1) in up to three 14-day home panels per year. The outcome used in this analysis, self-reported wheeze, was assessed after the morning spirometry measurement with the EasyOne question--did you wheeze after bedtime? The device was programmed such that morning spirometry and symptom questions could be completed only between 6-9 AM. FACES also collected data on the study participants’ physical and social environment: family income, household characteristics, indoor/outdoor environment, family demographics, and smoking habits, etc.
Air Pollution Monitoring and Modeling
During each panel day, air pollution data were collected at the California Air Resources Board (CARB) central monitoring site in Fresno that also served as an EPA Supersite. As part of the overall measurement of ambient pollutants, particle-bound PAH concentrations with three rings or greater were measured in real-time by the PAS2000 (EcoChem Analytics, League City, TX) at this site with an inlet approximately 10 meters above ground level from October 2000 to September 2008. The ambient levels of particle-bound PAH concentrations were measured at 1-minute intervals and aggregated into 24-hour daily averages (from 8 AM - 8 AM to coincide with the morning asthma symptom assessment).
In addition, we conducted ambient air pollution monitoring in Fresno outside 83 participant homes from February 2002 through February 2003 to supplement the central site estimates and understand the spatial variability of air pollution (Noth, Hammond, Biging and Tager, 2011). The homes were selected in relation to anticipated traffic exposure (low or high) and indoor sources, such as smoking status of the parents (non-smoking). The PAH samplers at the homes were placed in the backyard of the residence away from porches or overhangs and preferentially in the middle of the yard. Samplers were located approximately 1 meter from ground level. Samples were collected for 24 hours (8AM-8AM) on five days within each two-week panel study. There were 23 panels during the sub-study, and 28 of the 83 homes participated in more than one panel. Field blank filters were collected at the same time. Between one and seven homes were visited during each 2-week panel. These filter-based measurements (n=497) were 24-hour integrated samples collected on pre-baked quartz fiber filters (PallFlex Tissue Quartz) that were impregnated with XAD4 resin. The filter samples captured both particle-bound and vapor-phase PAHs and were analyzed for the 16 EPA Priority PAHs. For this analysis, we use two daily measurements of PAHs: phenanthrene and the sum of nine selected PAHs with 4-,5-, and 6-member rings (PAH456), which includes fluoranthene, benz[a]anthracene, chrysene, benzo[a]pyrene, benzo[b]fluoranthene, benzo[k]fluoranthene, benzo[ghi]perylene, indeno[1,2,3-cd]pyrene, and dibenz[a,h]anthracene. Some of the PAHs we analyzed are semi-volatile organic carbons, and the absorption processes and mechanisms of gas and particle pollutants are different. While the home sampling captured particle and vapor-phase PAH456 and phenanthrene, the central site only measured particle-bound PAHs with three or more aromatic rings. PAH456 and PAHs with three or more rings are not the same measures, but they are strongly correlated. PAH456 best resembles the central site PAHs with 3-rings or greater. Phenanthrene is one of the most common PAHs present in vapor-phase and it is a better surrogate for diesel exhaust exposure than particle-bound PAH (Pleil, Sobus, Madden, Funk, Hubbard and Rappaport, 2008).
We took two different approaches to assign daily PAH exposure to FACES participants. First, we used directly measured particle-bound PAH concentrations from the EPA Supersite as a proxy for PAH exposure in our cohort. These central-site measurements are not spatially resolved, i.e., each participant gets the same daily exposure.
Second, we applied LUR models with mixed effects linear regression to model individual-level exposures and estimate the spatial variability of PAHs (Noth, Hammond, Biging and Tager, 2011). The LUR models used the filter-based measurements from the 2-week FACES sub-study (sampling scattered through the year from 2002-2003) as the dependent variable and the particle-bound PAS 2000 measurement data at the EPA Supersite, meteorology data, and other spatial variables (e.g., traffic intensity, agricultural burning, and other source locations) as the independent variables (described in full in Noth, Hammond, Biging and Tager 2011). Two types of daily, individual PAH exposures were estimated for all FACES participants with LUR models: phenanthrene and PAH456. The LUR allowed us to incorporate both spatial and temporal information into our exposure models and exposure estimation. In the first LUR, individual-level phenanthrene was estimated by concentrations of PAHs at the EPA Supersite, distance to sources (major collector roads, railroad tracks), wind direction, neighborhood characteristics (type of home heating and age of homes in census blockgroup), frequency of agricultural burns within 5 miles, and season. Phenanthrene is primarily present in the vapor-phase. This model explained 46% of the between-house variability and 53% of the within-house variability as measured by the full mixed effects model variance components. In the second LUR, individual-level PAHs with 4-, 5-, or 6-member rings, PAH456, were estimated by concentrations of PAHs at the EPA supersite, distance to and intensity of source (minor collector roads, highway length within 500m), meteorological characteristics (wind direction, 24-hr wind recirculation, 24-hour relative humidity), neighborhood characteristics (home heating type by census blockgroup), and season. This model explained 81% of the between-house variability and 18% of the within-house variability as measured by the full mixed effects model variance components. More detail on field collection, measurement results and modeling can be found in Noth et al 2011.
To measure the relative model performance, we calculated a standardized unsystematic root mean square error (RMSEu) and total root mean square error (RMSEt), which is the RMSEu and RMSEt divided by the mean in the observed data for each of the three PAH estimates (Wilson and Zawar-Reza, 2006). Smaller values of RMSE indicate better model fit. RMSEu represents error related to model accuracy, whereas RMSEt includes both model accuracy and errors intrinsic to the model.
LUR estimates for PAH456 and phenanthrene were then calculated for each day of the FACES study for all participants using site- and time-specific values and model-derived coefficients. Using the common approach of a single central monitor that assumes a uniform spatial distribution throughout Fresno allows us to compare associations between exposure to PAHs and asthma outcomes to spatially resolved data (i.e., the LUR estimates). However, because of the difference in the type and species of PAHs, the central site measurements and the LUR estimates are not directly comparable.
Statistical Analysis
To be eligible for this analysis a child needed to complete a baseline field office visit and at least one home panel. We excluded children from the full cohort (n=315) who had no home visits, were lost to follow-up (n=16), or did not answer questions on the EasyOne spirometer when PAH was measured (n=2). Since morning wheeze was the primary outcome for this analysis, children who had cough-variant asthma and hence, no potential to wheeze were excluded (n=9). After these exclusions, some children were missing PAH central site data (n=1), PAH456 data (n=5), or phenanthrene data (n=4). After exclusions for eligibility, 283-287 children remained and provided 21 942 to 22 852 observations (the median observations per child = 75; range = 2 to 223 observations; and the inter-quartile interval = 82).
The model to describe the association between morning wheeze and PAH exposure was built in three steps (see Supplement Figure 1). First, regression analysis was used to explore bivariate associations with 92 candidate variables that we selected as confounders (see Supplement Table 2) and the outcome, morning wheeze. Confounders were selected a priori based on factors known to be associated with wheeze as well as factors known to be associated with PAHs and other ambient pollutants. Only those variables that had an association with wheeze with a p-value less than or equal to 0.30 were retained (variables 1-53 in Supplement Table 2).
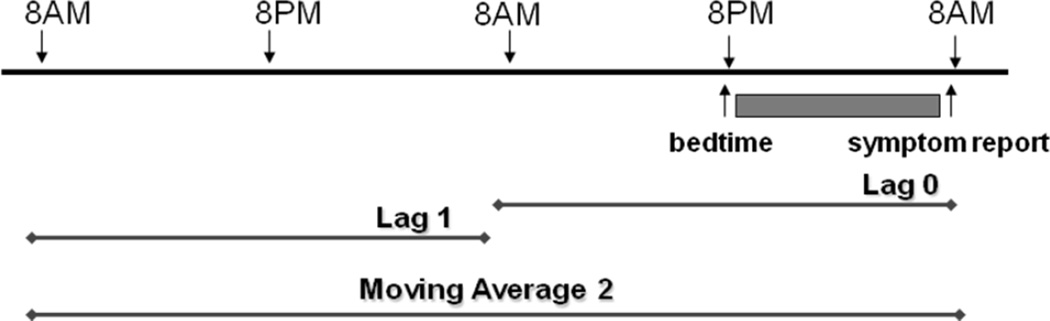
Next, a flexible model fitting algorithm was used to evaluate the association between the remaining candidate variables, and PAH at lag 0, and wheeze. Lag 0 is the average PAH exposure from 8 AM the previous day to 8 AM on the day that the child answered the wheeze question; and lag 1 is the 24 hour period that starts 48 hours prior to the outcome measurement, etc (Figure 1). We evaluated separate models for each of the different PAH estimates with the Deletion, Substitution, Addition (DSA) algorithm. The DSA, a data-driven, learning algorithm, searches for a model in the entire model space that best fits the user-supplied specifications (Sinisi and van der Laan, 2004). The DSA implements data-adaptive estimation through estimator selection based on a unified cross-validation methodology with the L2 loss function (observed minus expected) (van der Laan and Robins, 2002). This approach is far more intensive than usual model selection methodologies based on criteria such as Akaike information criterion (AIC) or Bayesian information criterion (BIC) to identify the “optimal” model, given the data. Candidate estimators are defined with polynomial generalized linear models generated under user-specified constraints (the degree of the polynomial, the maximum number of terms in the model, the maximum order of interactions and the maximum sum of powers in each interaction term).
Figure 1.
Description of lag and moving average designations
Every DSA model was set to include an indicator for potential seasonal effects, since PAH concentrations and exposures are, in general, higher during the cold weather months (November to February) mainly due to heightened wood burning and meteorological conditions in Fresno (e.g. lower photodegradation leads to higher stability) (Schauer and Cass, 2000). To further explore seasonal differences in the data, we performed a seasonal analysis on panel data obtained during winter months (November to February). However, this analysis yielded similar results to analyses based on the full data. To remove the seasonal fluctuations and secular trends in mean daily wheeze and PAH exposure, an Autoregressive, Integrated, Moving Average (ARIMA) model (Box, Jenkins and Reinsel, 1994) was implemented (Mann, et al., 2010). From this model, the fitted value for wheeze for each subject, for each day, was obtained. The inclusion of the fitted ARIMA wheeze term as an independent term in the regression models removes the observed temporal co-variation between the outcome (wheeze) and air pollution (PAH) exposure that occurs due to seasonal variation in meteorology, viral infections and aeroallergens as well as secular trends in both wheeze and PAH. In the DSA model selection, we forced the ARIMA term into each model to remove seasonality of wheeze. We performed a separate modeling procedure for each of the three PAH metrics: individual-level phenanthrene based on LUR estimates, individual-level PAH456 based on LUR estimates, and the central site PAH measurements. The DSA was run up to 10 times in each procedure, each time with a different user seed for all of the PAH metrics; we report only results for models that were identical in at least three out of the 10 runs.
The DSA was set to always include the ARIMA fitted term and the lag 0 PAH term. We allowed a maximum of 12 terms, no interactions, and second order polynomial terms for the model selection. The final model selected by the DSA included two variables in addition to the PAH lag 0 and ARIMA terms: annual household income under $35 000 and male sex. (Because these are nuisance variables we only present the coefficients in the Supplement Tables 3-5.) The DSA did not select the PAH lag 0 term in the final model; therefore, PAH was forced into the selection criteria. No other pollutants were entered into the model, because no other individual-level exposure estimates were available at the time.
This model was then used in a logistic regression based on the generalized estimated equation approach (GEE) to account for repeated measures. The GEE procedure in R version 2.12.1 (Vienna, Austria) was used for this step. The procedure allows for clustered data or repeated measures and a working correlation structure. The covariance matrix was set as exchangeable because, in this case, the coefficient of interest is defined by both the semi-parametric formula and a projection function that defines how the working model should approximate the true model. Based on earlier analyses with NO2, we chose to present models with lags for 0, 2, 5, 9, and 13 days, as well as, moving averages for 2, 5, 9, and 14 days. In each case, we used the same covariates as were used in the lag 0 model. These lags and moving averages cover the time intervals over which we observed associations between morning wheeze and NO2 (Mann, et al., 2010). Although results for all of the lags and moving averages are available, we bracketed the results to show only some of these values (Supplement Table 6).
To estimate the magnitude of the association between daily concentrations of PAHs, we calculated the difference between successive daily levels and used the 90th percentile value of the daily difference distribution of the PAH metrics for every lag and moving average. Thus, for each lag the daily difference is calculated as the absolute value of the 24 hour exposure estimate and the previous exposure estimate (e.g., the daily difference for lag 0 is derived from the 90th percentile distribution value of the difference between lag 0 and lag 1, similar distributions were generated for each successive lag difference distribution). For the individual metrics from the LUR models, the daily differences for the lags are an average of the differences for each eligible child on those days. The 90th percentiles of the lag daily differences are 1.79 – 2.77 ng/m3 for PAH456 based on LUR; 3.98 – 8.60 ng/m3 for the central-site measurements; and 1.53 – 2.38 ng/m3 for phenanthrene based on LUR.
For the moving averages, each PAH estimate was updated by adding the 90th percentile increment to the previous average and recalculating the previous average (i.e., odds ratios (ORs) represent the effect of increasing the moving average by these amounts: 0.32 – 1.60 ng/m3 for PAH456 based on LUR; 0.90 – 4.95 ng/m3 for the central-site measurements; and 0.27 – 1.24 ng/m3 for phenanthrene based on LUR). The 95% confidence intervals were calculated based on the non-parametric 25th and 975th estimates from 1000 bootstrapped samples using R.
Results
Compared to the full cohort (n=315), the subjects in this analysis (n=283-287) were somewhat more likely to have used inhaled steroids, to have an FEV1 <80% of predicted and to be classified with moderate to severe asthma (Table 1). The distribution of PAH values and correlations are presented in Tables 2 and 3. The median values of all of the PAH exposure estimates are similar, but the central site measurements have a greater variance. The PAH456 LUR and those from the central-site measurements are highly correlated (0.76), due to fact that central site data were included in the LUR. The phenanthrene LUR estimates are not as strongly correlated with either the PAH456 LUR estimates or the central-site measurements. The standardized RMSEu for PAH456, phenanthrene, and central site PAH are 0.27, 0.40, and 1.13, respectively. The standardized RMSEt for PAH456, phenanthrene and central site PAH are 0.59, 1.06, and 1.49, respectively.
Table 1.
Descriptive statistics for the FACES cohort
| Characteristic | Eligible for short-term analysis(n=283)a |
Full cohort (n=315)b |
|---|---|---|
| Mean Age at baseline[S.D.] | 8.6[1.7] | 8.5[1.7] |
| Male(%) | 56.9 | 56.5 |
| Income Less than $15,000(%) | 20.8 | 20.4 |
| Home Ownership(%) | 55.7 | 56.5 |
| Health Insurance(%) | 95.8 | 95.9 |
| Hispanic(%) | 39.9 | 39.7 |
| Non-hispanic white(%) | 41.0 | 41.9 |
| African American(%) | 15.9 | 16 |
| Mean # of Panel Visits Completed [s.d.] | 8.5[5.0] | 8.4[5.0] |
| Skin-test positive to at least one antigen(%)c | 62.8 | 62.7 |
| Mild intermittent asthma(%)d | 28.6 | 28.2 |
| Mild persistent asthma(%)d | 46.6 | 47.6 |
| Moderate or severe asthma(%)d | 24.7 | 24.1 |
| Use inhaled steroids(%) | 74.2 | 73.0 |
| Oral prednisone, last 12 months(%) | 37.9 | 37.5 |
| Reported morning wheezec(%) | 15 | - |
| %FEV1 < 80 % predictede | 18.2 | 17.5 |
| %FEF25−75 < 70 % predictede | 26.2 | 26.2 |
Abbreviations: FEV1, forced expiratory volume in 1 minute; FEF25−75, forced expiratory flow between 25% and 75% of forced vital capacity
The study subjects varied slightly for each PAH estimate. PAH456 had 283 subjects; PAH central site had 287 subjects; and phenanthrene had 284 subjects. This table shows results for the smallest group(n=283).
The full cohort contained children who were lost to follow-up, missing data or who had cough-variant asthma.
Positive to at least one antigen on allergy skin test panel or reported history of severe reaction to prior allergy skin test
Based on symptom severity guidelines in Global Initiative for Asthma
Pre-bronchodilator value
Table 2.
PAH exposure distribution based on panel days for eligible FACES participants October 2000-February 2007
| PAH Lag 0 (ng/m3) | Number of Observations |
Mean[S.D.] | Min, Median, Max | Interquartile Range |
|---|---|---|---|---|
| PAH456 LUR Individual Estimates |
21 942 | 3.74[2.98] | 0.57, 2.89, 55.02 | 2.21 |
| Central Site PAH measurements |
22 852 | 6.84[7.94] | 0.92, 3.45, 55.20 | 6.79 |
| Phenanthrene LUR Individual Estimates |
22 288 | 4.53[2.50] | 0.90, 3.88, 54.38 | 2.48 |
Abbreviations: PAH, polycyclic aromatic hydrocarbon; LUR, land use regression; Max, Maximum; Min, minimum; PAH456 polycyclic aromatic hydrocarbon with 4-,5-, or 6-rings
Table 3.
PAH estimate Pearson correlations based on all 24-hour daily averages from October 2000−February 2007 (n=50 293)
| PAH | PAH456 LUR | PAH Central Site measurements |
Phenanthrene LUR |
|---|---|---|---|
| PAH456 LUR | 1 | ||
| PAH Central Site measurements | 0.76 | 1 | |
| Phenanthrene LUR | 0.56 | 0.68 | 1 |
Abbreviations: LUR, land use regression; PAH, polycyclic aromatic hydrocarbon; PAH456 polycyclic aromatic hydrocarbon with 4-,5-, or 6-rings
Of the 92 candidate covariates that were considered as potential confounders (Supplement Table 2), 53 variables had a p-value ≤ 0.30 for the data reduction step. The 53 confounders were then considered for selection in the final model with the DSA. These variables can be grouped into several categories: meteorological and environmental conditions, social and demographic factors, allergens, and asthma symptomatic factors.
The model selected by the DSA from amongst the 53 candidate variables was chosen three or more times out of 10 runs of the DSA for each lag 0 PAH measurement. All models selected in the 10 runs contained similar combinations of the same variables. The final model is the same for all three lag 0 PAH exposure metrics, but the PAH exposure changes based on the lag, MA and PAH type. (The seasonal analysis based on the data from winter months November-February yielded similar PAH coefficients to the analysis on the full data, so the final model for the full data was used.) Coefficients for the final model and all of the covariates are presented in Supplement Table 3, 4, and 5 with ORs derived from GEE.
PAH exposure was evaluated at several different lags and moving averages (Table 4). In general, among all of the lags and moving averages, the odds of wheeze are increased with exposure to PAHs. The OR (95% confidence interval) was 1.02 (0.99, 1.06) for a 90th percentile daily difference of 1.79 ng/m3 increase in concentration of the lag 0 PAH456 LUR estimates; 1.04 (1.00, 1.07) for a 90th percentile daily difference of 1.53 ng/m3 increase in concentration of the lag 0 phenanthrene LUR estimates; and 1.04 (1.01, 1.07) for a 90th percentile daily difference of 3.98 ng/m3 increase in concentration of the lag 0 central-site measurements. Phenanthrene effects were generally greater but somewhat less precise than PAH456. Odds ratios for lags ranged from 1.01 to 1.10, although the 95% CI for several lag concentrations included 1. The highest odds of wheeze among all PAH metrics [1.10 (95% CI: 1.04-1.16)] was associated with the 1, 2, 3, 5, 6, 7, and 9-day lag concentrations for the central-site PAHs (see Supplement Table 6). The 90th percentile daily differences for each lag and MA are presented in Supplement Table 7.
Table 4.
ORs for wheeze: separate GEE models for DSA selected covariates for lags and moving averages of PAHs based on the 90th percentile daily differences in ng/m3
| Estimate | PAH456 LUR estimates |
Central Site PAH measurements |
Phenanthrene LUR estimates |
|||
|---|---|---|---|---|---|---|
| 90th percentile daily difference ng/m3 |
OR(95% CI)a | 90th percentile daily difference ng/m3 |
OR(95% CI)a | 90th percentile daily difference ng/m3 |
OR(95% CI)a | |
| Lag 0 | 1.79 | 1.02(0.99,1.06) | 3.98 | 1.04(1.01,1.07) | 1.53 | 1.04(1.00,1.07) |
| Lag 2 | 2.74 | 1.06(1.00,1.11) | 8.12 | 1.10(1.04,1.16) | 2.33 | 1.08(1.03,1.14) |
| Lag 5 | 2.67 | 1.03(0.98,1.07) | 8.15 | 1.10(1.03,1.16) | 2.28 | 1.06(1.00, 1.12) |
| Lag 9 | 2.73 | 1.04(0.99,1.10) | 8.42 | 1.10(1.04,1.17) | 2.35 | 1.04(0.99,1.09) |
| Lag 13 | 2.68 | 1.01(0.96,1.07) | 8.34 | 1.06(1.01,1.13) | 2.37 | 1.03(0.98,1.09) |
| 2-day moving average | 1.60 | 1.03(0.99,1.05) | 4.95 | 1.06(1.02,1.09) | 1.24 | 1.04(1.01,1.04) |
| 5-day moving average | 0.72 | 1.02(1.00,1.04) | 2.28 | 1.03(1.01,1.06) | 0.55 | 1.03(1.01,1.05) |
| 9-day moving average | 0.46 | 1.01(1.00,1.03) | 1.36 | 1.02(1.01,1.04) | 0.34 | 1.01(1.00, 1.03) |
| 14-day moving average | 0.33 | 1.01(1.00,1.02) | 0.90 | 1.01(1.01,1.02) | 0.27 | 1.01(1.00,1.02) |
Abbreviations: CI, confidence interval; Coeff., coefficient; DSA, Deletion Substitution Addition Algorithm; GEE, generalized estimating equation; LUR, land use regression; PAH, polycyclic aromatic hydrocarbon; OR, odds ratio; PAH456, polycyclic aromatic hydrocarbon with 4-,5-, or 6-rings
Non-parametric 95% confidence interval calculated with the 25th and 975th estimates from 1000 bootstrapped samples.
Discussion
We used central site data and land use regression to estimate daily exposure to PAHs in a cohort of asthmatic children and have shown that increases in daily concentrations of all exposure metrics--central site PAHs, PAH456 and phenanthrene--are associated with an increased odds of morning wheeze. The three exposure metrics are not identical, but they each represent a different approach to PAH exposure assessment. The central site captures particle-bound PAHs and temporal variation, but includes no spatial variation. The advantage of the addition of the LUR estimates is that they contain both temporal and spatial variation. PAH456 most closely resembles the central site PAH mixture, and the LUR applied to PAH456 adds spatial variability in addition to temporal variability. Phenanthrene is of intrinsic interest as its concentration is the second highest of the PAHs and it has been found to mimic the effects of diesel exhaust particles in vitro (Tsien, DiazSanchez, Ma and Saxon, 1997). Like the central site PAH measurements with which it is correlated, phenanthrene displays temporal variability; the dependence of its spatial variability on agricultural burning as well as traffic was included in the LUR model. The central site measurements, while they lack spatial variation, only contain Berkson’s error. The LUR estimates, while we gain spatial variation, are more heterogeneous and contain both classical and Berkson’s error.
The individual-level phenanthrene concentrations show statistical significance in lags 1-4 and moving averages for 2-day through 5-day. The point estimates for the associations based on measurements from the single central-site monitor in Fresno were slightly larger and the confidence intervals narrower than those based on individual-estimates for all lags and moving averages to 14 days. This could be due to the spatial variability and classical error included in the LUR estimates—for each panel day, participants no longer have the same exposure for PAH as they did with the central site measurements.
For all PAH metrics, odds ratios were larger for lags than moving averages; because the 90th percentile daily differences in the PAH metrics were greater for the lags than for the moving averages. These associations were independent of family income and gender (Supplemental Material Tables 3, 4, and 5). Unfortunately, we could not include concentrations of other pollutants in this analysis, since we did not have individual-level daily estimates for these exposures. The analysis of longer lags with PAH indicates that the effect of a single-day increase in PAH exposure is relatively stable over at least a 2-week period, as suggested by the similar odds with increasing length of the lag measurements (Supplement Table 6). Many of the coefficients for moving averages were larger than those for lags, however odds for moving averages were smaller in magnitude and decreased with more days out because the PAH concentration intervals evaluated were smaller and decreased with increased averaging times. For example, the ORs for the lags ranged from 1.01 to 1.10, while the moving averages ranged from 1.01 to 1.06.
Relatively few studies have evaluated the effects of short-term exposure to PAHs, despite the biologic importance of PAHs (Li, et al., 2003) and their substantial mass prevalence in the mixture of PM (Sioutas, Delfino and Singh, 2005). Miller and colleagues carried out personal monitoring for PAHs, in a cohort of pregnant women in New York City (Miller, et al., 2004). Most of the PAHs measured were the same as those in our study; although, the only association found was between the exposure to secondhand tobacco smoke (SHS) and the occurrence of cough and wheeze at age 12 months in children. In a study of longer term exposure to air pollutants in Southern California, variants of the PAH metabolizing enzyme microsomal epoxide hydrolase that results in increased reactive oxygen species were associated with an increased risk of asthma and, in addition, the risk increased with proximity of residences to freeways (Salam, Lin, Avol, Gauderman and Gilliland, 2007). A preliminary study in 70 FACES participants has demonstrated an association between the same PAH metrics used in this study and decreased FEV1, increased asthma severity, and suppression of regulatory T-cell function through methylation of the FoxP3 gene that up-regulates the function of these cells (Nadeau, et al., 2010). The suppression of regulatory T cells also was significantly associated with enhancement of the Th-2 phenotype that is associated with allergic asthma. The finding that PAH exposure was associated with methylation of FoxP3 is consistent with the observation that exposure to traffic-related air pollution can lead to DNA methylation (Baccarelli, et al., 2009). These associations with DNA methylation likely would enhance the documented ability of PAHs, particularly PHE to enhance IgE synthesis (Tsien, DiazSanchez, Ma and Saxon, 1997). Motor vehicles account for one third of PAH emissions in the US (Marr, Kirchstetter, Harley, Miguel, Hering and Hammond, 1999), and motor vehicles and residential wood combustion are the major sources of air pollution in Fresno (Chen, Watson, Chow and Magliano, 2007; Chow, et al., 2007).
Several caveats need to be considered in the interpretation of these data. We did not estimate individual-level exposures for other pollutants, so we did not evaluate other components of PM, secondhand smoke, or gaseous pollutants that are correlated with PAHs. (Although we explored family and home smoking, they were only weakly correlated with wheeze in this analysis and not selected by the DSA.) Thus, we cannot estimate the degree to which the associations we report here are independent of other pollutants. Based on the correlation structure of the other pollutants collected at the central site (Supplement Table 8), the PAHs in this analysis are correlated with PM2.5, CO, NO2 and elemental carbon. These strong correlations suggest that the PAHs we measured in Fresno are likely from traffic.
The general trend of the data reveal that PAHs, no matter the method used to measure the pollutant, are associated with increased wheeze in this cohort of asthmatics. While the data from the central site show greater odds for wheeze than the LUR metrics, this could be due to bias towards the null from the classical error present in the LUR models. The central site PAH exposures do not contain an error term because they are characterized by a Berkson error structure in which the true values for the individual exposures are independent from the observed values at the central site (Berkson, 1950). Berkson’s error adds little or no bias to the effect estimate, but it increases the standard deviation of the estimate. The central site measurements are statistically valid, but may not be as reliable. In other words, the effect measurements will not be biased from the central site, but the standard errors of the effects will be larger. A child’s true exposure to PAH lies within the observed measurement from Fresno’s central monitoring site. The central site is, in a sense, measuring the group’s average PAH exposure, and the group average is comprised of all of the individual exposures. While the central site is a good proxy for PAH exposure, it does not capture the spatial variability present in the study area.
The LUR models account for some of the spatial variability in the data, but the health effects are attenuated and biased towards the null. The LUR PAH exposure estimates contain both classical and Berkson’s error. However, we did not incorporate errors attached to the land use regression model (Noth, Hammond, Biging and Tager, 2011). We know these errors would bias our results, since there is likely to be variation in errors across strata in the model and unknown correlation of errors in the measurement of the other covariates. Because the error structure in our data is dominated by a classical error model, it is most likely, but not guaranteed, that any bias would be toward the null (Knuiman, Divitini, Buzas and Fitzgerald, 1998). Both the central site and LUR metrics show associations with wheeze that are likely valid, but the LUR effects appear to be biased towards the null. The LUR effects contain larger standard errors, which influence the level of attenuation of the effect estimate. The larger the standard errors, the greater the attenuation (Zeger, et al., 2000). While we expected the central site to have larger standard errors than the LUR effect estimates, we found the opposite. We interpret this to be due to a dominate classic error structure. Other researchers have found that more accurate exposure assessment, with the example of a land use regression model, might not lead to “improved health effect estimation” and furthermore, there can be a classic-like measurement error from the LUR estimates that can introduce variability in the estimated exposures, bias the health effect estimates, inflate the standard error, and be correlated in space (Szpiro, Paciorek and Sheppard, 2011). LUR models can be extremely site dependent and may not perform well with fewer than 100 monitoring sites; thus, the potential for exposure for misclassification is increased (Johnson, Isakov, Touma, Mukerjee and Ozkaynak, 2010).
One strength of our analysis is our use of the machine-learning DSA algorithm to determine which covariates, among those associated with morning wheeze, should be retained in the model. This algorithm requires no a priori model form and uses cross-validation to identify the optimal model across the entire model space of a linear polynomial whose parameters can be set by the user. Moreover, we report only models that gave the ideal request in at least three of 10 independent runs of the data. This approach reduces the probability of over-fitting the model. Future work with FACES will examine the effect of exposures to PAHs on other outcomes (e.g. lung function) among children with asthma and will include multiple pollutants in the analyses.
In summary, our results show that both PAHs with 4-, 5-, or 6-rings and phenanthrene are associated with increased wheeze among asthmatics. Regardless of whether the estimates were obtained via a fixed central-site monitor or a land use regression model, the results tell a similar story: exposure to polycyclic aromatic hydrocarbons is associated with an increased risk of wheeze in a susceptible population.
Supplementary Material
Acknowledgements
Boriana Pratt for data management and programming and the FACES team of investigators and field staff. The authors thank Fred Lurmann and colleagues at Sonoma Technology Inc. for the exposure assessment.
Grants: California Air Resources Board, Contract Nos. 99-322, 99-323 and -01-346
Division of Lung Diseases, National Heart Lung and Blood Institute Grant # R01 HL081521
Mickey Leland National Urban Air Toxics Research Program, RFA 2005-01
US Environmental Protection Agency Office of Transportation and Air Quality (P.O. No. 2A-0540-NASX)
The research described in this article was funded by the Mickey Leland National Urban Air Toxics Research Center (NUATRC), an organization jointly funded by the United States Environmental Protection Agency and private industry sponsors. The contents of this article do not necessarily reflect the views of NUATRC, or its sponsors, nor do they necessarily reflect the views and policies of the EPA or any of the private industry sponsors.
Abbreviations
- AIC
Akaike information criterion
- ARIMA
autoregressive integrated moving average
- BIC
Bayesian information criterion
- CARB
California Air Resources Board
- DSA
Deletion Substitution Addition algorithm
- EPA
Environmental Protection Agency
- FACES
Fresno Asthmatic Children’s Environment Study
- FEV1
forced expiratory volume in 1 minute
- FEF25-75
forced expiratory flow between 25% and 75% of forced vital capacity
- GEE
generalized estimating equation
- GINA
the Global Initiative for Asthma
- IgE
immunoglobulin E
- LUR
land use regression
- Max
maximum
- Min
minimum
- PAH
polycyclic aromatic hydrocarbon
- PAH456
sum of 9 polycyclic aromatic hydrocarbons with 4-,5-, or 6-member rings
- PM2.5
particulate matter with a diameter smaller than 2.5 microns
- PM10-2.5
particulate matter with a diameter between 2.5 and 10 microns
Footnotes
We declare no competing financial interests for this work.
The statements and conclusions in this article are those of the author and not necessarily those of the California Air Resources Board. The mention of commercial products, their source or their use in connection with material reported herein is not to be construed as actual or implied endorsement of such products.
References
- Baccarelli A, Wright RO, Bollati V, Tarantini L, Litonjua AA, Suh HH, Zanobetti A, Sparrow D, Vokonas PS, Schwartz J. Rapid DNA methylation changes after exposure to traffic particles. American journal of respiratory and critical care medicine. 2009;179(7):572–578. doi: 10.1164/rccm.200807-1097OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkson J. Are There 2 Regressions. Journal of the American Statistical Association. 1950;45(250):164–180. [Google Scholar]
- Box G, Jenkins G, Reinsel G. Time Series Analysis, Forecastings and Control. 3rd edn. Englewood Cliffs: Prentice Hall; 1994. [Google Scholar]
- Briggs DJ, de Hoogh C, Guiliver J, Wills J, Elliott P, Kingham S, Smallbone K. A regression-based method for mapping traffic-related air pollution: application and testing in four contrasting urban environments. Science of the Total Environment. 2000;253(1-3):151–167. doi: 10.1016/s0048-9697(00)00429-0. [DOI] [PubMed] [Google Scholar]
- Burton A. Children's health: methylation links prenatal PAH exposure to asthma. Environ Health Perspect. 2009;117(5):A195. doi: 10.1289/ehp.117-a195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LWA, Watson JG, Chow JC, Magliano KL. Quantifying PM2.5 source contributions for the San Joaquin Valley with multivariate receptor models. Environmental Science & Technology. 2007;41(8):2818–2826. doi: 10.1021/es0525105. [DOI] [PubMed] [Google Scholar]
- Chow JC, Watson JG, Lowenthal DH, Chen LWA, Zielinska B, Mazzoleni LR, Magliano KL. Evaluation of organic markers for chemical mass balance source apportionment at the Fresno Supersite. Atmospheric Chemistry and Physics. 2007;7(7):1741–1754. [Google Scholar]
- Delfino RJ. Epidemiologic evidence for asthma and exposure to air toxics: linkages between occupational, indoor, and community air pollution research. Environ Health Perspect. 2002;110(Suppl 4):573–589. doi: 10.1289/ehp.02110s4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delfino RJ, Staimer N, Tjoa T, Gillen DL, Polidori A, Arhami M, Kleinman MT, Vaziri ND, Longhurst J, Sioutas C. Air pollution exposures and circulating biomarkers of effect in a susceptible population: clues to potential causal component mixtures and mechanisms. Environ Health Perspect. 2009;117(8):1232–1238. doi: 10.1289/ehp.0800194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HEI Panel on the Health Effects of Traffic-Related Air Pollution Traffic-Related Air Pollution: A Critical Review of the Literature on Emissions, Exposure, and Health Effects. Boston, MA: Health Effects Institute; 2010. [Google Scholar]
- Holgate ST. Air pollution and health. San Diego, Calif.: Academic Press; 1999. pp. xiv–1065. [Google Scholar]
- Jedrychowski W, Galas A, Pac A, Flak E, Camman D, Rauh V, Perera F. Prenatal ambient air exposure to polycyclic aromatic hydrocarbons and the occurrence of respiratory symptoms over the first year of life. European Journal of Epidemiology. 2005;20(9):775–782. doi: 10.1007/s10654-005-1048-1. [DOI] [PubMed] [Google Scholar]
- Jedrychowski W, Perera FP, Whyatt R, Mroz E, Flak E, Jacek R, Penar A, Spengler J, Camman D. Wheezing and lung function measured in subjects exposed to various levels of fine particles and polycyclic aromatic hydrocarbons. Central European Journal of Medicine. 2007;2(1):66–78. [Google Scholar]
- Jerrett M, Burnett RT, Pope CA, 3rd, Ito K, Thurston G, Krewski D, Shi Y, Calle E, Thun M. Long-term ozone exposure and mortality. The New England journal of medicine. 2009a;360(11):1085–1095. doi: 10.1056/NEJMoa0803894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerrett M, Finkelstein MM, Brook JR, Arain MA, Kanaroglou P, Stieb DM, Gilbert NL, Verma D, Finkelstein N, Chapman KR, Sears MR. A cohort study of traffic-related air pollution and mortality in Toronto, Ontario, Canada. Environmental health perspectives. 2009b;117(5):772–777. doi: 10.1289/ehp.11533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M, Isakov V, Touma JS, Mukerjee S, Ozkaynak H. Evaluation of land-use regression models used to predict air quality concentrations in an urban area. Atmospheric Environment. 2010;44(30):3660–3668. [Google Scholar]
- Knuiman MW, Divitini ML, Buzas JS, Fitzgerald PE. Adjustment for regression dilution in epidemiological regression analyses. Ann Epidemiol. 1998;8(1):56–63. doi: 10.1016/s1047-2797(97)00107-5. [DOI] [PubMed] [Google Scholar]
- Krewski D, Jerrett M, Burnett RT, Ma R, Hughes E, Shi Y, Turner MC, Pope CA, 3rd, Thurston G, Calle EE, Thun MJ, Beckerman B, DeLuca P, Finkelstein N, Ito K, Moore DK, Newbold KB, Ramsay T, Ross Z, Shin H, Tempalski B. Extended follow-up and spatial analysis of the American Cancer Society study linking particulate air pollution and mortality. Res Rep Health Eff Inst. 2009;(140):5–114. discussion 115-136. [PubMed] [Google Scholar]
- Kunzli N, Kaiser R, Medina S, Studnicka M, Chanel O, Filliger P, Herry M, Horak F, Jr, Puybonnieux-Texier V, Quenel P, Schneider J, Seethaler R, Vergnaud JC, Sommer H. Public-health impact of outdoor and traffic-related air pollution: a European assessment. Lancet. 2000;356(9232):795–801. doi: 10.1016/S0140-6736(00)02653-2. [DOI] [PubMed] [Google Scholar]
- Lehndorff E, Schwark L. Biomonitoring of air quality in the Cologne Conurbation using pine needles as a passive sampler - Part II: polycyclic aromatic hydrocarbons (PAH) Atmospheric Environment. 2004;38(23):3793–3808. [Google Scholar]
- Levy JI, Houseman EA, Spengler JD, Loh P, Ryan L. Fine particulate matter and polycyclic aromatic hydrocarbon concentration patterns in Roxbury, Massachusetts: a community-based GIS analysis. Environ Health Perspect. 2001;109(4):341–347. doi: 10.1289/ehp.01109341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Sioutas C, Cho A, Schmitz D, Misra C, Sempf J, Wang M, Oberley T, Froines J, Nel A. Ultrafine particulate pollutants induce oxidative stress and mitochondrial damage. Environmental health perspectives. 2003;111(4):455–460. doi: 10.1289/ehp.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubitz S, Schober W, Pusch G, Effner R, Klopp N, Behrendt H, Buters JTM. Polycyclic Aromatic Hydrocarbons from Diesel Emissions Exert Proallergic Effects in Birch Pollen Allergic Individuals Through Enhanced Mediator Release from Basophils. Environmental Toxicology. 2010;25(2):188–197. doi: 10.1002/tox.20490. [DOI] [PubMed] [Google Scholar]
- Ma L, Shima M, Yoda Y, Yamamoto H, Nakai S, Tamura K, Nitta H, Watanabe H, Nishimuta T. Effects of airborne particulate matter on respiratory morbidity in asthmatic children. J Epidemiol. 2008;18(3):97–110. doi: 10.2188/jea.JE2007432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann JK, Balmes JR, Bruckner TA, Mortimer KM, Margolis HG, Pratt B, Hammond SK, Lurmann F, Tager IB. Short-Term Effects of Air Pollution on Wheeze in Asthmatic Children in Fresno, California. Environ Health Perspect. 2010 doi: 10.1289/ehp.0901292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis HG, Mann JK, Lurmann FW, Mortimer KM, Balmes JR, Hammond SK, Tager IB. Altered pulmonary function in children with asthma associated with highway traffic near residence. International journal of environmental health research. 2009;19(2):139–155. doi: 10.1080/09603120802415792. [DOI] [PubMed] [Google Scholar]
- Marr LC, Kirchstetter TW, Harley RA, Miguel AH, Hering SV, Hammond SK. Characterization of polycyclic aromatic hydrocarbons in motor vehicle fuels and exhaust emissions. Environmental Science & Technology. 1999;33(18):3091–3099. [Google Scholar]
- McConnell R, Islam T, Shankardass K, Jerrett M, Lurmann F, Gilliland F, Gauderman J, Avol E, Kunzli N, Yao L, Peters J, Berhane K. Childhood incident asthma and traffic-related air pollution at home and school. Environ Health Perspect. 2010;118(7):1021–1026. doi: 10.1289/ehp.0901232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller RL, Garfinkel R, Horton M, Camann D, Perera FP, Whyatt RM, Kinney PL. Polycyclic aromatic hydrocarbons, environmental tobacco smoke, and respiratory symptoms in an inner-city birth cohort. Chest. 2004;126(4):1071–1078. doi: 10.1378/chest.126.4.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau KC, McDonald-Hyman C, Noth EM, Pratt B, Hammond SK, Balmes JR, Tager IB. Ambient air pollution impairs T-cell function in asthma. J Allergy Clin Immunol. 2010;126(4):845–852. doi: 10.1016/j.jaci.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Services D.o.H.a.H., editor. National Institutes of Health. Morbidity and Mortality: 2009 Chart Book on Cardiovascular, Lung, and Blood Diseases. National Heart, Lung, and Blood Institutes; 2009. [Google Scholar]
- Nel AE, Diaz-Sanchez D, Ng D, Hiura T, Saxon A. Enhancement of allergic inflammation by the interaction between diesel exhaust particles and the immune system. Journal of Allergy and Clinical Immunology. 1998;102(4):539–554. doi: 10.1016/s0091-6749(98)70269-6. [DOI] [PubMed] [Google Scholar]
- Nel AE, Diaz-Sanchez D, Li N. The role of particulate pollutants in pulmonary inflammation and asthma: evidence for the involvement of organic chemicals and oxidative stress. Current opinion in pulmonary medicine. 2001;7(1):20–26. doi: 10.1097/00063198-200101000-00004. [DOI] [PubMed] [Google Scholar]
- Noth EM, Hammond SK, Biging GS, Tager IB. A spatial-temporal regression model to predict daily outdoor residential PAH concentrations in an epidemiologic study in Fresno, CA. Atmospheric Environment. 2011;45(14):2394–2403. [Google Scholar]
- Perera FP, Rauh V, Whyatt RM, Tsai WY, Tang DL, Diaz D, Hoepner L, Barr D, Tu YH, Camann D, Kinney P. Effect of prenatal exposure to airborne polycyclic aromatic hydrocarbons on neurodevelopment in the first 3 years of life among inner-city children. Environmental Health Perspectives. 2006;114(8):1287–1292. doi: 10.1289/ehp.9084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera FP, Li ZG, Whyatt R, Hoepner L, Wang SA, Camann D, Rauh V. Prenatal Airborne Polycyclic Aromatic Hydrocarbon Exposure and Child IQ at Age 5 Years. Pediatrics. 2009;124(2):E195–E202. doi: 10.1542/peds.2008-3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pleil JD, Sobus JR, Madden MC, Funk WE, Hubbard HF, Rappaport SM. Identification of Surrogate Measures of Diesel Exhaust Exposure in a Controlled Chamber Study. Environmental Science & Technology. 2008;42(23):8822–8828. doi: 10.1021/es800813v. [DOI] [PubMed] [Google Scholar]
- Pope CA., 3rd Respiratory disease associated with community air pollution and a steel mill, Utah Valley. Am J Public Health. 1989;79(5):623–628. doi: 10.2105/ajph.79.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenlund M, Berglind N, Pershagen G, Hallqvist J, Jonson T, Bellander T. Long-term exposure to urban air pollution and myocardial infarction. Epidemiology. 2006;17(4):383–390. doi: 10.1097/01.ede.0000219722.25569.0f. [DOI] [PubMed] [Google Scholar]
- Salam MT, Lin PC, Avol EL, Gauderman WJ, Gilliland FD. Microsomal epoxide hydrolase, glutathione S-transferase P1, traffic and childhood asthma. Thorax. 2007;62(12):1050–1057. doi: 10.1136/thx.2007.080127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauer JJ, Cass GR. Source apportionment of wintertime gas-phase and particle-phase air pollutants using organic compounds as tracers. Environmental Science & Technology. 2000;34(9):1821–1832. [Google Scholar]
- Sinisi SE, van der Laan MJ. Deletion/substitution/addition algorithm in learning with applications in genomics. Statistical applications in genetics and molecular biology. 2004;3 doi: 10.2202/1544-6115.1069. Article18. [DOI] [PubMed] [Google Scholar]
- Sioutas C, Delfino RJ, Singh M. Exposure assessment for atmospheric ultrafine particles (UFPs) and implications in epidemiologic research. Environmental health perspectives. 2005;113(8):947–955. doi: 10.1289/ehp.7939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szpiro AA, Paciorek CJ, Sheppard L. Does more accurate exposure prediction necessarily improve health effect estimates? Epidemiology (Cambridge, Mass. 2011;22(5):680–685. doi: 10.1097/EDE.0b013e3182254cc6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tager IB, Lurmann FW, Haight T, Alcorn S, Penfold B, Hammond SK. Temporal and Spatial Patterns of Ambient Endotoxin Concentrations in Fresno, California. Environ Health Perspect. 2010 doi: 10.1289/ehp.0901602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonne C, Melly S, Mittleman M, Coull B, Goldberg R, Schwartz J. A case-control analysis of exposure to traffic and acute myocardial infarction. Environmental health perspectives. 2007;115(1):53–57. doi: 10.1289/ehp.9587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien A, DiazSanchez D, Ma J, Saxon A. The organic component of diesel exhaust particles and phenanthrene, a major polyaromatic hydrocarbon constituent, enhances IgE production by IgE-secreting EBV-transformed human B cells in vitro. Toxicology and Applied Pharmacology. 1997;142(2):256–263. doi: 10.1006/taap.1996.8063. [DOI] [PubMed] [Google Scholar]
- van der Laan MJ, Robins JM. Unified Methods for Censored Longitudinal Data and Causality. New York, NY: Springer-Verlag; 2002. [Google Scholar]
- Ward DJ, Ayres JG. Particulate air pollution and panel studies in children: a systematic review. Occup Environ Med. 2004;61(4):e13. doi: 10.1136/oem.2003.007088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson JG, Kingham S, Pearce J, Sturman AP. A review of intraurban variations in particulate air pollution: Implications for epidemiological research. Atmospheric Environment. 2005;39(34):6444–6462. [Google Scholar]
- Wilson JG, Zawar-Reza P. Intraurban-scale dispersion modelling of particulate matter concentrations: Applications for exposure estimates in cohort studies. Atmospheric Environment. 2006;40(6):1053–1063. [Google Scholar]
- Zeger SL, Thomas D, Dominici F, Samet JM, Schwartz J, Dockery D, Cohen A. Exposure measurement error in time-series studies of air pollution: concepts and consequences. Environmental health perspectives. 2000;108(5):419–426. doi: 10.1289/ehp.00108419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.