Abstract
Glioblastoma multiforme (GBM) is a very aggressive and locally invasive tumor. The current standard of care is partial brain radiation therapy (60 Gy) concurrently with the alkylating agent temozolomide (TMZ). However, patients’ survival remains poor (6-12 months) mainly due to local and diffuse (distant) recurrence. The possibility to promote hyper radiosensitivity (HRS) with low dose radiation may contribute to improve outcome. Here, we evaluated the effect of VorinostatSAHA and TMZ on glioblastoma cells’ sensitivity to low dose radiation. Clonogenic survivals were performed on D54 (p53 and PTEN wild type) and U118 (p53 and PTEN mutants) cells exposed to clinically relevant doses of VorinostatSAHA and TMZ and increasing radiation doses. Apoptosis was measured by the activation of caspase-3 and the role of p53 and PTEN were evaluated with the p53 inhibitor pifithrin α and the PI3K/AKT pathway inhibitor LY29002. VorinostatSAHA promoted HRS at doses as low as 0.25 Gy in the D54 but not the U118 cells. Killing efficiency was associated with caspase-3 activation, delayed H2AX phosphorylation and abrogation of a radiation -induced G2 arrest. Inhibiting p53 function with pifithrin α prevented the promotion of HRS by VorinostatSAHA. Moreover, LY29002, a PI-3K inhibitor, restored promotion of HRS by VorinostatSAHA in the p53 mutant U118 cells to levels similar to the p53 wild type cells. TMZ also promoted HRS at doses as low as 0.15 Gy. These finding indicate that HRS can be promoted in p53 wild type glioblastoma cells through a functional PTEN to delay DNA repair and sensitize cells to low dose radiation. Promotion of HRS thus appears to be a viable approach for GBM that could be used as a basis to develop new Phase I/II studies.
Keywords: HDACI, Low dose fractionated radiation, Glioblastoma, p53; PTEN
INTRODUCTION
Partial brain radiation therapy (60 Gy) in combination with temozolomide (TMZ) is the current standard of care for malignant glioblastoma. The therapeutic effect of this treatment remains however poor with survival between 6 to 12 months. It is increasingly being recognized that diffuse and distant failures are important contributors to this prognosis [1]. Investigations to delineate new therapeutic approaches to improve this outcome are very much needed. The advent of “radiosensitizing” doses of chemotherapy to augment the effectiveness of standard fractionated external beam radiation therapy (1.0 - 3.0 Gy per fraction) has provided some of the most noteworthy advances in radiotherapy [2,3]. Recently, the histone deacetylase inhibitors (HDCAIs) have been described as a new class of radiosensitizers in several human cell lines and in a number of clinical trials [4]. These inhibitors prevent the deacetylation of histone and other proteins and consequently allow the targeted proteins to remain hyperacetylated. The general effect, as far as histones are concerned, is a more open, more accessible, chromatin structure [5]. Current evidence indicates that in order to produce a maximal radiosensitizing effect the HDACIs have to acetylate the histones at the time of radiation and in some cell lines after radiation as well. The mechanisms underlying HADCIs radiosensitizing effects are not fully understood but interference with dynamic local chromatin remodeling in the vicinity of the DNA double strands breaks apparently contribute to this effect [6].
Another important advent that is gaining momentum in radiation therapy is the use of Low Dose Fractionated Radiation Therapy (LDFRT). Potential benefits of using LDFRT as a chemopotentiator have only recently been investigated because it had long been assumed that doses less than 1.0 Gy per fraction would be ineffective for human tumor therapy. However, it is now becoming apparent that a number of cell lines are hyper sensitive to radiation doses well below 1.0 Gy. This phenomenon known as Hyperradiosensitivity (HRS) is characterized by statistically significant increased radiosensitivity at radiation doses below 1Gy as compared to the surviving fractions predicted by the linear quadratic model. Typically, HRS is followed by increased radioresistance due to induced DNA repair [7]. HRS is more prominent in proliferating malignant tissues than in quiescent normal tissues [7] and could thus potentially be exploited for therapeutic consideration. The mechanism (s) behind HRS could possibly include enhanced apoptosis and failure to fully arrest the progression of damaged G2-phase cells [7,8]. Although radiotherapy is the most effective non-surgical therapy for GBM patients, the intrinsic radioresistance of GBM cells allow tumor recurrence and ultimately treatment failures. A better understanding of the molecular mechanism leading to GBM cells radio sensitivity could contribute to develop new therapeutic approaches. In this study, we aimed at determining whether HDACI could promote HRS in glioblastoma cells and assessed the role of p53 and PTEN in this radiosensitizing effect
MATERIALS AND METHODS
Chemicals
Primary antibodies for Acetylated p53 (L373, L382), total p53 and actin were from Millipore, (Cat No 06-758), Oncogene Science (Cat No OP33) and EMD Chemicals (Darmstadtand, Germany) respectively. H3 and γH2AX antibodies were from Cell Signaling Technology (Beverly, Massachusetts) (Cat No 9715 and 9718). The PI3K inhibitor LY294002 was obtained from Cell Signaling Technology (Danvers, MA). The p53 inhibitor pifithrin a was obtained from EMD Chemicals (Darmstadt, Germany). Cell lyses were prepared as described before [9].
Cell Culture and treatments
The D54 and U118 cells were grown as described in [9]. Both cell lines are aggressive brain cancer cell lines isolated from patients with glioblastoma multiforme (WHO Grade IV). The U118 cells have a missense, point mutation in the p53 region that changes an arginine to a glutamine [10]. U118 also have a splicing defect in PTEN changing exon 8 to intron 8 [10]. The D54 cells have neither of these mutations and are rather wild type for both p53 and PTEN [10].
VorinostatSAHA (Exclusive Chemistry, Obninsk, Russia, Cat No: 149647-78-9) was used at the indicated concentrations for 4 hours, then the cells were replenished with fresh media. The rationale for choosing the VorinostatSAHA concentration and time of incubation is based on our previous studies showing increased sensitivity of the D54 cells under these conditions and the therapeutically relevant dose of VorinostatSAHA [9] [11]. For the LY294002 drug treatment, cells were exposed to 20 mM LY294002 for 1 hour and the media was replaced with fresh media. These conditions have been shown to inhibit AKT activity in glioblastoma cells [12]. For TMZ treatments, D54 cells were exposed to 51,5 μM (10 μg/ml) TMZ for 1h before radiation then the cells were replenished with fresh media containing 19.4 mM (3.75 μg/ml) TMZ or TMZ and 1.5 μM VorinostatSAHA for 4h. The TMZ concentration was chosen based on plasma concentrations reported for malignant glioma treatments [13] [14] and the 1h time point was in accordance with drug combination studies [9] [15] performed in GBM cells. The cells were irradiated with the indicated radiation dose with a Pantek Seifert X-ray machine with settings of 250 Kv and 13 mA at a constant rate of 0.34959 Gy/min when using a foam barrier or 2.4791 Gy/min without a foam barrier. The cells were grown at 37°C, irradiated at room temperature and immediately put back at 37°C as described in [16].
Clonogenic survival assay
Five hundred cells were plated the day before treatment and allowed to grow at 37 °C for 7-10 days after. Colonies (≥ 50 cells) were fixed and stained as described [9]. The colonies (100-150) were manually counted. Plating efficiency was 20-30%. Relative survival is expressed as a percentage of surviving colonies in reference to the mean plating efficiency of three sham-irradiated control plates. The Radiation Enhancement Ratio was calculated with the following formula: RER= Surviving Fractionradiation alone/Surviving Fractionradiation + drug. Ratio above 1 indicate radiation enhancement.
Apoptosis assay
Fluorometrtric Caspase-3 assay was performed with an assay kit from Promega (CaspACE-3) as described before [9]. Caspase-3 activation was measured at 12h and 72h post treatments but because no significant activation was observed at the 12h time point only data for the 72h time points are shown.
Statistical analysis
Statistical analysis was performed on the relative (Fluorescence Unit) ratios of Caspase-3 activity of cells exposed to VorinostatSAHA and radiation over cells exposed to radiation alone. Analysis was also performed on the relative (%) survival ratios of cells exposed to radiation over cells exposed to radiation and VorinostatSAHA. Calculations were performed by the Student t test. Probability values <0.05 are considered significant.
RESULTS
VorinostatSAHA promotes HRS in D54 but not U118 cells
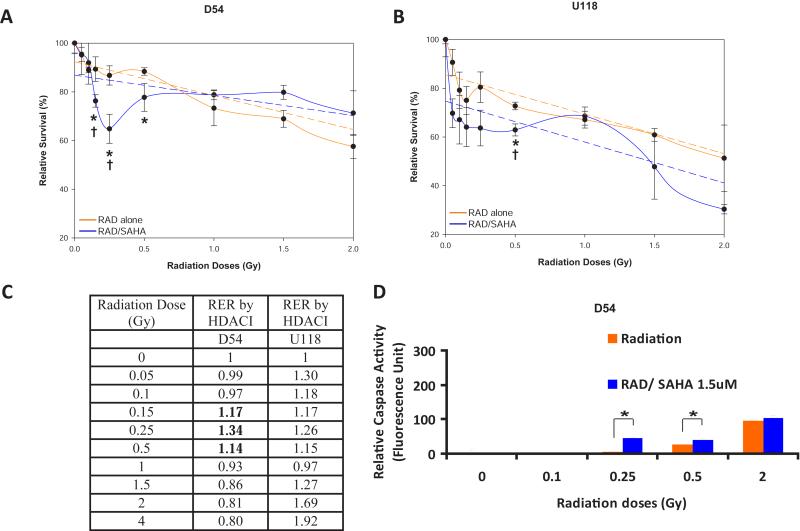
HDACIs are known radiosensitizers for conventional radiation doses [17] but their potential effects on low dose fractionated radiation therapy (LDFRT) are largely unexplored. Here, we used U118 and D54 cells, two aggressive glioblastoma cell lines isolated from patients with glioblastoma multiforme (WHO Grade IV) to assess the effect of HDACI on LDFRT. The U118 cells are p53 and PTEN mutants while the D54 cells are wild type for both genes [10]. We first treated D54 and U118 cells with clinically relevant doses of the HDACI VorinostatSAHA after low dose radiation. The data shown in Figure 1A indicate that VorinostatSAHA increased radiosensitivity in D54 cells at radiation dose as low as 0.15 Gy and had a more pronounced effect at 0.25 Gy. The percentage of cells killed at 0.25 Gy was nearly identical to the percentage of cells killed at eight times that dose (2.0 Gy) with radiation alone (Figure 1A). The increased killing efficiency at 0.25 Gy in the presence of HDACI represents a much higher level of cells killing than what the conventional quadratic linear model would have predicted (broken line Figure 1A) and is thus described as hyper-radiosensitivity (HRS). In fact, increased radiosensitivity was statistically significant from 0.15 to 0.5 Gy as compared to radiation alone and significant at 0.15 and 0.25Gy as compared to the surviving fractions predicted by the linear quadratic model (Figure 1A). This phenomenon, promotion of HRS by HDACI, was not observed in the p53 mutant cell line U118. Although the difference between radiation alone and enhanced radiosensitivity by HDCAI reached statistical significance at 0.5 Gy, no statistical differences between radiation alone or the predicted survival by the linear quadratic model were observed at lower radiation doses (Figure 1B). Moreover, calculations of the Radiation Enhancement Ratio by HDACI indicate that HDACI enhance radiation sensitivity (RER > 1) between 0.15 and 0.5 Gy in the D54 cells, in agreement with promotion of HRS as shown in Figure 1A, while in U118 cells the HDACI behave more like a classical radiosensitizer by enhancing radiation sensitivity at almost every radiation doses (Figure 1C).
Figure 1.
VorinostatSAHA promotes HRS in D54 but not U118 cells. A) Clonogenic survival assay measured in D54 cells after radiation followed by exposure to VorinostatSAHA (1.5 μM) for 4hr. Relative survival of cells exposed to radiation (RAD) is expressed as a percentage of the untreated cells or cells treated with VorinostatSAHA alone (RAD/SAHA). B) U118 cells treated as in A). Broken line is a graph of the linear quadratic equation with best-fit parameters calculated by SigmaPlot software. C) Radiation Enhancement Ratio (RER) by HDACI calculated with the following formula; RER= Surviving Fractionradiation alone/Surviving Fractionradiation + HDACI. D) Apoptosis assay. Caspase-3 activity was measured in D54 cells 72 hrs after the indicated radiation treatment followed by 4hr of VorinostatSAHA (1.5 μM). * = p<0.05 as compared to radiation alone. † = p<0.05 as compared to surviving fraction predicted by the linear quadratic model.
To determine whether promotion of HRS by HDACI resulted in increased apoptosis we measured the levels of caspase-3 activity. Data shown in Figure, 1D indicate that adding VorinostatSAHA following radiation doses as low as 0.25 Gy increased caspase-3 activity by almost 10 fold compared to radiation alone. VorinostatSAHA increased caspase-3 activity by 1.5 fold at 0.5 Gy and did not sensitize further D54 cells at 2 Gy. This indicates that indeed the promotion of HRS by HDACI in D54 cells is associated with increased apoptosis.
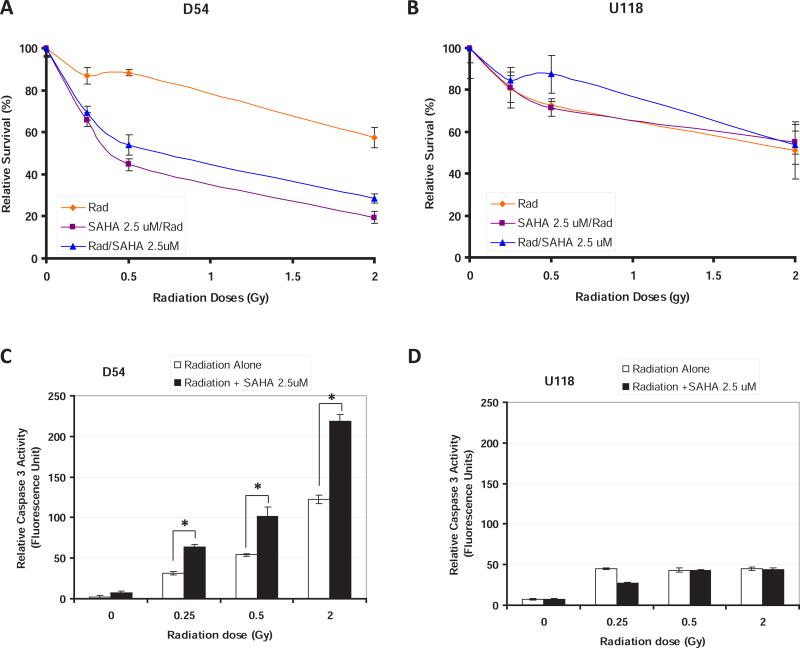
We then aimed at determining whether increasing VorinostatSAHA concentration or modifying the order of drug addiction could affect its radiosensitizing effect. Increasing VorinostatSAHA dose to 2.5 μM and adding it either before or after radiation increased D54 cells sensitivity to all doses of radiation (Figure 2A) but did not increase the sensitivity of U118 cells (Figure 2B). Interestingly, the promotion of the classical HRS phenomenon, increased radiosensitivity at radiation doses below 1Gy followed by increase resistance, as shown in Figure 1A, seems to be lost with higher HDACI concentration in favor of a more classical radiosensitizer effect where radiosensitivity is increased at every radiation doses (Figure 2A). To determine whether promotion of HRS by HDACI resulted in increased apoptosis we measured again the level of caspase-3 activity. The data shown in Figure 2C-D indicate that HDACI increased the level of apoptosis at each radiation doses in D54 but not U118 cells. The increase apoptosis was dose dependent and proportional to the surviving fractions (Figure 2A).
Figure 2.
VorinostatSAHA promotes radiosensitivity in D54 but not U118 cells. A) Clonogenic survival assay measured in D54 cells as in Figure 1A except that the dose of VorinostatSAHA was 2.5 μM. B) U118 cells treated as in A). C-D) Apoptosis assay. Caspase-3 activity was measured in D54 C) and U118, D) cells 72 hrs after the indicated radiation treatment followed by 4hr of VorinostatSAHA (2.5 μM). * = p<0.05.
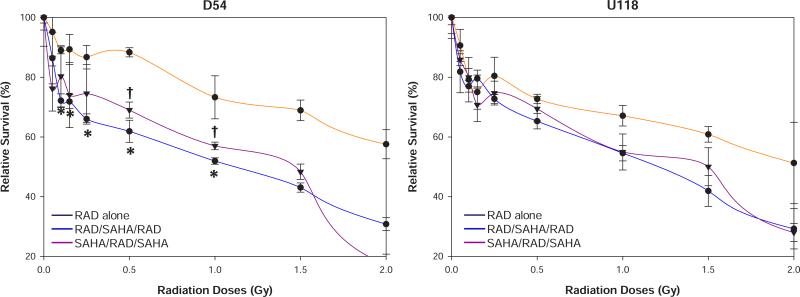
In an effort to elucidate the lack of HDACI sensitization in U118 cells we performed a series of additional survival assays with different sequences of drug and radiation exposures. Figure 3 indicates that adding VorinostatSAHA before and after radiation (SAHA/RAD/SAHA), or exposing the cells to radiation before and after VorinostatSAHA (RAD/SAHA/RAD), increased the sensitivity of both D54 and U118 cells to higher radiation doses (≥1 Gy) but only increased sensitivity to lower radiation doses in D54 cells. The increased sensitivity to lower radiation doses reached statistical significance from 0.1 to 1.0 Gy when the cells were exposed to radiation before and after VorinostatSAHA treatment (RAD/SAHA/RAD) but was only significant at or above 0.5 Gy when VorinostatSAHA preceded radiation in D54 cells (Figure 3A). However, none of these conditions reproduced the HRS phenomenon observed in Figure 1. It thus appears that the promotion of HRS by HDACI depends on low concentration (1.5 mM) of HDACI being added after radiation in p53 wild type D54 cells. Altering the order, sequence and/or drug concentrations may result in VorinostatSAHA behaving as a classical radiosensitizer rather than promoting HRS.
Figure 3.
Sequence of VorinostatSAHA addition affects cells sensitivity to radiation. A) Clonogenic survival assays performed as in Figure 1A) except that the cells were irradiated before and after the addition of VorinostatSAHA (1.5 μM, blue line) or that VorinostatSAHA was added before and after radiation (purple line). B) U118 cells treated as in A). * = p<0.05: RAD/SAHA/RAD as compared to RAD alone. † = p<0.05: SAHA/RAD/SAHA as compared to RAD alone.
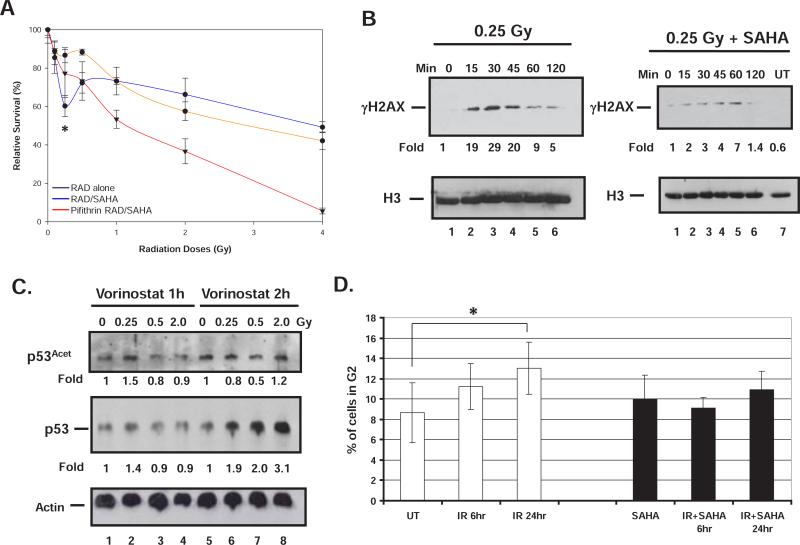
In order to determine whether a functional p53 is required to mediate promotion of HRS by HDACI we performed survival assays in the presence of the p53 inhibitor pifithrin α (Figure 4A). Figure 4A shows that D54 cells treated with pifithrin α are more sensitive to higher doses of radiation. However, inhibition of p53 resulted in a statistically significant loss of the promotion of HRS by HDACI observed earlier at 0.25 Gy (Figures 1A and 4A). For clarity purpose and a better comparison, the pifithrin a data were superimposed on the data from Figure 1A. These date thus indicate that the promotion of HRS by HDACI requires a functional p53.
Figure 4.
A) Promotion of HRS by HDACI requires a functional p53. D54 cells were treated with the p53 inhibitor pifithrin a (20 μM) 1h prior to exposure to the indicated dose of radiation or radiation followed by VorinostatSAHA (1.5 μM). B) Western blot analysis. D54 cells were exposed to radiation and VorinostatSAHA for the indicated period of time.
Histones were extracted and 10 μg were run on SDS PAGE and hybridized to the indicated antibody. Histone H3 was used as a loading control. C) Same as B) except that whole cell extracts were used and 100 μg were loaded. Actin was used as a loading control. Fold induction was measured by densitometry normalized to loading controls. D) D54 cells were treated (black boxes) or not (white boxes) with 1,5 μM VorinostatSAHA for 4 h and irradiated with 0.25 Gy. Cells were analyzed 6 or 24 hrs after radiation by FACS to measure the percentage of cells in G2.
* = p<0.05; RAD/SAHA as compared to pifithrin α RAD/SAHA
While a functional p53 is probably required to induce apoptosis (Figure 1C), HDACI could also promote HRS by preventing or delaying DNA repair. To verify this possibility, we measured the levels of γH2AX at different time points following 0.25 Gy of radiation in the presence or absence of VorinostatSAHA. The data shown on Figure 4B indicate that indeed the presence of VorinostatSAHA reduced and delay the up-regulation of γH2AX by several minutes and allowed a rapid acetylation of p53 (L373, L382) following exposure to 0.25 Gy (Figure 4C, lane 2). No effect on p53 phosphorylation was observed (data not shown). The pattern of p53 acetylation and upregulation observed at the 1h time point is consistent with the HRS phenomenon observed in Figure 1A where increase radiosensitivity is observed at 0.25 Gy followed by increase radioresistance at higher radiation doses. In addition, the data suggests that the p53 response to the combined regimen of 0.25Gy of radiation and VorinostatSAHA is sequential, where p53 is first acetylated (Figure 4C, lane 2) followed by stabilization (total p53, lane 6). Acetylation of p53 at residues L373, L382 has been shown to induce expression of p21 [18] and could consequently affect cell cycle progression. Although p53 and p21 can affect the G1 and G2 checkpoints [19], it is believed that a failure to arrest in G2 is contributing to HRS [20]. We thus measured the effect of VorinostatSAHA on the capacity of D54 cells to arrest in G2 following exposure to 0.25 Gy of radiation. The data shown in Figure 4D indicate that indeed VorinostatSAHA abrogate D54 cells capacity to arrest in G2 following exposure to 0.25 Gy of radiation.
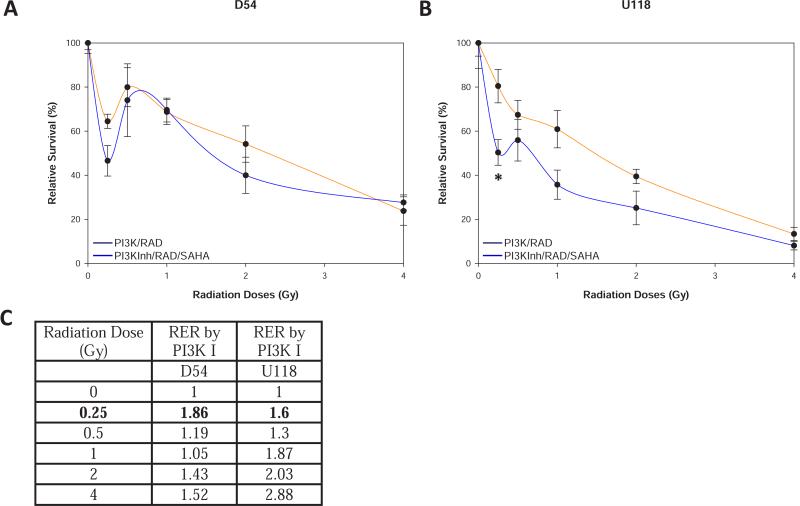
In addition to having a mutated p53, the U118 cells also have a mutation in the p53 regulated tumor suppressor PTEN. To determine whether a functional PTEN could restore HRS promoted by HDACI in a p53 mutated cell line, we treated the U118 cells with LY29002, an AKT inhibitor, to mimic a functional PTEN [21]. Figure 5A indicates that pre-treating D54 cells with LY29002 promoted HRS at 0.25 Gy and adding VorinostatSAHA after radiation further increased HRS by killing 20% more cells compared to LY29002 alone at the same radiation dose (0.25Gy). Most importantly, Figure 5B indicates that LY29002 restored the promotion of HRS by HDACI in U118 cells at 0.25 Gy to levels similar to what was observed in the p53 and PTEN wild type D54 cells. Restoration of HRS in U118 cells is also supported by the increased RER observed at 0.25 Gy (Figure 5C). It thus appears that by mimicking a functional PTEN in U118 cells with a PI3K inhibitor we can promote HRS in response to HDACI in U118 cells.
Figure 5.
Inhibiting PI3K restores promotion of HRS by HDACI in p53 mutant cells. A) Clonogenic survival assays as in Figure 1A except that D54 cells were treated with the PI3K inhibitor LY29002 (10 μM) 1h prior to exposure to radiation or radiation followed by VorinostatSAHA (1.5 μM). B) Same as A) except that U118 cells were used. * = p<0.05. C) Radiation Enhancement Ratio (RER) by PI3K Inhibitor (PI3K I) and or HDACI calculated as in Figure 1.
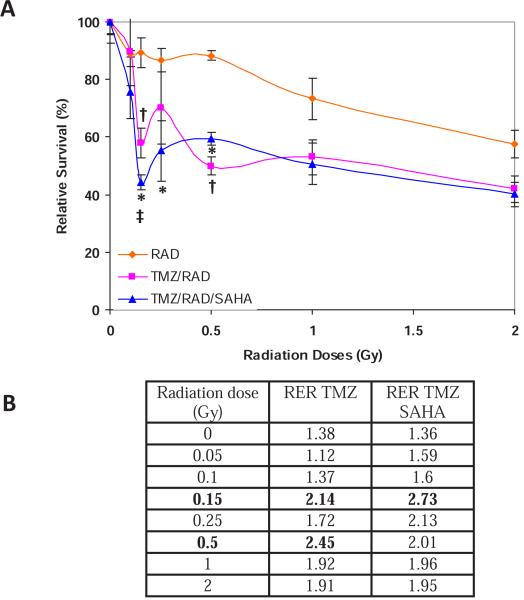
In order to determine the potential effect of HDACI on the current standard of care for glioblastoma, we performed additional clonogenic survival assays with VorinostatSAHA, TMZ and radiation. The data shown in Figure 6A indicate that TMZ can sensitize D54 cells to all doses of radiation used and promote a statistically significant HRS at 0.15 and 0.5 Gy. The promotion of HRS by TMZ is also supported by the increased RER at these two radiation doses (Figure 6B). Adding VorinostatSAHA after radiation significantly increased TMZ sensitization at every dose below 0.5Gy including a statistically significant more pronounced HRS at 0.15 Gy but had no additional effect as compared to TMZ alone at radiation doses above 0.25 Gy (Figure 6A). These observations are also in agreement with the RER (Figure 6B). These data thus indicate that TMZ and/or HDACI can promote HRS in glioblastoma cells and provide a rationale for further explorations of potential clinical applications.
Figure 6.
A) VorinostatSAHA enhances TMZ-induced radiosensitization. Clonogenic survival assay as in Figure 1A) except that the D54 cells were treated with TMZ (51.5 μM) for 1h prior to radiation or radiation and VorinostatSAHA (1.5 μM) exposure. * = p<0.05 : TMZ/RAD/SAHA as compared to RAD, ‡ = p<0.05: TMZ/RAD/SAHA as compared to TMZ/RAD, † = p<0.05: TMZ/RAD as compared to RAD. B) RER by TMZ and TMZ plus SAHA calculated as in Figure 1.
DISCUSSION
The data generated in this study further implicate HRS as a possible mode to improve glioblastoma treatments. HRS has been reported previously as an intrinsic characteristic of a number of malignant cell lines but the possibility to promote HRS in cells harboring no apparent sensitivity to low dose radiation offers the possibility to expand this phenomenon to new therapeutic applications. Our data (Figure 1A) indicate that forty percent more cells are killed at 0.25 Gy when the p53 wild-type D54 glioblastoma cells are treated with 1.5 μM VorinostatSAHA after radiation. A much higher radiation dose, about six times higher (1.5 Gy), is required to kill the same amount of cells with radiation alone. Our data indicate that promotion of HRS requires a functional p53 and activation of caspase-3 (Figure 1, 4). This is in good agreement with earlier studies on intrinsic HRS [8]. More recently it has also been shown that a failure to arrest in early G2 contributes to HRS [20]. Similarly our data indicate that treating the cells with VorinostatSAHA abolish the D54 cells capacity to arrest in G2 following exposure to 0.25Gy (Figure 4D). It thus appears that the molecular mechanisms underlying promotion of HRS are similar to the intrinsic HRS of proliferating cells. Nonetheless, these mechanisms are probably different than the conventional DNA damage response. Actually, the DNA damage response including activation of poly (ADP-ribose) polymerase 1 (PARP) [22], DNA-PK (DNA-dependent protein kinase) [23,24] and the ATM-dependent early G2-phase cell cycle checkpoint [25] are instrumental in overcoming HRS at higher radiation doses. It could also be argued that by relaxing the chromatin structure, the HDACIs could increase the number of DNA double strand breaks (DSBs) inflicted by ionizing radiation. However, this is not the case [26]. HDACIs rather prevent the refolding of the chromatin into a more condensed structure following repair [26]. HDACIs potentiate radiation-induced cell killing by preventing the rapid exchanges of epigenetic marks in the vicinity of the DSBs and prolonging the expression of γH2AX thus delaying or preventing DNA repair [27,28]. Our data are in good agreement with these earlier studies and indicate that VorinostatSAHA delayed the phosphorylation of H2AX in response to low dose radiation (Figure 4B). Another indication that HRS promoted by HDACI could be mediated at least in part by delayed DNA repair is provided by restoration of HRS in the p53 mutant cell line U118 with a PI3K inhibitor to mimic a functional PTEN. PTEN has been shown to increase radiosensitivity by delaying DSBs repair rather than affecting cell cycle redistribution [29]. Even so, as mentioned earlier we also observed that VorinostatSAHA prevented the G2 check point (Figure 4D), which could be PTEN independent, but required a functional p53 to promote HRS (Figure 4A). An alternative explanation for promotion of HRS by VorinostatSAHA could be the recently described inhibitory effect of VorinostatSAHA on Telomerase activity [30]. However, this effect is time and dose dependent and would probably not occur under the mild conditions (1.5 μM, 4h) used here. Moreover, this effect on Telomerase could not explain why HRS is lost when a p53 inhibitor is used in D54 cells or why mimicking a functional PTEN with an AKT inhibitor in U118 cells restored the phenomenon of HRS promoted by HDACI.
Regardless of the mechanisms involved, promotion of HRS by HDCAI appears to be a viable approach for GBM that could be used as a basis to develop new Phase I/II studies. Nonetheless, it is important to consider the potential effect of drug combination with radiation on normal tissue in order to maximize the potential therapeutic ratio. One of the great advantages of HDACIs is their selectivity for cancer cells and consequently sparing of normal tissues. We have previously shown that HDACIs increase anticancer drugs efficiency in cancer but not normal cells [9]. A similar phenomenon has been observed with radiation therapy where HDACIs radiosensitize cancer but not normal cells [27,31]. Similarly, no increase toxicity have been reported in a phase II trial involving the HDACI valproic acid, temozolomide (Temodar), and radiation for the treatment of glioblastoma multiforme as compared to what is reported for radiation and temozolomide alone [32]. Another appealing aspect of the HDACIs is that in addition to preferentially potentiate anticancer treatments in cancer cells they also apparently protect normal tissue from radiation-induced side effects. For example, the HDACI phenylbutyrate improve both DNA repair and cell survival in normal fibroblasts [31] and topical application of HDACI protect normal tissue from acute and long term effects of radiation [31,33, 6]. The HDACIs are therefore part of a rare class of agents that could provide a clear therapeutic advantage when combined with radiation therapy.
CONCLUSIONS
VorinostatSAHA can promote HRS by activating several branches of the p53 pathway including acetylation of p53, (Figure 4B) which could stabilize p53 [34] (Figure 4B) and lead to a failure to arrest in G2, and activation of PTEN to delay DNA repair. In addition, our data indicate that TMZ can also promote HRS in D54 cells (Figure 6). The molecular mechanisms underlying the cellular response to low (<1 Gy) but not high (>2 Gy) radiation doses are just beginning to be elucidated but are upholding promising possibilities for clinical applications such as whole organ irradiation including whole brain radiation radiotherapy as a basis to develop new Phase I/II studies.
ACKNOWLEDGEMENTS
Financial support was provided in part by a NIH/NCI, RO1 1CA116491-01 (FC) and an intramural grant (FC,YK) from the Radiation Oncology Department, School Medicine, University of Maryland, Baltimore.
Footnotes
Cite this article: Diss E, Nalabothula N, Nguyen D, Chang E, Kwok Y, et al. (2014) VorinostatSAHA Promotes Hyper-Radiosensitivity in Wild Type p53 Human Glioblastoma Cells. JSM Clin Oncol Res 2(1): 1004.
REFERENCES
- 1.Narayana A, Kelly P, Golfinos J, Parker E, Johnson G, Knopp E, et al. Antiangiogenic therapy using bevacizumab in recurrent high-grade glioma: impact on local control and patient survival. J Neurosurg. 2009;110:173–180. doi: 10.3171/2008.4.17492. [DOI] [PubMed] [Google Scholar]
- 2.Regine WF, Hanna N, Garofalo MC, Doyle A, Arnold S, Kataria R, et al. Low-dose radiotherapy as a chemopotentiator of gemcitabine in tumors of the pancreas or small bowel: a phase I study exploring a new treatment paradigm. Int J Radiat Oncol Biol Phys. 2007;68:172177. doi: 10.1016/j.ijrobp.2006.11.045. [DOI] [PubMed] [Google Scholar]
- 3.Connell PP, Hellman S. Advances in radiotherapy and implications for the next century: a historical perspective. Cancer Res. 2009;69:383392. doi: 10.1158/0008-5472.CAN-07-6871. [DOI] [PubMed] [Google Scholar]
- 4.Camphausen K, Tofilon PJ. Inhibition of histone deacetylation: a strategy for tumor radiosensitization. J Clin Oncol. 2007;25:40514056. doi: 10.1200/JCO.2007.11.6202. [DOI] [PubMed] [Google Scholar]
- 5.Nolan L, Johnson PW, Ganesan A, Packham G, Crabb SJ. Will histone deacetylase inhibitors require combination with other agents to fulfil their therapeutic potential? Br J Cancer. 2008;99:689–694. doi: 10.1038/sj.bjc.6604557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. http://mcpharmacol.com/index.php/Journals/article/view-File/193/182.
- 7.Krueger SA, Wilson GD, Piasentin E, Joiner MC, Marples B. The effects of G2-phase enrichment and checkpoint abrogation on low-dose hyper-radiosensitivity. Int J Radiat Oncol Biol Phys. 2010;77:15091517. doi: 10.1016/j.ijrobp.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Enns L, Bogen KT, Wizniak J, Murtha AD, Weinfeld M. Low-dose radiation hypersensitivity is associated with p53-dependent apoptosis. Mol Cancer Res. 2004;2:557–566. [PubMed] [Google Scholar]
- 9.Kim MS, Blake M, Baek JH, Kohlhagen G, Pommier Y, Carrier F. Inhibition of histone deacetylase increases cytotoxicity to anticancer drugs targeting DNA. Cancer Res. 2003;63:7291–7300. [PubMed] [Google Scholar]
- 10.Ishii N, Maier D, Merlo A, Tada M, Sawamura Y, Diserens AC, et al. Frequent co-alterations of TP53, p16/CDKN2A, p14ARF, PTEN tumor suppressor genes in human glioma cell lines. Brain Pathol. 1999;9:469–479. doi: 10.1111/j.1750-3639.1999.tb00536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gojo I, Tan M, Fang HB, Sadowska M, Lapidus R, Baer MR, et al. Translational phase I trial of vorinostat (suberoylanilide hydroxamic acid) combined with cytarabine and etoposide in patients with relapsed, refractory, or high-risk acute myeloid leukemia. Clin Cancer Res. 2013;19:1838–1851. doi: 10.1158/1078-0432.CCR-12-3165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Opel D, Westhoff MA, Bender A, Braun V, Debatin KM, Fulda S. Phosphatidylinositol 3-kinase inhibition broadly sensitizes glioblastoma cells to death receptor- and drug-induced apoptosis. Cancer Res. 2008;68:6271–6280. doi: 10.1158/0008-5472.CAN-07-6769. [DOI] [PubMed] [Google Scholar]
- 13.Friedman HS, Kerby T, Calvert H. Temozolomide and treatment of malignant glioma. Clin Cancer Res. 2000;6:2585–2597. [PubMed] [Google Scholar]
- 14.Agarwala SS, Kirkwood JM. Temozolomide, a novel alkylating agent with activity in the central nervous system, may improve the treatment of advanced metastatic melanoma. Oncologist. 2000;5:144–151. doi: 10.1634/theoncologist.5-2-144. [DOI] [PubMed] [Google Scholar]
- 15.Golding SE, Rosenberg E, Adams BR, Wignarajah S, Beckta JM, O'Connor MJ, et al. Dynamic inhibition of ATM kinase provides a strategy for glioblastoma multiforme radiosensitization and growth control. Cell Cycle. 2012;11:1167–1173. doi: 10.4161/cc.11.6.19576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernet M, Megnin-Chanet F, Hall J, Favaudon V. Control of the G2/M checkpoints after exposure to low doses of ionising radiation: implications for hyper-radiosensitivity. DNA Repair (Amst) 2010;9:48–57. doi: 10.1016/j.dnarep.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 17.Kim IA, Shin JH, Kim IH, Kim JH, Kim JS, Wu HG, et al. Histone deacetylase inhibitor-mediated radiosensitization of human cancer cells: class differences and the potential influence of p53. Clin Cancer Res. 2006;12:940–949. doi: 10.1158/1078-0432.CCR-05-1230. [DOI] [PubMed] [Google Scholar]
- 18.Zhao Y, Lu S, Wu L, Chai G, Wang H, Chen Y, et al. Acetylation of p53 at lysine 373/382 by the histone deacetylase inhibitor depsipeptide induces expression of p21(Waf1/Cip1). Mol Cell Biol. 2006;26:27822790. doi: 10.1128/MCB.26.7.2782-2790.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deckbar D, Jeggo PA, Lobrich M. Understanding the limitations of radiation-induced cell cycle checkpoints. Crit Rev Biochem Mol Biol. 2011;46:271–283. doi: 10.3109/10409238.2011.575764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Krueger SA, Joiner MC, Weinfeld M, Piasentin E, Marples B. Role of apoptosis in low-dose hyper-radiosensitivity. Radiat Res. 2007;167:260–267. doi: 10.1667/RR0776.1. [DOI] [PubMed] [Google Scholar]
- 21.Leevers SJ, Vanhaesebroeck B, Waterfield MD. Signalling through phosphoinositide 3-kinases: the lipids take centre stage. Curr Opin Cell Biol. 1999;11:219–225. doi: 10.1016/s0955-0674(99)80029-5. [DOI] [PubMed] [Google Scholar]
- 22.Chalmers A, Johnston P, Woodcock M, Joiner M, Marples B. PARP-1, PARP-2, and the cellular response to low doses of ionizing radiation. Int J Radiat Oncol Biol Phys. 2004;58:410–419. doi: 10.1016/j.ijrobp.2003.09.053. [DOI] [PubMed] [Google Scholar]
- 23.Marples B, Cann NE, Mitchell CR, Johnston PJ, Joiner MC. Evidence for the involvement of DNA-dependent protein kinase in the phenomena of low dose hyper-radiosensitivity and increased radioresistance. Int J Radiat Biol. 2002;78:1139–1147. doi: 10.1080/09553000210166606. [DOI] [PubMed] [Google Scholar]
- 24.Vaganay-Juery S, Muller C, Marangoni E, Abdulkarim B, Deutsch E, Lambin P, et al. Decreased DNA-PK activity in human cancer cells exhibiting hypersensitivity to low-dose irradiation. Br J Cancer. 2000;83:514–518. doi: 10.1054/bjoc.2000.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marples B, Wouters BG, Joiner MC. An association between the radiation-induced arrest of G2-phase cells and low-dose hyper-radiosensitivity: a plausible underlying mechanism? Radiat Res. 2003;160:38–45. doi: 10.1667/rr3013. [DOI] [PubMed] [Google Scholar]
- 26.Falk M, Lukasova E, Gabrielova B, Ondrej V, Kozubek S. Chromatin dynamics during DSB repair. Biochim Biophys Acta. 2007;1773:1534–1545. doi: 10.1016/j.bbamcr.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 27.Munshi A, Kurland JF, Nishikawa T, Tanaka T, Hobbs ML, Tucker SL, et al. Histone deacetylase inhibitors radiosensitize human melanoma cells by suppressing DNA repair activity. Clin Cancer Res. 2005;11:4912–4922. doi: 10.1158/1078-0432.CCR-04-2088. [DOI] [PubMed] [Google Scholar]
- 28.Camphausen K, Burgan W, Cerra M, Oswald KA, Trepel JB, Lee MJ, et al. Enhanced radiation-induced cell killing and prolongation of gammaH2AX foci expression by the histone deacetylase inhibitor MS-275. Cancer Res. 2004;64:316–321. doi: 10.1158/0008-5472.can-03-2630. [DOI] [PubMed] [Google Scholar]
- 29.Kao GD, Jiang Z, Fernandes AM, Gupta AK, Maity A. Inhibition of phosphatidylinositol-3-OH kinase/Akt signaling impairs DNA repair in glioblastoma cells following ionizing radiation. J Biol Chem. 2007;282:21206–21212. doi: 10.1074/jbc.M703042200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li CT, Hsiao YM, Wu TC, Lin YW, Yeh KT, Ko JL. Vorinostat, SAHA, represses telomerase activity via epigenetic regulation of telomerase reverse transcriptase in non-small cell lung cancer cells. J Cell Biochem. 2011;112:3044–3053. doi: 10.1002/jcb.23229. [DOI] [PubMed] [Google Scholar]
- 31.Chung YL, Lee MY, Pui NN. Epigenetic therapy using the histone deacetylase inhibitor for increasing therapeutic gain in oral cancer: prevention of radiation-induced oral mucositis and inhibition of chemical-induced oral carcinogenesis. Carcinogenesis. 2009;30:1387–1397. doi: 10.1093/carcin/bgp079. [DOI] [PubMed] [Google Scholar]
- 32.Kamrava M, Citrin D, Sproull M, Lita E, Smith S, Sears-Crouse N, Cooley-Zgela T, Fine H, Camphausen K. Acute toxicity in a phase II clinical trial of valproic acid in combination with temodar and radiation therapy in patients with glioblastoma multiforme. International journal of radiation oncology, biology, physics. 2008;72:2094. [Google Scholar]
- 33.Chung YL, Wang AJ, Yao LF. Antitumor histone deacetylase inhibitors suppress cutaneous radiation syndrome: Implications for increasing therapeutic gain in cancer radiotherapy. Molecular cancer therapeutics. 2004;3:317–325. [PubMed] [Google Scholar]
- 34.Li M, Luo J, Brooks CL, Gu W. Acetylation of p53 inhibits its ubiquitination by Mdm2. J Biol Chem. 2002;277:50607–50611. doi: 10.1074/jbc.C200578200. [DOI] [PubMed] [Google Scholar]