SUMMARY
Background
Despite improving survival in cystic fibrosis (CF) patients, there is a mortality peak in early adulthood. Defining risk factors that predict significant worsening of lung disease in young adulthood may identify opportunities to improve outcomes in adults.
Methods
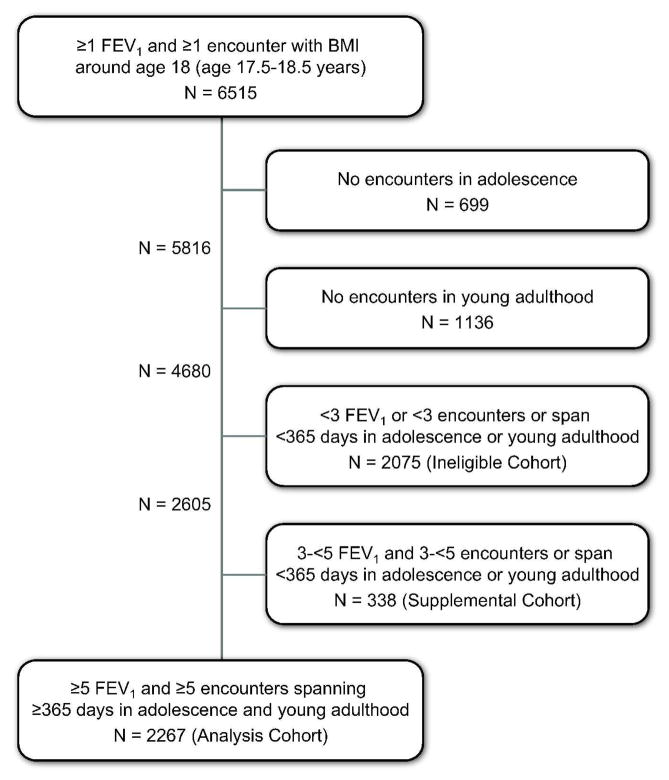
We identified 4680 patients in the Epidemiologic Study of Cystic Fibrosis 1994–2005 with data in both adolescence (age 14.0–17.4 years) and young adulthood (age 18.5–22.0 years) and analyzed 2267 who had ≥5 encounters and ≥5 measurements of forced expiratory volume in 1 second (FEV1) spanning ≥1 year during both adolescence and young adulthood, and ≥1 encounter with weight and height and ≥1 FEV1 measurement age 17.5–18.5 years. We compared the annualized rates of decline in FEV1 during adolescence and young adulthood stratified by best FEV1 around age 18. Logistic regression was used to identify risk factors associated with substantial decline (>20 points) in FEV1% predicted in young adulthood.
Results
Annual rate of decline was greater in young adulthood than in adolescence. Risk factors for substantial decline included slower rate of FEV1 decline, greater FEV1 variability, faster body mass index (BMI) decline, male sex, chronic inhaled antibiotics, Haemophilus influenzae detection, and absence of multidrug-resistant Pseudomonas aeruginosa in adolescence, and lower than expected FEV1 and BMI around age 18.
Conclusions
Decline in lung function accelerates in young adults with CF, especially in those with early stage lung disease. Adolescents at risk for substantial decline in lung function in young adulthood have higher FEV1 and worse nutritional status, among other identifiable risk factors.
Keywords: Transition, nutrition, risk factors, youth, H. influenzae, P. aeruginosa
INTRODUCTION
Young adulthood is an important and vulnerable period for patients with cystic fibrosis (CF). Often around age 18, many CF patients have taken over responsibility for managing their disease and are transitioning their care from pediatric to adult health care providers.1 It is generally recognized that young adulthood is also a time of significant acceleration of morbidity and mortality in CF. The median age at death for patients with CF in the U.S. in 2009 was 26.1 years according to the Cystic Fibrosis Foundation Patient Registry.2 The median forced expiratory volume in 1 second (FEV1) in 13- to 18-year-old CF patients was relatively preserved at 93.6 % predicted, whereas it was 65.3 % in those over 18 years. In addition, there was a difference in the percentage of patients with full adherence to recommended CF care guidelines for clinic attendance and recommended health screenings across age groups: 72.9% for 6- to 18-year-old CF patients and 58.4% for CF patients over 18 years.2 We hypothesized that there are identifiable risk factors in adolescence associated with accelerated lung function decline during early adulthood that can direct intervention and care. Our first aim was to compare the rate of pulmonary function decline during adolescence and young adulthood. The second aim was to identify adolescent risk factors for substantial pulmonary function decline in young adulthood.
METHODS
Data from the Epidemiologic Study of Cystic Fibrosis (ESCF), a large, prospective observational study of CF patients in the United States and Canada between 1994 and 2005, were used for this analysis.3 The study was approved by the Copernicus Group institutional review board (tracking number OVA 1-03-008) and local institutional review boards as applicable. Participants or their guardians provided informed consent. Patients were eligible for this analysis if they had at least five encounters and five FEV1 measurements spanning at least 365 days during both adolescence (defined as age 14.0–17.5 years) and young adulthood (defined as age 18.5–22.0 years), plus at least one additional encounter that recorded weight, height, and at least one FEV1 measurement between ages 17.5 and 18.5 years. All FEV1 measurements were included in the analyses, regardless of whether a pulmonary function test was taken at the time of clinical stability.
We estimated the annualized rate of FEV1 decline during adolescence and young adulthood using a mixed-effects model with random slopes and intercepts for each period. This is similar to the approach used in Ren et al4 and Konstan et al.5 The selection of 3.5 years for the “before” and “after” periods was based on a desire to have patients with enough data to establish trend lines over a reasonably long span of time (necessitating a longer time) and wanting to keep the time reasonably short so that we could evaluate “local” effects that could be approximated by a linear trend. Patients were stratified into four categories based on best FEV1 around age 18 (age 17.5 to 18.5 years) <40% predicted, 40 to <70% predicted, 70 to <100% predicted, and ≥100% predicted. Values for FEV1% predicted were calculated from the equations of Hankinson.6
To define a substantial decline in FEV1, the decline during adolescence was first extrapolated to obtain a fitted (predicted) value at age 18. This was compared to a fitted value at age 22 extrapolated from the rate of decline during young adulthood (Figure 1). A substantial decline was defined as a decrease of 20 or more points in FEV1% predicted between the fitted value at age 18 and the fitted value at age 22.
Fig. 1.
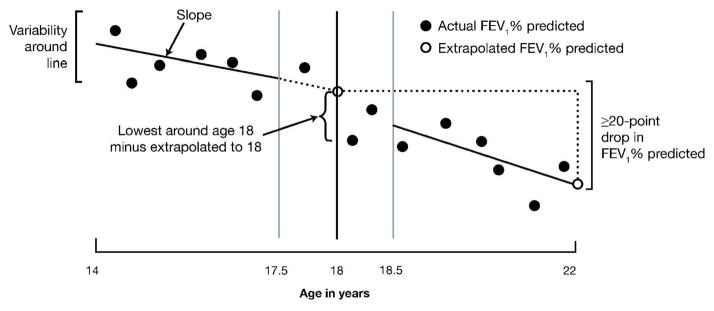
Timeline and FEV1 assessments.
Multivariable logistic analysis was used to assess risk factors during adolescence associated with substantial decline in FEV1 in young adulthood. The analytical approach was to select a priori a handful of key variables to be included in the model regardless of statistical significance and then to test other variables for statistical significance at the α=0.05 level. Once the model using α=0.05 was developed, we then used backward stepwise analysis with α=0.01 to determine the sensitivity of the remaining variables to the presence of the variables that were not significant or had borderline significance. The variables selected for inclusion regardless of statistical significance were a history of cirrhosis or the presence of cough, sputum production, or crackles at any visit during adolescence. Other clinical, demographic, and health care utilization variables were tested statistically. These included sex, race and ethnicity, socioeconomic status (using state insurance status at any time during the years studied as proxy), medical conditions, FEV1 during adolescence and around age 18, nutritional status based on body mass index (BMI) z-score, microorganisms detected by respiratory tract culture, prescribed routine therapies, and annualized number of clinic visits and intravenous (IV) pulmonary exacerbations. The time-varying variables were summarized over the adolescent period (age 14.0–17.5 years) or around age 18 (age 17.5–18.5 years). The evaluation of number of pulmonary exacerbations was limited to patients treated by IV antibiotics and did not include patients treated by inhaled or oral antibiotics, in part due to data limitations in ESCF. Outlier or clearly erroneous values for FEV1 and for the various predictors were set to missing before the analysis. (Appendix A in the online data supplement presents and defines all of the variables.)
Some additional sensitivity analyses were performed. One sensitivity analysis evaluated the effect of including in the model patients who had at least three data values during adolescence and young adulthood (in addition to those who met the original criterion of at least five data values). ESCF covers more than a decade and there have been a number of changes in the treatment of CF during that period, although the use of longitudinal patient-specific data mitigates the effect of any possible secular trend. As a separate sensitivity analysis, the year of the 18th birthday was added as an explanatory variable.
Analyses were performed using SAS Version 9.1 or later (SAS Institute, Inc., Cary, NC).
RESULTS
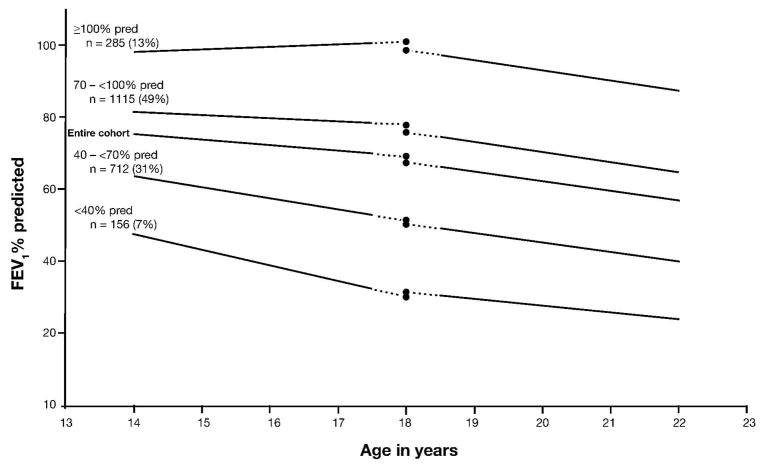
A total of 6515 ESCF patients had at least one pulmonary function test and one encounter that included assessment of BMI in the year around the 18th birthday (17.5–18.5 years). Of these, 1835 had no encounters in either the adolescence or young adulthood period, and an additional 2413 did not meet the data requirements (≥5 encounters and ≥5 FEV1 measurements spanning ≥365 days) for adolescence or for young adulthood, leaving 2267 patients for the analysis cohort (Figure 2). However, 338 of the excluded patients did have sufficient data to allow inclusion in a supplemental cohort for a sensitivity analysis (≥3 encounters and ≥3 FEV1 measurements spanning ≥365 days). Patient demographics and clinical characteristics during adolescence and around age 18 are shown in Table 1 for the patients in the analysis cohort, the 338 patients evaluated in the supplemental cohort, and the 2075 who had some data in adolescence and young adulthood but could not be included in the model (the ineligible cohort). In the analysis cohort, the annualized rate of FEV1 decline was greater in young adulthood (−2.68% predicted per year) than during adolescence (−1.59% predicted per year). The more rapid decline in young adulthood was attributable to patients with FEV1 ≥70% predicted at age 18, who constituted 61.7% of the cohort. Patients with lower FEV1% predicted showed a slower rate of decline in young adulthood than in adolescence (Figure 3 and Table 2).
Fig. 2.
Patient disposition.
TABLE 1.
Patient Demographics and Clinical Characteristics1
| Demographics | Analysis Cohort (≥5) (2267) N (%) |
Supplemental Cohort (≥3) (338) N (%) |
Ineligible Cohort (≥1) (2075) N (%) |
|
|---|---|---|---|---|
| Gender2 | Male | 1172 (52) | 205 (61) | 1107 (53) |
| Female | 1095 (48) | 133 (39) | 968 (47) | |
|
| ||||
| Race3 | Caucasian | 2127 (94) | 314 (93) | 1914 (92) |
| African American | 59 (3) | 9 (3) | 56 (3) | |
| Hispanic | 66 (3) | 11 (3) | 81 (4) | |
| Other (mixed) | 14 (1) | 4 (1) | 23 (1) | |
|
| ||||
| State insurance | Yes | 695 (31) | 64 (19) | 433 (21) |
| No | 1023 (45) | 170 (50) | 742 (36) | |
| Unknown | 549 (24) | 104 (31) | 900 (43) | |
|
| ||||
|
Clinical characteristics during adolescence
| ||||
| Comorbidities during adolescence | Allergic bronchopulmonary aspergillosis | 197 (9) | 11 (3) | 132 (6) |
| Asthma | 927 (41) | 80 (24) | 637 (31) | |
| Atypical mycobacteria4 | 49 (2) | 1 (0) | 29 (1) | |
| Insulin or oral hypoglycemic use (CFRD) | 305 (13) | 22 (7) | 231 (11) | |
| Nasal polyps5 | 821 (36) | 110 (33) | 546 (26) | |
| Sinusitis6 | 1034 (46) | 123 (36) | 723 (35) | |
| Pneumothorax3 | 27 (1) | 3 (1) | 20 (1) | |
| Hemoptysis | 206 (9) | 9 (3) | 144 (7) | |
| Cirrhosis3 | 79 (3) | 8 (2) | 69 (3) | |
|
| ||||
| Best FEV1 in the year around the 18th birthday | <40% predicted | 156 (7) | 15 (4) | 233 (11) |
| 40%–69% predicted | 712 (31) | 84 (25) | 615 (30) | |
| 70%–99% predicted | 1115 (49) | 178 (53) | 941 (45) | |
| ≥100% predicted | 284 (13) | 61 (18) | 286 (14) | |
|
| ||||
| Best BMI percentile in the year around the 18th birthday | >25th percentile | 585 (26) | 67 (20) | 604 (29) |
| 10th–25th percentile | 452 (20) | 72 (21) | 413 (20) | |
| <10th percentile | 1230 (54) | 198 (59) | 1053 (51) | |
|
| ||||
| Clinical symptoms at any encounter during adolescence | Cough5 | 2265 (100) | 337 (100) | 2030 (98) |
| Sputum production6 | 2233 (99) | 322 (95) | 1919 (92) | |
| Crackles | 1721 (76) | 172 (51) | 1261 (61) | |
|
| ||||
| Microbiology during adolescence | 8 or fewer cultures during adolescence | 1597 (70) | 299 (88) | 1654 (80) |
| Pseudomonas aeruginosa | 1945 (86) | 236 (70) | 1560 (75) | |
| P. aeruginosa, mucoid6 | 1607 (71) | 182 (54) | 1200 (58) | |
| P. aeruginosa, non-mucoid6 | 1786 (79) | 205 (61) | 1356 (65) | |
| P. aeruginosa, multidrug resistant | 362 (16) | 22 (7) | 251 (12) | |
| Haemophilus influenzae5 | 631 (28) | 99 (29) | 478 (23) | |
| Staphylococcus aureus5 | 1572 (69) | 247 (73) | 1319 (64) | |
| S. aureus, methicillin resistant | 138 (6) | 8 (2) | 164 (8) | |
| Stenotrophomonas maltophilia | 318 (14) | 21 (6) | 246 (12) | |
| Burkholderia cepacia3 | 121 (5) | 12 (4) | 110 (5) | |
|
| ||||
| Maintenance therapy use in adolescence | Airway clearance | 2210 (97) | 307 (91) | 1795 (87) |
| Dornase alfa | 1706 (75) | 176 (52) | 1359 (65) | |
| Inhaled bronchodilator6 | 2157 (95) | 284 (84) | 1810 (87) | |
| Inhaled mast cell stabilizer6 | 791 (35) | 67 (20) | 484 (23) | |
| Oral antibiotics2 | 702 (31) | 59 (17) | 629 (30) | |
| Inhaled antibiotics | 1305 (58) | 114 (34) | 976 (47) | |
| Enteral supplements2 | 250 (11) | 19 (6) | 267 (13) | |
|
| ||||
|
Health care interventions during adolescence7
| ||||
| Routine encounters | Mean annualized rate ± SD/year | 3.5 ± 1.6 | 2.5 ± 1.5 | 3.3 ± 2.2 |
| Exacerbations requiring IV antibiotics during adolescence | Mean annualized rate ± SD/year2 | 0.8 ± 1.1 | 0.3 ± 0.7 | 0.8 ± 1.2 |
| No exacerbations in adolescent period | 726 (32) | 219 (65) | 1021 (49) | |
CFRD, cystic fibrosis–related diabetes; SD, standard deviation
Except as noted, cohort differences are statistically significant at P < 0.05 by chi-square test (for frequency variables) or by Tukey-adjusted mean comparisons (for continuous variables).
The ineligible cohort is not statistically different from the analysis cohort.
No cohorts are statistically different.
The ineligible cohort is not statistically different from the supplemental or analysis cohorts.
The supplemental cohort is not statistically different from the analysis cohort.
The ineligible cohort is not statistically different from the supplemental cohort.
For the ineligible cohort, very short time spans led to unreasonable extrapolated rates, so encounter rates >15/yr (n=28) and IV exacerbation rates >10/yr (n=11) were set to missing.
Fig. 3.
FEV1 % predicted by age and CF severity.
TABLE 2.
Intercepts (FEV1 % Predicted at Age 18), Slopes (FEV1 % Predicted/Year), and 95% Confidence Intervals for Figure 3
| FEV1 % predicted | Adolescence | Young Adulthood | ||
|---|---|---|---|---|
|
| ||||
| Intercept at age 18 yr | Slope (% pred/yr) | Intercept at age 18 yr | Slope (% pred/yr) | |
| Entire cohort | 69.02 (68.48, 69.57) | −1.09 (−1.36, −0.82) | 67.48 (66.95, 68.01) | −2.68 (−2.88, −2.49) |
| ≥100 | 100.65 (99.10, 102.20) | −3.39 (−4.16, −2.61) | 98.29 (96.76, 99.83) | −2.76 (−3.34, −2.19) |
| 70–<100 | 77.81 (77.03, 78.58) | −1.92 (−2.31, −1.54) | 75.67 (74.90, 76.43) | −2.78 (−3.06, −2.50) |
| 40–<70 | 51.15 (50.19, 52.12) | 0.38 (−0.09, 0.85) | 50.28 (49.34, 51.22) | −2.67 (−3.01, −2.32) |
| <40 | 30.24 (28.21, 32.27) | 2.34 (1.31, 3.36) | 31.35 (29.34, 33.37) | −1.88 (−2.64, −1.11) |
FEV1, forced expiratory volume in 1 second
Of the 2267 patients, 667 (29%) had a substantial decline in FEV1% predicted (20 points) from age 18 to age 22. The logistic regression model predicting a substantial decline in lung function was developed iteratively. In preliminary modeling, the aspect of lung function around age 18 that best predicted the likelihood of a significant drop in young adulthood was the difference between the lowest measured FEV1 between ages 17.5 and 18.5 and the extrapolated FEV1 value at age 18 (using the line fitted from age 14.0 to 17.5). The rate of decline in FEV1 during adolescence and the variability of FEV1 around that fitted line were both also strong predictors. BMI was also a strong predictor, even after adjusting for these three aspects of FEV1. The most significant predictor related to BMI was obtained by subtracting the extrapolated BMI z-score value at age 18 from the highest (rather than the lowest) measured value around age 18. At this juncture, we enforced parallelism between BMI and FEV1 by including the rate of decline in BMI during adolescence and the variability around the fitted BMI line regardless of statistical significance.
Risk factors in adolescence associated with substantial decline in young adulthood included higher FEV1 around age 18 (relative to the extrapolated value), slower rate of decline in FEV1% predicted, greater variability in FEV1% predicted, and/or a faster rate of decline in BMI. Additionally, FEV1 and/or BMI around age 18 that were lower than expected based on fitted values were also associated with substantial decline (Table 3). Neither variability in BMI nor the variables selected a priori (cough, sputum production, crackles, and cirrhosis) were statistically significant. Only four additional variables were significant predictors of substantial decline: male sex, the presence of Haemophilus influenzae on respiratory tract culture, and the percentage of encounters at which chronic inhaled antibiotics were prescribed (i.e., indicated as used for prophylaxis or for chronic suppressive therapy). The presence of multidrug-resistant Pseudomonas aeruginosa was associated with a lower likelihood of substantial decline. Other demographic, clinical, and health care utilization characteristics described in Appendix A were not predictors of substantial decline; these included the use of insulin/oral hypoglycemic agents (a proxy for CF-related diabetes).
TABLE 3.
Multivariate Risk Model of Factors in the Adolescent Period Influencing Substantial Decline in the Adult Period (N=2267)
| Odds Ratio | 95% Confidence Interval | Risk of a substantial decline is higher if … | |
|---|---|---|---|
|
Variables (P <0.01)
| |||
| Difference between fitted FEV1 % predicted at age 18 and lowest measured FEV1 around age 18 | 0.95 | 0.94–0.96 | Worst FEV1 around age 18 is low compared with fitted value at 18 |
| Slope of FEV1 (% predicted per year) during adolescence | 1.06 | 1.04–1.09 | Rate of decline during adolescence is less severe |
| Difference between fitted BMI z-score at age 18 and highest measured BMI z-score around age 18 | 0.62 | 0.50–0.78 | Best BMI around age 18 is low compared with fitted value at 18 |
| Slope of BMI (z-score per year) during adolescence | 0.48 | 0.30–0.78 | Rate of BMI decline during adolescence is more severe |
| Female | 0.74 | 0.60–0.90 | Patient is male |
| Presence of Haemophilus influenzae on any culture during adolescence | 1.40 | 1.12–1.73 | H. influenzae is ever present |
|
| |||
|
Variables (P <0.05)
| |||
| Percentage of encounters recording inhaled antibiotics during adolescence | 1.71 | 1.03–2.82 | Inhaled antibiotics are recorded more often |
| FEV1% predicted variability (SD around fitted line) during adolescence | 1.04 | 1.01–1.07 | Variability around the adolescent trend line is greater |
| Presence of Pseudomonas aeruginosa (multidrug resistant) on any culture during adolescence | 0.74 | 0.56–0.99 | P. aeruginosa (multidrug resistant) is never present |
|
| |||
|
Variables (not statistically significant)
| |||
| BMI z-score variability (SD around fitted line) during adolescence | 0.99 | 0.52–1.90 | Variability around the BMI trend line is greater (NS) |
| Cirrhosis reported at any encounter during adolescence | 1.35 | 0.82–2.24 | Cirrhosis is ever reported (NS) |
| Percentage of encounters with occasional or daily cough during adolescence | 2.28 | 0.93–5.61 | Cough is recorded more often (NS) |
| Percentage of encounters with crackles during adolescence | 0.84 | 0.54–1.29 | Crackles are recorded less often (NS) |
| Percentage of encounters with occasional or daily sputum during adolescence | 0.87 | 0.53–1.44 | Sputum is recorded less often (NS) |
BMI, body mass index; FEV1, forced expiratory volume in 1 second; NS, not significant; SD, standard deviation
Backward stepping using α=0.01 removed the nonsignificant variables first, then the chronic inhaled antibiotics and P. aeruginosa variables, and finally the variability of FEV1. The remaining variables were all statistically significant at α=0.01 and had odds ratios nearly the same as when the other variables were included in the model (data not shown).
The sensitivity analysis that included the 338 patients with at least three data values during adolescence and young adulthood in addition to the original 2267 patients resulted in no material changes. The sensitivity analysis that included year of the 18th birthday as an additional covariate also resulted in no material changes. The effect of chronic inhaled antibiotic therapy was strengthened slightly; other coefficients were essentially unchanged.
DISCUSSION
In this large cohort of adolescent CF patients, we have demonstrated an acceleration of lung function decline in early adulthood. This decline is attributable to adolescents with early stage lung disease by FEV1 criteria, i.e., those with FEV1 ≥70% predicted around age 18. This is consistent with our previous finding that higher FEV1 is a risk factor for FEV1 decline in children and adolescents.7 Furthermore, patients at greatest risk of a substantial, clinically meaningful FEV1 decline in young adulthood can be identified by specific risk factors, most notably several aspects of FEV1 and BMI during adolescence.
Adolescents with a slower rate of FEV1 decline had a greater risk of substantial decline in young adulthood. In addition, if a patient’s lowest FEV1 around age 18 was lower than expected based on the estimated value at age 18 from the fitted line during adolescence, the patient was at further risk of a substantial decline. This suggests that acceleration in decline detectable at age 18 precedes and predicts a substantial drop in lung function by age 22. Even after accounting for the slope of FEV1 and the FEV1 around age 18, increased variability in FEV1 was predictive of substantial decline. Patients with a faster rate of BMI decline had a greater risk of substantial decline in lung function. Acceleration in BMI decline, defined by the difference between measured highest BMI at age 18 and the extrapolated trend line of BMI, also predicted a substantial drop in lung function. Thus, estimating the risk for substantial decline between age 18 and 22 years requires assessment of several aspects of both FEV1 and BMI during adolescence.
The finding that adolescent patients with higher FEV1 are at risk for accelerated decline in FEV1 in young adulthood is important new information for both pediatric and adult CF care providers and suggests opportunities for improving care. It has been previously shown that patients with higher FEV1 receive fewer therapies, including therapies that have been shown to increase or maintain lung function and reduce the frequency of pulmonary exacerbations.8 Moreover, patients with fewer symptoms and normal or near-normal pulmonary function are less likely to receive vigilant monitoring or intervention,8 and may be less attentive to self-care. Normal developmental patterns of adolescence, including striving for normality by disregarding or avoiding treatment recommendations, may be reinforced by measurably early stage lung disease.9 These factors are difficult to study, but the implications for clinical practice are highly relevant. Practitioners, families, and patients should be aware that high FEV1 and minimal decline in FEV1 during adolescence do not predict the same stable course during young adulthood, but are actually risk factors for increasing decline and substantial loss of lung function in the ensuing years.
The other two aspects of FEV1 found to be independent risk factors relate to variability. The difference between the lowest FEV1 around age 18 and the extrapolated FEV1 at age 18 encompasses both curvature and short-term variability. A longer-term measure of variability is the standard deviation of FEV1 measures around the trend line during adolescence. Both are independent risk factors for substantial decline in FEV1 in young adulthood. After controlling for these variables, we found that the frequency of IV-treated pulmonary exacerbations was not an independent predictor of substantial decline in FEV1. This is somewhat surprising given that pulmonary exacerbation is a known risk factor for lung function decline.7,10 A long-standing issue in CF clinical care has been the lack of a commonly held definition of pulmonary exacerbation. Mirroring this conundrum, data collection at the many study sites demonstrated variability in making the clinical diagnosis of pulmonary exacerbation; only treated exacerbations were recorded in ESCF. We assume that exacerbations, treated or untreated, account for much of the variability in FEV1. Thus the standard deviation of FEV1 measures around the trend line may in fact be largely a measure of frequency and severity of pulmonary exacerbations, whether or not identified or treated. This could explain why a measure of treated exacerbations was not an independent risk factor.
In a recent analysis using ESCF, Liou et al11 demonstrated that a substantial number of patients have relatively large individual year-to-year changes in FEV1% predicted from early adolescence through early adulthood that are not accurately represented in median FEV1 population data for this group. The ages of greatest risk were the early teen years rather than the early adulthood we chose to evaluate in this study. It would be valuable to see if the risk factors in those earlier teen years are similar to those we report here.
Nutritional status is a strong, independent predictor of survival in CF in both children and adults12 and is also correlated with FEV1% predicted values.13 We found that the most relevant nutritional status predictors of substantial decline in FEV1% predicted were a faster rate of decline in BMI during adolescence or a lower than expected BMI around the 18th birthday compared with the extrapolated BMI based on slope of decline during adolescence. These variables are related, but have independent effects on the likelihood of substantial decline even after adjusting for several aspects of FEV1. These findings, taken together, suggest more vigorous monitoring of nutritional status and more nutritional intervention during adolescence, even in those with early stage lung disease, could reduce FEV1 decline in the following years.
Having more encounters documenting use of chronic inhaled antibiotics during adolescence was associated with a greater risk of substantial decline in young adulthood. Inhaled antibiotics are indicated for chronic suppressive treatment of P. aeruginosa; however, P. aeruginosa infection was not, in itself, associated with risk of substantial decline. Chronic use of inhaled antibiotics may be a marker of more advanced disease.
H. influenzae is frequently present in respiratory tract cultures of young patients with CF; prevalence peaks at over 30% in the preschool years but then decreases to less than 10% during adulthood.2 H. influenzae can be part of normal respiratory flora in healthy children, but is a significant pathogen in other disorders, notably chronic obstructive pulmonary disease. H. influenzae has not been previously reported to be associated with more rapid decline in lung function in CF. However, this is the first report to specifically study pulmonary function changes in discrete time periods during late adolescence and early adulthood and demonstrates that presence of this organism predicts decline in lung function. We do not believe that H. influenzae is simply a marker of milder disease, as it remained significant in the predictive model even after adjustment for FEV1 and its derived variables. H. influenzae has a number of properties that would suggest the potential for significant pathogenicity in the CF lung, including biofilm formation.14 This finding, which may have implications for antimicrobial selection and recommendations in CF, deserves further evaluation.
The presence of P. aeruginosa in respiratory tract cultures has been previously reported to be associated with more rapid decline in lung function, especially when mucoidy is present.15,16 However, we did not find that presence or absence of P. aeruginosa or of mucoid P. aeruginosa was predictive. This may reflect the very high rate of P. aeruginosa infection (86%) in this adolescent cohort. We did find that substantial lung function decline in the adult period was less likely to occur in patients with multidrug-resistant P. aeruginosa. We previously reported that ESCF sites with FEV1 in the highest quartile had a higher use of IV antibiotics and a higher rate of multidrug-resistant P. aeruginosa on respiratory cultures than sites in the lowest FEV1 quartile.17 Thus, the decreased risk for decline seen in the group with multidrug-resistant P. aeruginosa might be a marker of more frequent antibiotic therapy leading to better overall outcomes.
Male sex was associated with an increased risk of substantial decline in the early adult period. This contrasts with earlier reports indicating that female sex is a risk factor for morbidity and mortality in CF in patients 1–20 years of age.18 However, the effect of sex has been seen primarily in patients up to age 10,18 and several recent reports have suggested the resolution of the gender gap in CF mortality.19–21 Young men with CF use fewer prescribed therapies;22,23 while ESCF did not measure adherence, this sex-related difference is a plausible reason for this finding.
This study has several limitations. Only patients who had at least five visits in the 3.5 years before and after the year around the 18th birthday were included in the analysis cohort. This allowed more rigorous assessment of longitudinal patterns of pulmonary function, nutritional status, and other clinical factors, but reduced the available cohort. These data may not reflect the clinical course of patients who are seen less frequently. In addition, the requirement of data during young adulthood introduces at least some potential survivor bias. The fact that the sensitivity analysis using the supplemental cohort gave similar results provides some reassurance on these points. In addition, the sensitivity analysis addressing the possibility of a secular trend did not materially change the results.
In conclusion, the rate of FEV1 decline accelerates in young adults with CF, most notably in those with high pulmonary function (FEV1 ≥70% predicted). Other risk factors in adolescence associated with substantial decline in young adulthood include lower BMI and variability in either FEV1 or BMI. This combination of risk factors is of concern given our understanding of adolescent cognition and behavior in regard to health. CF clinicians should have a heightened awareness for adolescents with these risk factors, while implementing strategies to assist these patients in understanding risk, embracing preventative strategies, and adopting positive behaviors that may slow progression of disease as they transition to adulthood.
Acknowledgments
This study was supported by Genentech, Inc., South San Francisco, CA. All Sources of support for the ESCF in the form of grants, case report forms, and data analysis were provided by Genentech, Inc., South San Francisco, CA. The authors gratefully acknowledge the patients, parents, investigators, and coordinators of the Epidemiologic Study of Cystic Fibrosis (ESCF).
ABBREVIATIONS
- BMI
body mass index
- CF
cystic fibrosis
- ESCF
Epidemiologic Study of Cystic Fibrosis
- FEV1
forced expiratory volume in 1 second
- H. influenzae
Haemophilus influenzae
- IV
intravenous
- P. aeruginosa
Pseudomonas aeruginosa
Appendix A: Variables Considered in the Development of the Model
Demographic characteristics (age at diagnosis, gender, race in four categories, genotype, mother’s highest level of education completed, median household income based on zip code, and insurance status)
Comorbid conditions (defined as whether the patient has ever had any of the following conditions, and if so, the number of encounters and percentage of encounters at which they were present in adolescence and around age 18: allergic bronchopulmonary aspergillosis, asthma, atypical mycobacteria, cirrhosis, elevated liver function test, hemoptysis, use of insulin/oral hypoglycemics, nasal polyps, pneumothorax, portal hypertension, and sinusitis)
Pulmonary function tests (defined using FEV1% predicted as a 4-group categorization of best value around age 18, the best or worst or mean value in adolescence, the best or worst or mean value around age 18, ever/never or percentage of tests not marked stable in adolescence, difference between best and worst value around age 18, difference between best or worst value around age 18 and the fitted age 18 value from adolescence, difference between best or worst value around age 18 and the value nearest to 18th birthday, slope of values in adolescence, and residual standard deviation in adolescence)
Clinical signs and symptoms (defined as whether the patient has ever had any of the following conditions, and if so, the number of encounters and percentage of encounters at which they were present in adolescence and around age 18: cough, crackles, and sputum production)
Height/weight (defined as best/worst and mean/median in adolescence and around age 18: height-for-age percentile/z-score, and weight-for-age percentile/z-score)
Body mass index (defined as best/worst, first/last, median, mean, slope [adolescence only], and residual standard deviation [adolescence only] for actual, percentile, and z-score in adolescence and around/nearest age 18 and differences in these measures between adolescence and around age 18; last and first, worst/best/mean and predicted at age 18; and nearest to and predicted at age 18)
Microorganisms (defined as >8 cultures in adolescence and ever cultured, number of cultures, and percentage of cultures in adolescence and around age 18 for Burkholderia cepacia, Haemophilus influenzae, Pseudomonas aeruginosa (PA) [multidrug-resistant, mucoid, non-mucoid, and any], Stenotrophomonas maltophilia, Staphylococcus aureus (SA), methicillin-resistant SA (MRSA), and combinations of these occurring in the period and concurrently, including SA without PA, multidrug-resistant PA without SA, multidrug-resistant PA with concurrent SA, non–multidrug-resistant PA without SA, non–multidrug-resistant PA with concurrent SA)
Use of therapies (defined as whether the following therapies were ever used, and if so, the number of encounters and percentage of encounters at which they were prescribed in adolescence and around age 18: airway clearance techniques, enteral supplements, chronic inhaled antibiotics, chronic oral antibiotics, inhaled bronchodilators, oral bronchodilators, mast cell stabilizers, inhaled corticosteroids, oral corticosteroids, and dornase alfa)
Health care resource utilization (defined as total counts in adolescence and around age 18 for encounters, stable encounters, sick encounters, cultures, and categorization of pulmonary function tests per year in adolescence)
IV exacerbations (defined as intravenous antibiotic treatment for pulmonary exacerbation ever having occurred, number of occurrences, and rate in adolescence and around age 18)
Footnotes
All authors contributed to the concept and design, analysis and interpretation of data, drafting of the manuscript, and final approval of the manuscript.
Disclosure of Conflict of Interest
This study is sponsored by Genentech, Inc. Ann McMullen, Michael Schechter, Michael Konstan, Jeffrey Wagener, Wayne Morgan, and Susanna McColley have received honoraria from Genentech, Inc. for serving as members of the Scientific Advisory Group for the Epidemiologic Study of Cystic Fibrosis (ESCF). Stacy VandenBranden, Ann McMullen, Michael Konstan, Wayne Morgan, Jeffrey Wagener, and Susanna McColley have served as consultants to Genentech. No compensation was provided to these authors in exchange for production of this manuscript. David Pasta and Rory Michaelis are employees of ICON Clinical Research, which was paid by Genentech for providing analytical services for this study. Jeffrey Wagener was previously an employee of Genentech.
References
- 1.Parker HW. Transition and transfer of patients who have cystic fibrosis to adult care. Clin Chest Med. 2007;28:423–432. doi: 10.1016/j.ccm.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 2.2009 Annual Data Report. Bethesda, MD: Cystic Fibrosis Foundation; 2010. Cystic Fibrosis Foundation Patient Registry. [Google Scholar]
- 3.Morgan WJ, Butler SM, Johnson CA, Colin AA, FitzSimmons SC, Geller DE, Konstan MW, Light MJ, Rabin HR, Regelmann WE, Schidlow DV, Stokes DC, Wohl ME, Kaplowitz H, Wyatt MM, Stryker S. Epidemiologic Study of Cystic Fibrosis: design and implementation of a prospective, multicenter, observational study of patients with cystic fibrosis in the U.S and Canada. Pediatr Pulmonol. 1999;28:231–241. doi: 10.1002/(sici)1099-0496(199910)28:4<231::aid-ppul1>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 4.Ren CL, Pasta DJ, Rasouliyan L, Wagener JS, Konstan MW, Morgan WJ. Relationship between inhaled corticosteroid therapy and rate of lung function decline in children with cystic fibrosis. J Pediatr. 2008;153:746–751. doi: 10.1016/j.jpeds.2008.07.010. [DOI] [PubMed] [Google Scholar]
- 5.Konstan MW, Wagener JS, Pasta DJ, Millar SJ, Jacobs JR, Yegin A, Morgan WJ. Clinical use of dornase alfa is associated with a slower rate of FEV1 decline in cystic fibrosis. Pediatr Pulmonol. 2011 doi: 10.1002/ppul.21388. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 7.Konstan MW, Morgan WJ, Butler SM, Pasta DJ, Craib ML, Silva SJ, Stokes DC, Wohl ME, Wagener JS, Regelmann WE, Johnson CA Scientific Advisory Group and the Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis. Risk factors for rate of decline in forced expiratory volume in one second in children and adolescents with cystic fibrosis. J Pediatr. 2007;151:134–139. doi: 10.1016/j.jpeds.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Konstan MW, Butler SM, Schidlow DV, Morgan WJ, Julius JR, Johnson CA. Patterns of medical practice in cystic fibrosis: part II. Use of therapies. Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis. Pediatr Pulmonol. 1999;28:248–254. doi: 10.1002/(sici)1099-0496(199910)28:4<248::aid-ppul3>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 9.Sawyer SM, Rosier MJ, Phelan PD, Bowes G. The self-image of adolescents with cystic fibrosis. J Adolesc Health. 1995;16:204–208. doi: 10.1016/1054-139X(94)00071-L. [DOI] [PubMed] [Google Scholar]
- 10.Packe GE, Hodson ME. Changes in spirometry during consecutive admissions for infective pulmonary exacerbation in adolescent and adult cystic fibrosis. Respir Med. 1992;86:45–48. doi: 10.1016/s0954-6111(06)80147-2. [DOI] [PubMed] [Google Scholar]
- 11.Liou TG, Elkin EP, Pasta DJ, Jacobs JR, Konstan MW, Morgan WJ, Wagener JS. Year-to-year changes in lung function in individuals with cystic fibrosis. J Cyst Fibros. 2010;9:250–256. doi: 10.1016/j.jcf.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma R, Florea VG, Bolger AP, Doehner W, Florea ND, Coats AJ, Hodson ME, Anker SD, Henein MY. Wasting as an independent predictor of mortality in patients with cystic fibrosis. Thorax. 2001;56:746–750. doi: 10.1136/thorax.56.10.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liou TG, Adler FR, Fitzsimmons SC, Cahill BC, Hibbs JR, Marshall BC. Predictive 5-year survivorship model of cystic fibrosis. Am J Epidemiol. 2001;153:345–352. doi: 10.1093/aje/153.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Starner TD, Zhang N, Kim G, et al. Haemophilus influenzae forms biofilms on airway epithelia: implications in cystic fibrosis. Am J Respir Crit Care Med. 2006;174:213–220. doi: 10.1164/rccm.200509-1459OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henry RL, Mellis CM, Petrovic L. Mucoid Pseudomonas aeruginosa is a marker of poor survival in cystic fibrosis. Pediatr Pulmonol. 1992;12:158–161. doi: 10.1002/ppul.1950120306. [DOI] [PubMed] [Google Scholar]
- 16.Emerson J, Rosenfeld M, McNamara S, Ramsey B, Gibson RL. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr Pulmonol. 2002;34:91–100. doi: 10.1002/ppul.10127. [DOI] [PubMed] [Google Scholar]
- 17.Johnson C, Butler SM, Konstan MW, Morgan W, Wohl ME. Factors influencing outcomes in cystic fibrosis: a center-based analysis. Chest. 2003;123:20–27. doi: 10.1378/chest.123.1.20. [DOI] [PubMed] [Google Scholar]
- 18.Rosenfeld M, Davis R, Fitzsimmons S, Pepe M, Ramsey B. Gender gap in cystic fibrosis mortality. Am J Epidemiol. 1997;145:794–803. doi: 10.1093/oxfordjournals.aje.a009172. [DOI] [PubMed] [Google Scholar]
- 19.Moran A, Dunitz J, Nathan B, Saeed A, Holme B, Thomas W. Cystic fibrosis-related diabetes: current trends in prevalence, incidence, and mortality. Diabetes Care. 2009;32:1626–1631. doi: 10.2337/dc09-0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verma N, Bush A, Buchdahl R. Is there still a gender gap in cystic fibrosis? Chest. 2005;128:2824–2834. doi: 10.1378/chest.128.4.2824. [DOI] [PubMed] [Google Scholar]
- 21.Courtney JM, Bradley J, Mccaughan J, O’Connor TM, Shortt C, Bredin CP, Bradbury I, Elborn JS. Predictors of mortality in adults with cystic fibrosis. Pediatr Pulmonol. 2007;42:525–532. doi: 10.1002/ppul.20619. [DOI] [PubMed] [Google Scholar]
- 22.Britto MT, Garrett JM, Dugliss AJ, Daeschner CW, Jr, Johnson CA, Leigh MW, Majure JM, Schultz WH, Konrad TR. Risky behavior in teens with cystic fibrosis or sickle cell disease: a multicenter study. Pediatrics. 1998;101:250–256. doi: 10.1542/peds.101.2.250. [DOI] [PubMed] [Google Scholar]
- 23.Berge JM, Patterson JM, Goetz D, Milla C. Gender differences in young adults’ perceptions of living with cystic fibrosis during the transition to adulthood: a qualitative investigation. Fam Syst Health. 2007;25:190–203. [Google Scholar]