Abstract
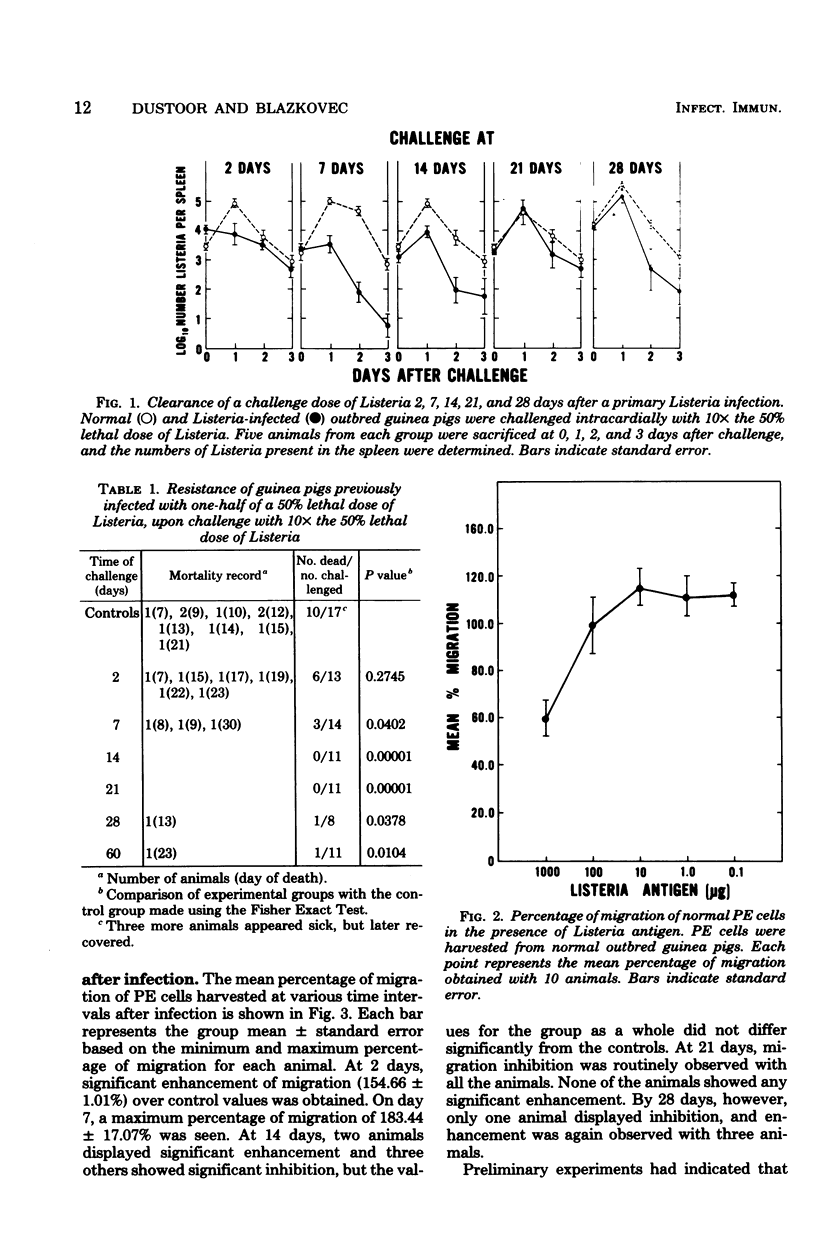
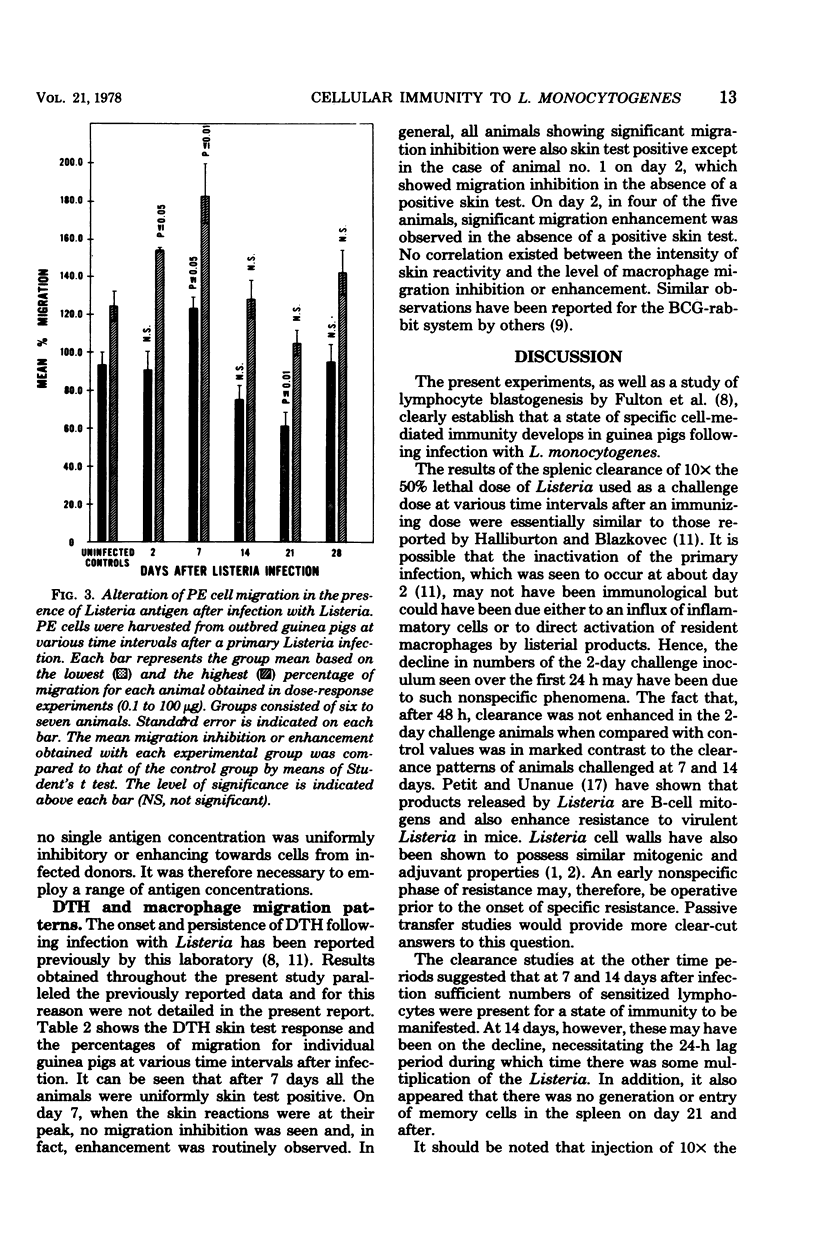
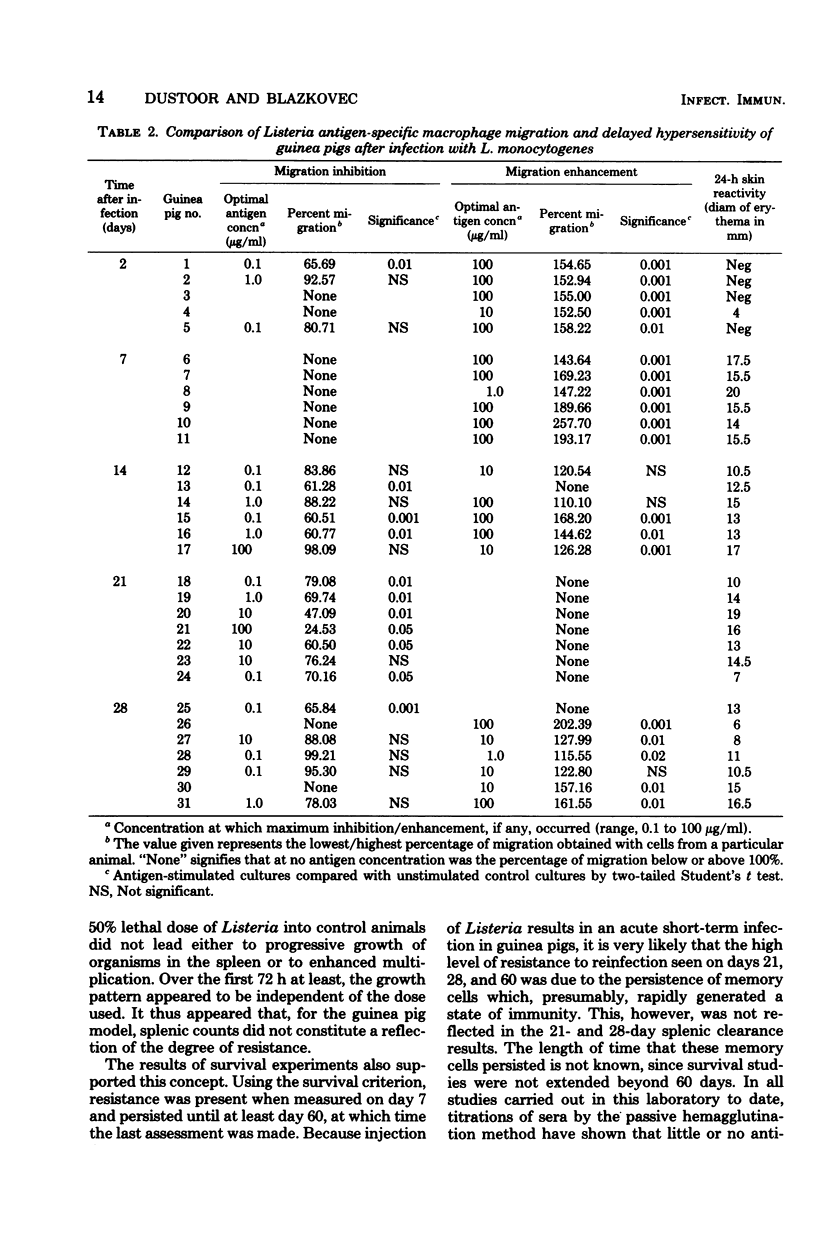
A Listeria monocytogenes infection in guinea pigs was used to study the interrelationship between antigen-induced macrophage migration inhibition, delayed-type hypersensitivity, and acquired cellular resistance. Early after infection (at 2 and 7 days), very significant enhancement of macrophage migration was observed. Migration inhibition was detected beginning on day 14 and was uniformly observed only on day 21 of the infection, after which a shift again to enhancement was seen. The early detection (by day 2) of migration enhancement suggested that this assay may be more sensitive than assessment of delayed type hypersensitivity in vivo, which in this system was first detectable only on day 4. Acquired cellular resistance, as measured by enhanced survival following a high dose challenge with Listeria, was present from day 7 after infection until at least day 60. By splenic clearance studies, however, acquired cellular resistance was present only until day 14 after infection, suggesting that in this system splenic clearance was not a very reliable criterion for measuring acquired cellular resistance.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campbell P. A., Schuffler C., Rodriguez G. E. Listeria cell wall fraction: a B cell adjuvant. J Immunol. 1976 Mar;116(3):590–594. [PubMed] [Google Scholar]
- Cohen J. J., Rodriguez G. E., Kind P. D., Campbell P. A. Listeria cell wall fraction: a B cell mitogen. J Immunol. 1975 Mar;114(3):1132–1134. [PubMed] [Google Scholar]
- Collins F. M., Mackaness G. B. The relationship of delayed hypersensitivity to acquired antituberculous immunity. I. Tuberculin sensitivity and resistance to reinfection in BCG-vaccinated mice. Cell Immunol. 1970 Sep;1(3):253–265. doi: 10.1016/0008-8749(70)90047-x. [DOI] [PubMed] [Google Scholar]
- Collins F. M., Mackaness G. B. The relationship of delayed hypersensitivity to acquired antituberculous immunity. II. Effect of adjuvant on the allergenicity and immunogenicity of heat-killed tubercle bacilli. Cell Immunol. 1970 Sep;1(3):266–275. doi: 10.1016/0008-8749(70)90048-1. [DOI] [PubMed] [Google Scholar]
- Collins F. M. Mechanisms in antimicrobial immunity. J Reticuloendothel Soc. 1971 Jul;10(1):58–99. [PubMed] [Google Scholar]
- David J. R. Macrophage activation by lymphocyte mediators. Fed Proc. 1975 Jul;34(8):1730–1736. [PubMed] [Google Scholar]
- Fox R. A., Gregory D. S., Feldman J. D. Migration inhibition factor (MIF) and migration stimulation factor (MSF) in fetal calf serum. J Immunol. 1974 May;112(5):1861–1866. [PubMed] [Google Scholar]
- Fulton A. M., Dustoor M. M., Kasinski J. E., Blazkovec A. A. Blastogenesis as an in vitro correlate of delayed hypersensitivity in guinea pigs infected with Listeria monocytogenes. Infect Immun. 1975 Sep;12(3):647–655. doi: 10.1128/iai.12.3.647-655.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GEORGE M., VAUGHAN J. H. In vitro cell migration as a model for delayed hypersensitivity. Proc Soc Exp Biol Med. 1962 Nov;111:514–521. doi: 10.3181/00379727-111-27841. [DOI] [PubMed] [Google Scholar]
- Galindo B., Myrvik Q. N. Migratory response of granulomatous alveolar cells from BCG-sensitized rabbits. J Immunol. 1970 Jul;105(1):227–237. [PubMed] [Google Scholar]
- Halliburton B. L., Blazkovec A. A. Delayed hypersensitivity and acquired cellular resistance in guinea pigs infected with Listeria monocytogenes. Infect Immun. 1975 Jan;11(1):1–7. doi: 10.1128/iai.11.1.1-7.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinsdill R. D., Berman D. T. Antigens of Brucella abortus. I. Chemical and immunoelectrophoretic characterization. J Bacteriol. 1967 Feb;93(2):544–549. doi: 10.1128/jb.93.2.544-549.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. The immunology of antituberculous immunity. Am Rev Respir Dis. 1968 Mar;97(3):337–344. doi: 10.1164/arrd.1968.97.3.337. [DOI] [PubMed] [Google Scholar]
- Osebold J. W., Pearson L. D., Medin N. I. Relationship of antimicrobial cellular immunity to delayed hypersensitivity in Listeriosis. Infect Immun. 1974 Feb;9(2):354–362. doi: 10.1128/iai.9.2.354-362.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit J. C., Unanue E. R. Effects of bacterial products on lymphocytes and macrophages: their possible role in natural resistance to listeria infetion in mice. J Immunol. 1974 Sep;113(3):984–992. [PubMed] [Google Scholar]
- Rocklin R. E., MacDermott R. P., Chess L., Schlossman S. F., David J. R. Studies on mediator production by highly purified human T and B lymphocytes. J Exp Med. 1974 Nov 1;140(5):1303–1316. doi: 10.1084/jem.140.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenstreich D. L., Blake J. T., Rosenthal A. S. The peritoneal exudate lymphocyte. I. Differences in antigen responsiveness between peritoneal exudate and lymph node lymphocytes from immunized guinea pigs. J Exp Med. 1971 Nov 1;134(5):1170–1186. doi: 10.1084/jem.134.5.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandok P. L., Hinsdill R. D., Albrecht R. M. Alterations in mouse macrophage migration: a function of assay systems, lymphocyte activation product preparation, and fractionation. Infect Immun. 1975 May;11(5):1100–1109. doi: 10.1128/iai.11.5.1100-1109.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soborg M. In citro migration of peripheral human leucocytes in cellular hypersensitivity. Acta Med Scand. 1968 Jul-Aug;184(1-2):135–139. [PubMed] [Google Scholar]
- Tubergen D. G., Feldman J. D., Pollock E. M., Lerner R. A. Production of macrophage migration inhibition factor by continuous cell lines. J Exp Med. 1972 Feb 1;135(2):255–266. doi: 10.1084/jem.135.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisbart R. H., Bluestone R., Goldberg L. S., Pearson C. M. Migration enhancement factor: a new lymphokine. Proc Natl Acad Sci U S A. 1974 Mar;71(3):875–879. doi: 10.1073/pnas.71.3.875. [DOI] [PMC free article] [PubMed] [Google Scholar]



