Abstract
Importance
Chorioamnionitis is strongly linked to preterm birth and to neonatal infection. The association between histological and clinical chorioamnionitis and cognitive, behavioral and neurodevelopmental outcomes among extremely preterm neonates is less clear. We evaluated the impact of chorioamnionitis on 18-22 month neurodevelopmental outcomes in a contemporary cohort of extremely preterm neonates.
Objective
To compare the neonatal and neurodevelopmental outcomes of three groups of extremely-low-gestational-age infants with increasing exposure to perinatal inflammation: no chorioamnionitis, histological chorioamnionitis alone, or histological plus clinical chorioamnionitis.
Design
Longitudinal observational study.
Setting
Sixteen centers of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network.
Participants
2390 extremely preterm infants born <27 weeks' gestational age between January 1, 2006 and December 31, 2008 with placental histopathology and 18-22 months' corrected age follow-up data were eligible.
Main exposure
Chorioamnionitis
Main Outcome Measures
Outcomes included cerebral palsy, gross motor functional limitation, behavioral scores (according to the Brief Infant-Toddler Social and Emotional Assessment), cognitive and language scores (according to the Bayley Scales of Infant Development, 3rd-Edition) and composite measures of death/neurodevelopmental impairment. Multivariable logistic and linear regression models were developed to assess the association between chorioamnionitis and outcomes while controlling for important variables known at birth.
Results
Neonates exposed to chorioamnionitis had a lower gestational age (GA) and had higher rates of early-onset sepsis and severe periventricular-intraventricular hemorrhage as compared with unexposed neonates. In multivariable models evaluating death and neurodevelopmental outcomes, inclusion of gestational age in the model diminished the association between chorioamnionitis and adverse outcomes. Still, histological+clinical chorioamnionitis was associated with increased risk of cognitive impairment as compared with no chorioamnionitis (Adjusted OR 2.4, [1.3- 4.3] without GA; Adjusted OR 2.0, [1.1-3.6] with GA as a covariate). Histological chorioamnionitis alone was associated with lower odds of death/neurodevelopmental impairment as compared with histological+clinical chorioamnionitis (Adjusted OR 0.68, [0.52-0.89] without GA; 0.66, [0.49-0.89] with GA). Risk of behavioral problems did not differ statistically between groups.
Conclusions and Relevance
Antenatal exposure to chorioamnionitis is associated with altered odds of cognitive impairment and death/neurodevelopmental impairment in extremely preterm infants.
Keywords: chorioamnionitis, preterm, neurodevelopmental impairment, outcome
Introduction
The increased survival of extremely-low-gestational-age neonates (ELGAN) has focused attention on the importance of assessing and improving long-term neurodevelopmental outcomes. It is estimated that as many as 11-15% of extremely preterm infants develop cerebral palsy (CP) and approximately half have other adverse outcomes including abnormalities in cognition, language development and behavior.1-3 Chorioamnionitis and perinatal inflammation are thought to play a causal role in inciting preterm birth and white matter injury.4-6 The association of chorioamnionitis and cognitive and neurobehavioral deficits remains unclear. Published studies in extremely preterm neonates offer inconclusive findings with reports suggesting associations with cerebral palsy and PVL7-9, lower mental developmental index (MDI) scores10 and, alternatively, reports of no association between chorioamnionitis and neurodevelopmental outcomes.11-14 Disparate results may be related to differences in the definitions of chorioamnionitis (histological vs. clinical), the gestational age range studied, the age at follow-up, the outcomes selected (e.g., brain lesions, neurological outcomes or composite death/impairment), and to issues of sample size and single versus multiple-center recruitment. GA is particularly important as it not only conveys maturity, but also conveys information on the immune response15-18 and other developmentally regulated phenomena19; some studies have grouped ELGAN with moderate or late preterm infants.
In the present study, we evaluate whether histological and clinical chorioamnionitis are associated with increased risk of in-hospital morbidities, mortality and neurodevelopmental impairment (NDI) at 18-22 months' corrected age among extremely preterm neonates <27 weeks. At early gestations (prior to CNS myelination), inflammatory injury may be diffuse and result in unique pathological and clinical manifestations. Beyond periventricular leukomalacia and CP, we aimed to report on neurocognitive and behavioral outcomes. We hypothesized that (1) extremely preterm infants exposed to chorioamnionitis would have a higher risk for neonatal morbidities, mortality and poor neurodevelopmental outcomes compared to infants with no exposure, and (2) more severe, clinically manifest chorioamnionitis would be associated with worse outcomes.
Methods
This is a retrospective analysis of prospectively collected data on a cohort of extremely preterm neonates with and without exposure to histological or clinical chorioamnionitis. Preterm infants <27 weeks born between January 1, 2006 and December 31, 2008 at centers of the Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network (NRN) were included in the study if they had placental histopathology data and follow-up to 18-22 months' corrected age. Infants with congenital or chromosomal anomalies were excluded. Routine placental pathology is recommended for premature and high-risk deliveries by the Placental Pathology Task Force of the College of American Pathologists20; and this recommendation is generally followed by the NRN centers (performed in 82% of inborn infants during the study period). Neonates were divided into three exposure groups: no chorioamnionitis, histological chorioamnionitis alone and histological+clinical chorioamnionitis. Clinically apparent chorioamnionitis was deemed the highest exposure group; a strong association with fetal inflammatory response syndrome has been reported by other investigators.8,21 Data from the NICHD NRN Generic data base22 and Follow-up studies23 were utilized. The studies were approved by the institutional review board (IRB) of each participating NRN center and written informed consent was obtained from the parents or guardians for longitudinal follow-up per requirement of the individual IRBs.
Study definitions
Neonatal and maternal data were collected prospectively for all live-born infants from birth until death, hospital discharge, transfer or 120 days, whichever occurred first.22 Infant follow-up data were collected during a comprehensive follow-up evaluation at 18-22 months' corrected age. Maternal and neonatal demographic characteristics included: age, race, prenatal care, maternal complications, preterm premature rupture of membranes (PPROM)>18 hours, antenatal antibiotics, antenatal corticosteroids, chorioamnionitis, Cesarean-delivery, birth weight, GA, gender, small for gestational age status24, delivery room resuscitation, Apgar scores, cord pH and base deficit.
Histopathological chorioamnionitis was recorded if chorioamnionitis was noted on the placental pathology report or if acute, subacute chorioamnionitis or chronic chorionitis (as defined by the Stillbirth Collaborative Research Network Pathology Protocol25) were documented. Assessment of histopathological chorioamnionitis was based on local reports made by individual site pathologists. Clinical chorioamnionitis (typically characterized by two or more of: maternal fever, uterine tenderness, malodorous amniotic fluid, maternal or fetal tachycardia or evidence of inflammation) also was noted if recorded in the mother's medical record by the treating clinicians and confirmed histopathologically. Cases of clinical chorioamninonitis without histopathological confirmation were excluded to avoid misclassification.
In-hospital morbidities included: mortality, early (EOS) and late-onset sepsis (LOS), bronchopulmonary dysplasia (BPD)26, periventricular-intraventricular hemorrhage (PIVH)27, ventriculomegaly, cystic periventricular leukomalacia (PVL), necrotizing enterocolitis (NEC)28 and retinopathy of prematurity (ROP) stage 3 or greater as defined in prior publications.22
As part of the NICHD NRN Follow-up Study, surviving infants underwent a comprehensive follow-up assessment at 18-22 months' corrected age performed by certified examiners trained to reliability on an annual basis.23 Psychometric testing was performed using the cognitive and language subscales of the Bayley Scales of Infant and Toddler Development-III (Bayley-III).29 A score of 100+15 on the Bayley III represents the mean +1SD; a score <70 represents 2SD below the mean. Children who were so severely developmentally delayed that they could not be assessed were assigned scores (54 for severe cognitive delay and 46 for severe language delay). Behavioral screening was performed using the Brief Infant-Toddler Social and Emotional Assessment (BITSEA) administered to the primary caregiver in the form of a structured interview.30 The BITSEA is a nationally standardized behavioral screener used to determine the need for diagnostic assessment for socioemotional and behavioral problems. Total Problem Scores can be compared with specific percentile rankings of normative populations; low percentile rankings (≤25%) are associated with higher problem scores (externalizing, internalizing, dysregulation problems and behaviors seen in autism spectrum disorders).
CP was defined as a non-progressive CNS disorder with abnormal muscle tone in at least one extremity and abnormal control of movement and posture that interfered with age-appropriate activities.3 Disabling CP was classified based on the modified Gross Motor Function Classification System (≥Level II).31 Neurodevelopmental impairment was defined by one or more of: disabling CP, Bayley-III cognitive score <70, Gross Motor Function Level≥ II, blindness or permanent hearing loss that did not permit the child to understand or communicate despite amplification.
Statistical analysis
Children with and without exposure to chorioamnionitis defined histologically or clinically were compared with respect to maternal and neonatal baseline characteristics and outcome measures. Demographic characteristics were analyzed for the entire sample, as well as separately for infants excluded or lost to follow-up. For outcome measures, three exposure groups were compared: no chorioamnionitis, histological chorioamnionitis alone, and histological+clinical chorioamnionitis. Multivariable logistic and linear regression models were developed to assess the primary (death/NDI) and secondary outcomes adjusting for important covariates available at the time of birth that were selected a priori (maternal age, multiple birth, parity, antenatal steroids, maternal hypertension, antepartum hemorrhage, gender, GA, SGA status, insurance, race and center). Models including GA at delivery were compared to those without any GA at delivery variable considering the fact that while GA at delivery lies in the causal pathway between chorioamnionitis and outcomes, it also provides important information about the risks of adverse outcomes in extremely preterm newborns.
Results
3082 infants were assessed for eligibility and the following groups were excluded: infants with chromosomal or congenital anomalies (n=55), infants with ‘clinical chorioamnionitis’ with normal histopathology (n=82) and infants lacking placental histopathology (n=555). The final study cohort consisted of 2390 inborn infants.(Figure 1) Infants with and without placental histopathology had similar baseline characteristics except for 5-minute-Apgar score (Apgar score <3 occurred in 20.8% among those with placental histopathology vs. 26.4% without) and base deficit (4.99 mmol/L among those with histopathology vs. 4.28 mmol/L without). Mothers whose placentas were examined were more likely to have prepartum hemorrhage (22.9% vs. 17.6%), to receive antibiotics (66.9% vs. 58%) or antenatal steroids (75.4% vs. 66.9%), and to deliver by C-section (54% vs. 46.8%).(Table 1) Of the eligible participants, 38% were born to mothers with histological chorioamnionitis alone and 19% were born to mothers with histological+clinical chorioamnionitis. Primary outcome (death/NDI) was available for 2235 (93.5%) of all eligible infants.
Figure 1. Participants.
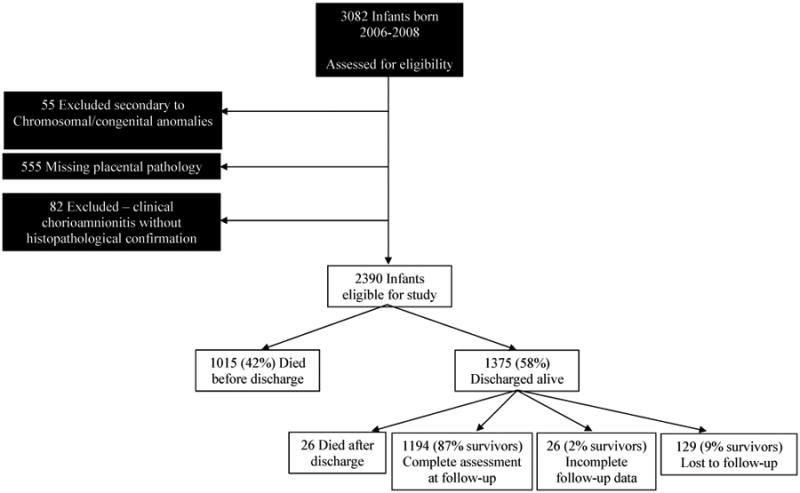
Table 1. Maternal and neonatal characteristics among neonates with and without placental pathology data.
| Characteristics | Neonates with placental pathology | Neonates with missing placental pathology (excluded) | P-value |
|---|---|---|---|
|
| |||
| Maternal | N = 2390 | N = 555 | |
|
| |||
| Mother's age, years | 27.2 (6.42) | 26.8 (6.39) | 0.20 |
| Mean (SD), Median (Q1, Q3) | 27 (22, 32) | 26 (22, 32) | |
|
| |||
| Maternal Race/ethnicity, % | |||
| Black | 39.5 | 36.3 | |
| White | 35.7 | 37.8 | 0.44 |
| Hispanic | 19.8 | 21.5 | |
| Other | 5.0 | 4.4 | |
|
| |||
| ≥ 1 Prenatal visit, % | 93.8 | 94.0 | 0.95 |
|
| |||
| Diabetes mellitus, % | 3.9 | 5.1 | 0.23 |
|
| |||
| Parity, % | |||
| 0 or 1 | 38.6 | 38.9 | 0.089 |
| 2 or 3 | 46.0 | 49.2 | |
| > 3 | 15.4 | 11.9 | |
|
| |||
| Hypertension, % | |||
| Chronic | 8.3 | 8.0 | 0.54 |
| Pregnancy-induced | 9.8 | 11.3 | |
| None | 81.9 | 80.7 | |
|
| |||
| Prepartum hemorrhage, % | 22.9 | 17.6 | 0.0077** |
|
| |||
| PPROM >18h, % | 25.7 | 25.6 | 0.10 |
|
| |||
| Duration of PPROM, hrs | 34.4 (103.2) | 43.6 (152.7) | |
| Mean (SD), Median (Q1, Q3) | 0 (0, 8.33) | 0 (0, 7.95) | 0.67 |
|
| |||
| Antenatal antibiotics, % | 66.9 | 58.0 | <.0001** |
|
| |||
| Antenatal steroids, % | 75.4 | 66.9 | <.0001** |
|
| |||
| Multiple birth, % | 25.9 | 21.8 | 0.054 |
|
| |||
| Cesarean delivery, % | 54.0 | 46.8 | 0.0025** |
|
| |||
| Mother's education: high school graduate, % | 74.2 | 72.0 | 0.41 |
|
| |||
| Insurance, % | |||
| Medicaid | 46.9 | 43.6 | 0.21 |
| Private | 39.5 | 40.4 | |
| Self/Other/Unins./Uknown. | 13.6 | 16.0 | |
|
| |||
| Neonatal | |||
|
| |||
| Birth weight, % | |||
| 401-500 g | 12.9 | 113.9 | 0.34 |
| 501-750 g | 54.1 | 50.6 | |
| 751-1000 g | 33.1 | 35.5 | |
|
| |||
| Gestational age, wk | 24.3 (1.35) | 24.3 (1.42) | |
| Mean (SD), Median (Q1, Q3) | 25 (23, 25) | 24 (23, 26) | 0.74 |
|
| |||
| Male, % | 51.4 | 54.6 | 0.19 |
|
| |||
| SGA at birth, % | 5.8 | 5.8 | 1.00 |
|
| |||
| Delivery room resuscitation, % | 71.4 | 67.2 | 0.053 |
| Intubation, % | 71.2 | 67.2 | 0.067 |
| Epinephrine, % | 5.8 | 6.9 | 0.41 |
| Chest compression, % | 10.0 | 9.2 | 0.62 |
|
| |||
| Apgar score < 3, % | |||
| At 1 minute | 43.5 | 47.2 | 0.13 |
| At 5 minutes | 20.8 | 26.4 | 0.0050** |
|
| |||
| Cord pH1 | 7.28 (0.12) | 7.29 (0.12) | |
| Mean (SD), Median (Q1, Q3) | 7.31 (7.24, 7.36) | 7.31 (7.24, 7.36) | 0.47 |
|
| |||
| Base deficit1, mmol/L | |||
| Mean (SD) | 4.99 (4.98) | 4.28 (4.34) | |
| Median (Q1, Q3) | 4 (2, 7) | 3 (1, 5) | 0.020** |
Percentages were tested with a chi-square test. Medians were tested with a Wilcoxon rank sum test.
P-value is significant at alpha = 0.05 level of significance.
Cord pH and base deficit were found for the 1,648 that had cord blood gas (1,360 with placental pathology information, 288 without placental pathology information).
Mothers with histological or histological+clinical chorioamnionitis were more likely to identify with Black race, to have PPROM >18 hours, to deliver vaginally, to receive intrapartum antibiotics, and to be normotensive as compared with mothers without chorioamnionitis.(Table 2) Neonates exposed to antenatal chorioamnionitis had a lower GA and had lower Apgar scores as compared with unexposed neonates (despite a higher cord pH). A smaller proportion of neonates exposed to chorioamnionitis was SGA as compared to infants with no exposure.
Table 2. Baseline maternal and neonatal characteristics by exposure to chorioamnionitis.
| Characteristics | No chorioamnionitis | Histological chorioamnionitis alone | Histological and clinical chorioamnionitis | P-value |
|---|---|---|---|---|
|
| ||||
| Maternal | N = 1,014 | N = 910 | N = 466 | |
|
| ||||
| Mother's age, years | ||||
| Mean (SD), Median | 27.2 (6.48) | 26.9 (6.29) | 27.8 (6.53) | |
| (Q1, Q3) | 27 (22,32) | 26.5 (22,32) | 27 (23,32) | 0.12 |
|
| ||||
| Race/ethnicity, % | ||||
| Black | 35.4 | 42.2 | 43.2 | |
| White | 39.4 | 34.7 | 29.9 | 0.0042* |
| Hispanic | 20.6 | 18.4 | 20.9 | |
| Other | 4.7 | 4.7 | 6.0 | |
|
| ||||
| Education, % high school graduate | 72.3 | 75.2 | 76.5 | 0.24 |
|
| ||||
| ≥ 1 Prenatal visit, % | 93.1 | 93.9 | 95.3 | 0.26 |
|
| ||||
| Diabetes mellitus, % | 4.7 | 2.9 | 3.9 | 0.10 |
|
| ||||
| Hypertension, % Chronic | 13.7 | 4.1 | 4.9 | |
| Pregnancy-induced | 20.5 | 2.4 | 0.6 | <.0001* |
| None | 65.8 | 93.5 | 94.4 | |
|
| ||||
| PPROM >18h, % | 9.3 | 30.2 | 53.2 | <.0001* |
|
| ||||
| Duration of PPROM, hrs | ||||
| Mean (SD), | 18.5 (102) | 36.4 (95) | 70.8 (114) | |
| Median (Q1, Q3) | 0 (0, 0.05) | 0.03 (0, 17.7) | 12.9 (0.02, 94.4) | <.0001* |
|
| ||||
| Antenatal antibiotics, % | 50.6 | 73.4 | 89.7 | <.0001* |
|
| ||||
| Antenatal steroids, % | 75.4 | 75.9 | 74.6 | 0.88 |
|
| ||||
| Cesarean delivery, % | 67.9 | 45.1 | 41.2 | <.0001* |
|
| ||||
| Neonatal | ||||
|
| ||||
| Birth weight, % | ||||
| 401-500 g | 14.0 | 11.1 | 13.7 | |
| 501-750 g | 54.6 | 54.4 | 52.4 | 0.25 |
|
| ||||
| 751-1000 g | 31.4 | 34.5 | 33.9 | |
| Gestational age, wk | ||||
| Mean (SD) | 24.6 (1.29) | 24.2 (1.36) | 24.1 (1.39) | |
| Median (Q1, Q3) | 25 (24, 26) | 24 (23, 25) | 24 (23, 25) | <.0001* |
|
| ||||
| Male, % | 53.5 | 49.0 | 51.7 | 0.15 |
|
| ||||
| SGA at birth, % | 10.9 | 2.0 | 2.2 | <.0001* |
|
| ||||
| Delivery room resuscitation, % | 72.0 | 71.0 | 71.0 | 0.85 |
| Intubation | 71.6 | 70.9 | 71.0 | 0.92 |
| Epinephrine | 5.3 | 5.3 | 8.0 | 0.09 |
| Chest compression | 9.5 | 9.8 | 11.6 | 0.43 |
|
| ||||
| Apgar score < 3, % | ||||
| At 1 minute | 40.5 | 41.8 | 53.1 | <.0001* |
| At 5 minutes | 17.3 | 21.1 | 27.9 | <.0001* |
|
| ||||
| Cord pH | ||||
| Mean (SD), | 7.26 (0.13) | 7.30 (0.11) | 7.29 (0.10) | |
| Median (Q1, Q3) | 7.29 (7.21, 7.35) | 7.33 (7.26, 7.37) | 7.31 (7.24, 7.36) | <.0001* |
|
| ||||
| Base deficit, mmol/L | ||||
| Mean (SD), | 5.35 (5.56) | 4.48 (4.18) | 5.13 (4.88) | |
| Median (Q1, Q3) | 4.0 (2.0, 7.0) | 3.0 (1.0, 6.0) | 4.0 (2.0, 7.0) | 0.08 |
Percentages were tested with a chi-square test. Medians were tested with a Wilcoxon rank sum test.
P-value is significant at alpha = 0.05 level of significance.
Of the 1376 infants exposed to any chorioamnionitis, 44.8% with histological chorioamnionitis died by 18-22 months' corrected age compared with 51.2% with histological+clinical chorioamnionitis. In comparison, 46.1% with no exposure to chorioamnionitis died. A greater proportion of infants born to mothers with histological or clinical chorioamnionitis had early onset sepsis (3.6% and 5.9% respectively vs. 1.3% without chorioamnionitis) and a greater proportion received IV antibiotics (55% and 76.5% vs. 45.3%). Adjusted risks of sepsis and antibiotic administration were elevated for the histological+clinical chorioamnionitis group as compared with the other two groups. Among infants with histological+clinical chorioamnionitis, adjusted risk of severe PIVH also was increased (p=0.033)(Table 3). No statistically significant differences in the risks of late-onset sepsis, BPD, central nervous system ventriculomegaly, cystic PVL, NEC or ROP were observed between groups.
Table 3. Adjusted neonatal and 18-22 months' corrected age outcomes by exposure to chorioamnionitis.
| Outcomes | No chorioamnionitis | Histological chorioamnionitis alone | Histological and clinical chorioamnionitis | Adjusted4 P-value Histological chorioamnionitis alone vs None | Adjusted4 P-value Histological+Clinical Chorioamnionitis vs None | Adjusted4 P-value Histological alone vs. Histological+Clinical Chorioamnionitis |
|---|---|---|---|---|---|---|
| Neonatal/early death | N = 1,014 | N = 910 | N = 466 | |||
| Death within 12 hrs, % | 15.7 | 20.6 | 27.0 | 0.47 | 0.0097* | 0.044* |
| Death prior to discharge, % | 41.7 | 41.4 | 46.1 | 0.0023* | 0.16 | 0.23 |
| In-hospital outcome | N = 855 | N = 723 | N = 340 | |||
| Early-onset sepsis, % | 1.3 | 3.6 | 5.9 | 0.0035* | <.0001* | 0.067 |
| Physiologic BPD, % | 56.0 | 54.1 | 56.8 | 0.43 | 0.99 | 0.50 |
| PIVH grade 3 or 4, % | 20.9 | 22.0 | 29.3 | 0.67 | 0.033* | 0.0097* |
| (Grade 3) | (7.0) | (10.1) | (12.5) | |||
| (Grade 4) | (13.8) | (11.9) | (16.8) | |||
| Cystic PVL, % | 4.6 | 4.8 | 6.1 | 0.77 | 0.53 | 0.37 |
| Ventriculomegaly, % | 6.0 | 4.4 | 5.8 | 0.24 | 0.90 | 0.39 |
| Late-onset sepsis, % | 43.7 | 47.3 | 41.3 | 0.15 | 0.50 | 0.057 |
| Neonatal antibiotics, % | 45.3 | 55.0 | 76.5 | 0.0049* | <.0001* | <.0001* |
| NEC, % | 11.1 | 15.1 | 15.3 | 0.082 | 0.18 | 0.92 |
| ROP stage 3 or greater, % | 24.7 | 28.3 | 30.9 | 0.88 | 0.41 | 0.45 |
| 18-month outcome | N = 512 | N = 473 | N = 209 | |||
| Cognitive score1 < 85 | 27.5 | 30.5 | 35.4 | 0.42 | 0.055 | 0.17 |
| Cognitive score1 < 70 | 7.1 | 8.1 | 14.4 | 0.81 | 0.024* | 0.029* |
| Cognitive score1 < 55 | 2.0 | 3.0 | 3.8 | 0.48 | 0.36 | 0.73 |
| Cognitive score- | ||||||
| Mean (SD), | 89.4 (14.4) | 87.9 (13.6) | 86.1 (16.2) | |||
| Median (Q1, Q3) | 90 (80, 100) | 90 (80, 95) | 90 (75, 95) | 0.45 | 0.0060* | 0.027* |
| Language score1 < 85 | 50.2 | 52.6 | 52.4 | 0.50 | 0.92 | 0.65 |
| Language score1 < 70 | 18.5 | 18.1 | 21.8 | 0.96 | 0.30 | 0.29 |
| Language score1 < 55 | 4.6 | 6.0 | 7.3 | 0.34 | 0.26 | 0.73 |
| Language score- | ||||||
| Mean (SD), | 84.7 (17.4) | 83.4 (16.7) | 82.7 (18.7) | |||
| Median (Q1, Q3) | 83 (74, 97) | 83 (74, 94) | 83 (71, 97) | 0.70 | 0.38 | 0.22 |
| GMF Level > II, % | 6.3 | 6.6 | 9.6 | 0.58 | 0.52 | 0.24 |
| Cerebral palsy, moderate to severe, % | 4.5 | 4.9 | 8.1 | 0.50 | 0.38 | 0.12 |
| Spastic diplegia, moderate to severe, % | 0.6 | 1.1 | 1.4 | 0.70 | 0.53 | 0.33 |
| Behavioral problems2 | ||||||
| Rating < 26th pctile, % | 39.3 | 43.6 | 36.9 | 0.44 | 0.39 | 0.12 |
| Parental worry ≥ 3, % | 7.9 | 10.8 | 6.7 | 0.11 | 0.70 | 0.10 |
| Hearing impairment, % | 1.2 | 2.1 | 1.9 | NA | NA | NA |
| Vision impairment, % | 1.8 | 1.3 | 0.5 | 0.32 | 0.15 | 0.36 |
| NDI3, % | 10.9 | 11.6 | 18.2 | 0.64 | 0.14 | 0.041* |
| N = 950 | N = 857 | N = 428 | ||||
| Death prior to 18 months, % | 46.1 | 44.8 | 51.2 | 0.011* | 0.71 | 0.075 |
| Composite outcome | N = 950 | N = 857 | N = 428 | |||
| Death or NDI, % | 52.0 | 51.2 | 60.1 | 0.011* | 0.57 | 0.0061* |
Scores from Bayley Scales of Infant Development, Third Edition.
Scores from the Brief Infant Toddler Socioemotional Adjustment Scale (BITSEA). Parental worry ≥ 3 means the parent is either “worried” or “very worried” about the child's behaviors, emotions and relationships.
Neurodevelopmental impairment.
P-values for the significance of chorioamnionitis type were obtained from either logistic regression or linear regression models of the outcome in each row, adjusting for covariates for gender, antenatal steroids, small for gestational age (SGA), hypertension, and gestational age. A covariate for centers was added to models for death within 12 hours and death prior to discharge. The SGA covariate was excluded from the model for convergence reasons for early on-set sepsis. P-values for NDI, NDI or death, and death prior to 18 months were taken from the full models as described in the odds ratio tables for those outcomes. Because of sparseness in the outcome data, it was not possible to model hearing impairment or spastic diplegia. The p-value for hearing impairment is from a chi-square test. The p-value for diplegia is from an exact test.
P-value is significant at alpha = 0.05 level of significance.
Survivors to 18-22 months' corrected age included 512 infants whose mothers had no chorioamnionitis and 682 whose mothers had chorioamnionitis. Children with neurodevelopmental assessments at 18-22 months' corrected age and children lost to follow-up were comparable for all baseline characteristics except for mode of delivery and maternal age and race (proportionally more children born vaginally and proportionally more children born to younger mothers and mothers of Hispanic race were lost to follow-up).(Supplemental eTable 1) Of infants followed to 18-22 months' corrected age, infants exposed to histological+clinical chorioamnionitis had a significantly higher risk of cognitive impairment (defined as a cognitive score <70) as compared with the no chorioamnionitis (p=0.02) and histological chorioamnionitis alone (p=0.03) groups.(Table 2) Adjusted risks of CP were not significantly different despite increasing rates with increasing exposure to inflammation: 4.5% among unexposed infants, 4.9% among infants exposed to histological chorioamnionitis alone and 8.1% among those exposed to histological+clinical chorioamnionitis. Similarly, there were no significant differences in the risks of spastic diplegia, gross motor functional limitation, language delay or behavioral problems between groups.
On multivariable analyses, the association between chorioamnionitis and outcome was significantly impacted by inclusion of GA in the model. With GA as a covariate (along with other important covariates impacting outcome), the association between chorioamnionitis and adverse outcomes was diminished.(Table 4) Sequential analysis of each variable in the logistic regression models revealed that only GA impacted the association between chorioamnionitis and outcome in this manner. Compared with no chorioamnionitis, histological+clinical chorioamnionitis was associated with a heightened risk of low cognitive score <70 (Adjusted OR 2.38, [1.32-4.28] without GA; Adjusted OR 2.00, [1.10-3.64] with GA) and a decreased continuous Cognitive Score (β, -3.87 [-6.19 to -1.54] without GA and β, -3.25 [-5.56 to -0.93] with GA). Compared with histological+clinical chorioamnionitis, histological chorioamnionitis alone was associated with reduced odds of death/NDI (Adjusted OR=0.68, [0.52-0.89] without GA; Adjusted OR=0.66, [0.49-0.89] with GA) and NDI (Adjusted OR=0.57, [0.35-0.93] without GA; Adjusted OR=0.59, [0.36-0.98] with GA). Histological chorioamnionitis also was associated with reduced risk of death/NDI as compared with the no chorioamnionitis group, but only in models that included GA as a covariate (Adjusted OR 1.00, [0.80-1.26] without GA; Adjusted OR 0.72, [0.56-0.92] with GA). Direct comparison of the unadjusted rates of death and neurodevelopmental outcomes by week of gestation revealed the same patterns of association.(Supplemental eTable 2) The rate of death was lower in the histological chorioamnionitis group than in the no chorioamnionitis or histological+clinical chorioamnionitis groups for each week <27. The rate of cognitive impairment was highest in the clinical plus histological chorioamnionitis group for each gestational week at delivery and nearly two-fold higher than the other exposure groups overall. We further tested for an interaction between histological or clinical chorioamnionitis and GA in models for NDI or death, death (alone), and NDI (alone), and there was no significant interaction effect in any of the models. (The smallest interaction p-value was 0.19 and it occurred in the NDI model).
Table 4. Comparison of multivariable models including gestational age at delivery to those without any gestational age at delivery variable.
| Death and neurodevelopmental outcomes by chorioamnionitis including gestational age | |||
|---|---|---|---|
| Outcome | Histo Chorio vs. No | Histo+Clinical vs. No | Histo vs. Histo+Clinical |
| Death/NDIa | 0.72 (0.56, 0.93) | 1.09 (0.80, 1.49) | 0.66 (0.49, 0.89) |
| Deatha | 0.72 (0.56, 0.93) | 0.94 (0.69, 1.28) | 0.76 (0.57, 1.03) |
| NDIa | 0.89 (0.56, 1.42) | 1.51 (0.88, 2.59) | 0.59 (0.36, 0.98) |
| CPa | 0.80 (0.42, 1.53) | 1.39 (0.67, 2.87) | 0.58 (0.29, 1.16) |
| Cognitive Scoreb | -0.72 (-2.59, 1.14) | -3.25 (-5.56, -0.93) | 2.52 (0.29, 4.76) |
| <70a | 1.07 (0.62, 1.85) | 2.00 (1.10, 3.64) | 0.53 (0.31, 0.94) |
| <85a | 1.15 (0.82, 1.60) | 1.50 (0.99, 2.28) | 0.76 (0.52, 1.12) |
| Death and neurodevelopmental outcomes by chorioamnionitis without gestational age | |||
| Outcome | Histo Chorio vs. No | Histo+Clinical vs. No | Histo vs. Histo+Clinical |
| Death/NDIa | 1.00 (0.80, 1.26) | 1.47 (1.11, 1.93) | 0.68 (0.52, 0.89) |
| Deatha | 1.01 (0.81, 1.27) | 1.32 (1.00, 1.74) | 0.77 (0.59, 1.00) |
| NDIa | 1.02 (0.65, 1.61) | 1.79 (1.05, 3.04) | 0.57 (0.35, 0.94) |
| CPa | 0.89 (0.47, 1.69) | 1.60 (0.78, 3.28) | 0.56 (0.28, 1.11) |
| Cognitive Scoreb | -1.30 (-3.17, 0.57) | -3.87 (-6.19, -1.54) | 2.57 (0.31, 4.82) |
| <70a | 1.21 (0.71, 2.08) | 2.38 (1.32, 4.28) | 0.51 (0.29, 0.89) |
| <85a | 1.25 (0.90, 1.74) | 1.66 (1.10, 2.51) | 0.75 (0.51, 1.10) |
Abbreviations: NDI, neurodevelopmental impairment; CP, cerebral palsy
For categorical outcomes, the adjusted OR (95% CI) from logistic regression models is presented
For continuous outcomes (e.g., Cognitive score), the regression coefficient is presented (β, 95% CI) from linear regression models. This reflects the adjusted difference in score with exposure to chorioamnionitis. A positive parameter estimate indicates that the average score of the treatment in the first position is relatively higher compared to the average score of the treatment in the second position. A negative parameter estimate indicates that the average score of the treatment in the first position is relatively lower than the average score of the treatment in the second position.
Note: Death/NDI, Death, NDI, and Cognitive Score <85 were adjusted by the covariates used for finding adjusted odds ratios of the primary outcome. The remaining outcomes were adjusted by reduced models which contained covariates for center, gender, antenatal steroids, small for gestational age, and hypertension. Because of sparseness of some outcomes, it became necessary to combine some centers.
Discussion
Conflicting reports on neonatal outcomes following exposure to chorioamnionitis among extremely premature neonates makes prediction of outcomes difficult. This study evaluated the neonatal and neurodevelopmental outcomes of <27 week GA neonates exposed to no chorioamnionitis, histological chorioamnionitis alone and histological+clinical chorioamnionitis. Compared to unexposed neonates, neonates exposed to any chorioamnionitis had a lower GA and had higher rates of EOS and severe PIVH. In multivariable models evaluating death and neurodevelopmental outcomes, histological+clinical chorioamnionitis was associated with increased risk of cognitive impairment as compared with no chorioamnionitis. Histological chorioamnionitis alone was associated with lower odds of death/neurodevelopmental impairment as compared with histological+clinical chorioamnionitis. Histological chorioamnionitis alone also was associated with lower odds of death/NDI as compared with the no chorioamnionitis group, but only when GA at delivery was included in the multivariable model. Both composite and non-composite outcomes are presented as non-composite outcomes ignore censoring from infant deaths.
Our findings are consistent with the results of Hendson et al who reported an association between chorioamnionitis and lower Bayley-II mental-developmental-index scores.10 We reported on cognitive impairment specifically using Bayley-III cognitive scores. Histological chorioamnionitis was found to be protective for death/NDI with GA included as a covariate. As GA may lie in the causal pathway between chorioamnionitis and death and neurodevelopmental outcomes, one may argue whether adjustment for GA is appropriate. Regardless, other causes of prematurity may impact survival more profoundly (e.g., aberrant placentation, hypoxia-ischemia). Additionally, a regulated maternal or fetal immune response that triggers parturition and removes the fetus from an inflammatory intra-uterine environment may be protective.32,33 Histological chorioamnionitis is consistently less detrimental than histological+clinical chorioamnionitis. In a study evaluating all fetal outcomes, Lahra et al reported a survival advantage for neonates with histological chorioamnionitis; surviving premature neonates <30 weeks were more likely to have histological chorioamnionitis or histological evidence of a fetal response as compared to fetuses who were stillborn or preterm neonates who died within 28 days.34 We collected no data on fetal deaths.
Studies on chorioamnionitis in older GA neonates report a strong association between chorioamnionitis and white matter injury and CP6,35 alluding to possible differences in critical GA windows or immune maturity.15,18 Studies in very preterm infants report mixed results7,8,11-13,36,37 and are limited by retrospective assessment of outcomes, incongruent definitions of chorioamnionitis, single center design (necessitating study over many years, when perinatal care may have changed) and small or inadequate sample sizes over broader GA ranges. Few prospective studies have addressed the potential association of histological chorioamnionitis with the outcomes of extremely preterm neonates. One noteworthy exception is the study by Leviton et al that evaluated <28 week neonates (n=899 with placental data and neurological assessment at 24 months' corrected age).9 Histological chorioamnionitis, defined using stricter criteria than in our study, was associated with an increased risk of ventriculomegaly and diparetic CP. The risk of ventriculomegaly was further increased by recovery of a single or multiple placental organisms, whereas the risk of CP was not. We found no association of ventriculomegaly with chorioamnionitis, but measurements of ventricular size were not ascertained. Our study also found no statistically significant association with cerebral palsy, though the percentage of infants with CP increased with increasing severity of perinatal inflammation (4.5% with no chorioamnionitis vs. 8.1% with histological+clinical chorioamnionitis). Strunk et al. reported a reduced risk of late-onset sepsis following chorioamnionitis.38 We found no difference in the rate of LOS among neonates with and without exposure to chorioamnionitis. Our study also evaluated behavioral outcomes based on screening using the BITSEA. Though we found no significant association with chorioamnionitis, this does not rule out possible effects that would be detected later in childhood or with other behavioral instruments.
Studies that assess chorioamnionitis without assessing fetal inflammatory response (including our own) lack important data in that fetal involvement is likely a more important predictor of neonatal outcomes than isolated maternal or intrauterine inflammation. Further, inflammatory biomarkers (e.g., measurements of cytokines, chemokines, damage markers) or amniotic or placental microbiological studies are an important omission. Early biomarkers of clinically significant disease may provide more rapid and accurate risk characterization. Though the NRN centers used the Stillbirth Collaborative Research Network Pathology Protocol to classify chorioamnionitis, the NRN registry provided no information on the details of these assessments. Additionally, histopathological assessments were performed by multiple pathologists, raising the possibility of inter-observer and center variability; we had no ratings of inter-rater reliability. Center variation also may have impacted the decision to obtain placental histopathology and the care provided. Ideally, placental histopathology would have been performed for all neonates born during the study period. Additionally, as in other studies targeting preterm infants, a healthy comparison group is lacking. Prematurity in the absence of histological chorioamnionitis is likely a result of other pathological processes that may alter the risk of death/impairment. Evaluation of inflammation over time (not only throughout gestation but in the postnatal period) is important as well.39 Preclinical models have shown that the timing of an inflammatory exposure may alter the course of injury (e.g., preconditioning or sensitization).40-43 Subacute exposure to lipopolysaccharide (LPS) 24h prior to a second noxious exposure lessened brain injury in a rodent model compared with exposure to the second insult alone. In contrast, very acute (6h prior) or remote exposures (72h prior) intensified injury following the second exposure.40 It is possible that histological chorioamnionitis may correspond to this subacute exposure or that combining all forms of histological chorioamnionitis blurs the distinction between acute, sub-acute and chronic intrauterine inflammation. We have not considered the impact of postnatal inflammation.
The strengths of our study include the large sample size of 2390 neonates born prior to 27 weeks (including 1376 exposed to chorioamnionitis), the multicenter study design over a three-year period and the detailed follow-up and careful neurodevelopmental assessments that included cognitive and behavioral outcomes. Studying a narrow GA cohort of extremely preterm neonates allowed us to focus on the impact of chorioamnionitis on very immature neonates, while much of the literature has combined neonates of broader gestational ranges.
Conclusions
Antenatal exposure to chorioamnionitis is associated with altered odds of cognitive impairment and death/neurodevelopmental impairment in extremely preterm infants.
Supplementary Material
Acknowledgments
The National Institutes of Health, the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the National Center for Research Resources, and the National Center for Advancing Translational Sciences provided grant support for the Neonatal Research Network's Generic Database and Follow-up Studies.
Data collected at participating sites of the NICHD Neonatal Research Network (NRN) were transmitted to RTI International, the data coordinating center (DCC) for the network, which stored, managed and analyzed the data for this study. On behalf of the NRN, Dr. Abhik Das (DCC Principal Investigator) and Mr. Douglas E. Kendrick (DCC Statistician) had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study. The following investigators, in addition to those listed as authors, participated in this study:
NRN Steering Committee Chair: Michael S. Caplan, MD, University of Chicago, Pritzker School of Medicine.
Alpert Medical School of Brown University and Women & Infants Hospital of Rhode Island (U10 HD27904) – Betty R. Vohr, MD; Angelita M. Hensman, RN BSN; Robert Burke, MD; Melinda Caskey, MD; Katharine Johnson, MD; Barbara Alksninis, PNP; Theresa M. Leach, MEd CAES; Victoria E. Watson, MS CAS.
Case Western Reserve University, Rainbow Babies & Children's Hospital (U10 HD21364, M01 RR80) – Avroy A. Fanaroff, MD; Deanne E. Wilson-Costello, MD; Bonnie S. Siner, RN; Monika Bhola, MD; Gulgun Yalcinkaya, MD; Harriet G. Friedman, MA.
Cincinnati Children's Hospital Medical Center, University Hospital, and Good Samaritan Hospital (U10 HD27853, M01 RR8084) – Kurt Schibler, MD; Edward F. Donovan, MD; Kate Bridges, MD; Barbara Alexander, RN; Cathy Grisby, BSN CCRC; Holly L. Mincey, RN BSN; Jody Hessling, RN; Teresa L. Gratton, PA; Jean J. Steichen, MD; Kimberly Yolton, PhD.
Duke University School of Medicine, University Hospital, Alamance Regional Medical Center, and Durham Regional Hospital (U10 HD40492, M01 RR30) – Ronald N. Goldberg, MD; C. Michael Cotten, MD MHS; Ricki F. Goldstein, MD; Kathy J. Auten, MSHS; Kimberley A. Fisher, PhD FNP-BC IBCLC; Sandra Grimes, RN BSN; Kathryn E. Gustafson, PhD; Melody B. Lohmeyer, RN MSN.
Emory University, Children's Healthcare of Atlanta, Grady Memorial Hospital, and Emory University Hospital Midtown (U10 HD27851, M01 RR39) – David P. Carlton, MD; Ira Adams-Chapman, MD.
Eunice Kennedy Shriver National Institute of Child Health and Human Development – Stephanie Wilson Archer, MA.
Indiana University, University Hospital, Methodist Hospital, Riley Hospital for Children, and Wishard Health Services (U10 HD27856, M01 RR750) – Brenda B. Poindexter, MD MS; Anna M. Dusick, MD; Leslie Dawn Wilson, BSN CCRC; Faithe Hamer, BS; Carolyn Lytle, MD MPH; Heike M. Minnich, PsyD HSPP.
RTI International (U10 HD36790) – W. Kenneth Poole, PhD; Dennis Wallace, PhD; Jamie E. Newman, PhD MPH; Jeanette O'Donnell Auman, BS; Margaret M. Crawford, BS CCRP; Carolyn M. Petrie Huitema, MS CCRP; Kristin M. Zaterka-Baxter, RN BSN CCRP.
Stanford University, Dominican Hospital, El Camino Hospital, and Lucile Packard Children's Hospital (U10 HD27880, M01 RR70) – Krisa P. Van Meurs, MD; David K. Stevenson, MD; Susan R. Hintz, MD MS Epi, Alexis S. Davis, MD MS Epi; M. Bethany Ball, BS CCRC; Andrew W. Palmquist, RN; Melinda S. Proud, RCP; Elizabeth Bruno, PhD; Maria Elena DeAnda, PhD; Anne M. DeBattista, RN PNP; Jean G. Kohn, MD MPH; Hali E.Weiss, MD.
Tufts Medical Center, Floating Hospital for Children (U10 HD53119, M01 RR54) – Ivan D. Frantz III, MD; John M. Fiascone, MD; Brenda L. MacKinnon, RNC; Anne Furey, MPH; Ellen Nylen, RN BSN; Elisabeth C. McGowan, MD.
University of Alabama at Birmingham Health System and Children's Hospital of Alabama (U10 HD34216, M01 RR32) – Waldemar A. Carlo, MD; Namasivayam Ambalavanan, MD; Myriam Peralta-Carcelen, MD MPH; Monica V. Collins, RN BSN MaEd; Shirley S. Cosby, RN BSN; Fred J. Biasini, PhD; Kristen C. Johnston, MSN CRNP; Kathleen G. Nelson, MD; Cryshelle S. Patterson, PhD; Vivien A. Phillips, RN BSN; Sally Whitley, MA OTR-L FAOTA.
University of California – San Diego Medical Center and Sharp Mary Birch Hospital for Women and Newborns (U10 HD40461) – Neil N. Finer, MD; Yvonne E. Vaucher, MD MPH; David Kaegi, MD; Maynard R. Rasmussen, MD; Kathy Arnell, RNC; Clarence Demetrio, RN; Martha G. Fuller, RN MSN; Wade Rich, BSHS RRT.
University of Iowa, Children's Hospital (U10 HD53109, M01 RR59) – Michael J. Acarregui, MD; Karen J. Johnson, RN BSN; Diane L. Eastman, RN CPNP MA.
University of Miami, Holtz Children's Hospital (U10 HD21397, M01 RR16587) – Shahnaz Duara, MD; Charles R. Bauer, MD; Ruth Everett-Thomas, RN MSN; Sylvia Fajardo-Hiriart, MD; Arielle Rigaud, MD; Maria Calejo, MS; Silvia M. Frade Eguaras, MA; Michelle Harwood Berkowits, PhD; Andrea Garcia, MS; Helina Pierre, BA; Alexandra Stoerger, BA.
University of New Mexico Health Sciences Center (U10 HD53089, M01 RR997) – Kristi L. Watterberg, MD; Jean R. Lowe, PhD; Janell F. Fuller, MD; Robin K. Ohls, MD; Conra Backstrom Lacy, RN; Rebecca Montman, BSN.
University of Rochester Medical Center, Golisano Children's Hospital (U10 HD40521, UL1 RR24160, M01 RR44) – Dale L. Phelps, MD; Gary J. Myers, MD; Linda J. Reubens, RN CCRC; Erica Burnell, RN; Diane Hust, MS RN CS; Julie Babish Johnson, MSW; Rosemary L. Jensen; Emily Kushner, MA; Joan Merzbach, LMSW; Kelley Yost, PhD; Lauren Zwetsch, RN MS PNP.
University of Texas Health Science Center at Houston Medical School, Children's Memorial Hermann Hospital, and Lyndon Baines Johnson General Hospital/Harris County Hospital District (U10 HD21373) – Kathleen A. Kennedy, MD MPH; Jon E. Tyson, MD MPH; Nora I. Alaniz, BS; Patricia W. Evans, MD; Charles Green, PhD; Beverly Foley Harris, RN BSN; Margarita Jiminez, MD MPH; Anna E. Lis, RN BSN; Sarah Martin, RN BSN; Georgia E. McDavid, RN; Brenda H. Morris, MD; M. Layne Poundstone, RN BSN; Saba Siddiki, MD; Maegan C. Simmons, RN; Patti L. Pierce Tate, RCP; Sharon L. Wright, MT(ASCP).
University of Texas Southwestern Medical Center at Dallas, Parkland Health & Hospital System, and Children's Medical Center Dallas (U10 HD40689, M01 RR633) – Pablo J. Sánchez, MD; Roy J. Heyne, MD; Walid A. Salhab, MD; Charles R. Rosenfeld, MD; Alicia Guzman; Melissa H. Leps, RN; Nancy A. Miller, RN; Gaynelle Hensley, RN; Sally S. Adams, MS RN CPNP; Linda A. Madden, RN CPNP; Elizabeth T. Heyne, MS MA PA-C PsyD; Janet S. Morgan, RN; Catherine Twell Boatman, MS CIMI; Lizette E. Torres, RN.
University of Utah Medical Center, Intermountain Medical Center, LDS Hospital, and Primary Children's Medical Center (U10 HD53124, M01 RR64, UL1 RR25764) – Roger G. Faix, MD; Bradley A. Yoder, MD; Karen A. Osborne, RN BSN CCRC; Cynthia Spencer, RNC; Kimberlee Weaver-Lewis, RN BSN; Shawna Baker, RN; Karie Bird, RN; Jill Burnett, RNC; Michael Steffen, MS CPM.
Wake Forest University, Baptist Medical Center, Forsyth Medical Center, and Brenner Children's Hospital (U10 HD40498, M01 RR7122) – T. Michael O'Shea, MD MPH; Robert G. Dillard, MD; Lisa K. Washburn, MD; Barbara G. Jackson, RN, BSN; Nancy Peters, RN; Korinne Chiu, MA; Deborah Evans Allred, MA LPA; Donald J. Goldstein, PhD; Raquel Halfond, MA; Carroll Peterson, MA; Ellen L. Waldrep, MS; Cherrie D. Welch, MD MPH; Melissa Whalen Morris, MA; Gail Wiley Hounshell, PhD.
Wayne State University, Hutzel Women's Hospital and Children's Hospital of Michigan (U10 HD21385) – Rebecca Bara, RN BSN; Laura A. Goldston, MA.
Yale University, Yale-New Haven Children's Hospital, and Bridgeport Hospital (U10 HD27871, UL1 RR24139, M01 RR125) – Richard A. Ehrenkranz, MD; Harris Jacobs, MD; Christine G. Butler, MD; Patricia Cervone, RN; Sheila Greisman, RN; Monica Konstantino, RN BSN; JoAnn Poulsen, RN; Janet Taft, RN BSN; Joanne Williams, RN BSN; Elaine Romano, MSN.
Funding Source: The National Institutes of Health and the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) provided grant support for the Neonatal Research Network Generic Data Base and Follow-up Studies.
List of abbreviations
- Bayley-III
Bayley Scales of Infant and Toddler Development-III
- BITSEA
Brief Infant-Toddler Social and Emotional Assessment
- BPD
bronchopulmonary dysplasia
- BW
birth weight
- CI
confidence interval
- CNS
central nervous system
- ELGAN
extremely low-gestational-age neonate
- EOS
early-onset sepsis
- FIRS
fetal inflammatory response syndrome
- GA
gestational age
- Histo
histological chorioamnionitis
- PIVH
periventricular-intraventricular hemorrhage
- LOS
late-onset sepsis
- LPS
lipopolysaccharide
- MDI
mental developmental index
- NDI
neurodevelopmental impairment
- NEC
necrotizing enterocolitis
- NICHD
National Institute of Child Health and Human Development
- NRN
Neonatal Research Network
- OR
odds ratio
- PDA
patent ductus arteriosus
- PROM
premature rupture of membranes
- PVL
periventricular leukomalacia
- ROP
retinopathy of prematurity
References
- 1.Duncan AF, Watterberg KL, Nolen TL, et al. Effect of ethnicity and race on cognitive and language testing at age 18-22 months in extremely preterm infants. The Journal of pediatrics. 2012 Jun;160(6):966–971 e962. doi: 10.1016/j.jpeds.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hack M, Wilson-Costello D, Friedman H, Taylor GH, Schluchter M, Fanaroff AA. Neurodevelopment and predictors of outcomes of children with birth weights of less than 1000 g: 1992-1995. Archives of pediatrics & adolescent medicine. 2000 Jul;154(7):725–731. doi: 10.1001/archpedi.154.7.725. [DOI] [PubMed] [Google Scholar]
- 3.Vohr BR, Stephens BE, Higgins RD, et al. Are outcomes of extremely preterm infants improving? Impact of Bayley assessment on outcomes. The Journal of pediatrics. 2012 Aug;161(2):222–228 e223. doi: 10.1016/j.jpeds.2012.01.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nelson KB. Infection in pregnancy and cerebral palsy. Developmental medicine and child neurology. 2009 Apr;51(4):253–254. doi: 10.1111/j.1469-8749.2008.03256.x. [DOI] [PubMed] [Google Scholar]
- 5.Gotsch F, Romero R, Kusanovic JP, et al. The fetal inflammatory response syndrome. Clinical obstetrics and gynecology. 2007 Sep;50(3):652–683. doi: 10.1097/GRF.0b013e31811ebef6. [DOI] [PubMed] [Google Scholar]
- 6.Wu YW, Colford JM., Jr Chorioamnionitis as a risk factor for cerebral palsy: A meta-analysis. JAMA : the journal of the American Medical Association. 2000 Sep 20;284(11):1417–1424. doi: 10.1001/jama.284.11.1417. [DOI] [PubMed] [Google Scholar]
- 7.Suppiej A, Franzoi M, Vedovato S, Marucco A, Chiarelli S, Zanardo V. Neurodevelopmental outcome in preterm histological chorioamnionitis. Early human development. 2009 Mar;85(3):187–189. doi: 10.1016/j.earlhumdev.2008.09.410. [DOI] [PubMed] [Google Scholar]
- 8.Rovira N, Alarcon A, Iriondo M, et al. Impact of histological chorioamnionitis, funisitis and clinical chorioamnionitis on neurodevelopmental outcome of preterm infants. Early human development. 2011 Apr;87(4):253–257. doi: 10.1016/j.earlhumdev.2011.01.024. [DOI] [PubMed] [Google Scholar]
- 9.Leviton A, Allred EN, Kuban KC, et al. Microbiologic and histologic characteristics of the extremely preterm infant's placenta predict white matter damage and later cerebral palsy. the ELGAN study. Pediatric research. 2010 Jan;67(1):95–101. doi: 10.1203/PDR.0b013e3181bf5fab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hendson L, Russell L, Robertson CM, et al. Neonatal and neurodevelopmental outcomes of very low birth weight infants with histologic chorioamnionitis. The Journal of pediatrics. 2011 Mar;158(3):397–402. doi: 10.1016/j.jpeds.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 11.Mu SC, Lin CH, Sung TC, et al. Neurodevelopmental outcome of very-low-birth-weight infants with chorioamnionitis. Acta paediatrica Taiwanica = Taiwan er ke yi xue hui za zhi. 2007 Jul-Aug;48(4):207–212. [PubMed] [Google Scholar]
- 12.Polam S, Koons A, Anwar M, Shen-Schwarz S, Hegyi T. Effect of chorioamnionitis on neurodevelopmental outcome in preterm infants. Archives of pediatrics & adolescent medicine. 2005 Nov;159(11):1032–1035. doi: 10.1001/archpedi.159.11.1032. [DOI] [PubMed] [Google Scholar]
- 13.Botet F, Figueras J, Carbonell-Estrany X, Narbona E. The impact of clinical maternal chorioamnionitis on neurological and psychological sequelae in very-low-birth weight infants: a case-control study. Journal of perinatal medicine. 2011 Mar;39(2):203–208. doi: 10.1515/jpm.2011.005. [DOI] [PubMed] [Google Scholar]
- 14.Andrews WW, Cliver SP, Biasini F, et al. Early preterm birth: association between in utero exposure to acute inflammation and severe neurodevelopmental disability at 6 years of age. American journal of obstetrics and gynecology. 2008 Apr;198(4):466 e461–466 e411. doi: 10.1016/j.ajog.2007.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yoon BH, Romero R, Moon J, et al. Differences in the fetal interleukin-6 response to microbial invasion of the amniotic cavity between term and preterm gestation. The journal of maternal-fetal & neonatal medicine : the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2003 Jan;13(1):32–38. doi: 10.1080/jmf.13.1.32.38. [DOI] [PubMed] [Google Scholar]
- 16.Azizia M, Lloyd J, Allen M, Klein N, Peebles D. Immune status in very preterm neonates. Pediatrics. 2012 Apr;129(4):e967–974. doi: 10.1542/peds.2011-1579. [DOI] [PubMed] [Google Scholar]
- 17.Rozycki HJ, Comber PG, Huff TF. Cytokines and oxygen radicals after hyperoxia in preterm and term alveolar macrophages. American journal of physiology Lung cellular and molecular physiology. 2002 Jun;282(6):L1222–1228. doi: 10.1152/ajplung.00230.2001. [DOI] [PubMed] [Google Scholar]
- 18.Brochu ME, Girard S, Lavoie K, Sebire G. Developmental regulation of the neuroinflammatory responses to LPS and/or hypoxia-ischemia between preterm and term neonates: An experimental study. Journal of neuroinflammation. 2011;8:55. doi: 10.1186/1742-2094-8-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leviton A, Blair E, Dammann O, Allred E. The wealth of information conveyed by gestational age. The Journal of pediatrics. 2005 Jan;146(1):123–127. doi: 10.1016/j.jpeds.2004.09.028. [DOI] [PubMed] [Google Scholar]
- 20.Langston C, Kaplan C, Macpherson T, et al. Practice guideline for examination of the placenta: developed by the Placental Pathology Practice Guideline Development Task Force of the College of American Pathologists. Archives of pathology & laboratory medicine. 1997 May;121(5):449–476. [PubMed] [Google Scholar]
- 21.Chaiworapongsa T, Romero R, Kim JC, et al. Evidence for fetal involvement in the pathologic process of clinical chorioamnionitis. American journal of obstetrics and gynecology. 2002 Jun;186(6):1178–1182. doi: 10.1067/mob.2002.124042. [DOI] [PubMed] [Google Scholar]
- 22.Stoll BJ, Hansen NI, Bell EF, et al. Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010 Sep;126(3):443–456. doi: 10.1542/peds.2009-2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newman JE, Bann CM, Vohr BR, Dusick AM, Higgins RD. Improving the neonatal research network annual certification for neurologic examination of the 18-22 month child. The Journal of pediatrics. 2012 Dec;161(6):1041–1046 e1042. doi: 10.1016/j.jpeds.2012.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstetrics and gynecology. 1996 Feb;87(2):163–168. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 25.Pinar H, Koch MA, Hawkins H, et al. The Stillbirth Collaborative Research Network (SCRN) Placental and Umbilical Cord Examination Protocol. American journal of perinatology. 2011 Dec;28(10):781–792. doi: 10.1055/s-0031-1281509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walsh MC, Yao Q, Gettner P, et al. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics. 2004 Nov;114(5):1305–1311. doi: 10.1542/peds.2004-0204. [DOI] [PubMed] [Google Scholar]
- 27.Payne AH, Hintz SR, Hibbs AM, et al. Neurodevelopmental Outcomes of Extremely Low-Gestational-Age Neonates With Low-Grade Periventricular-Intraventricular Hemorrhage. JAMA pediatrics. 2013 Mar 4;:1–9. doi: 10.1001/jamapediatrics.2013.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walsh MC, Kliegman RM. Necrotizing enterocolitis: treatment based on staging criteria. Pediatric clinics of North America. 1986 Feb;33(1):179–201. doi: 10.1016/S0031-3955(16)34975-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bayley N. Bayley scales of infant development-3rd edition. San Antonio, TX: The Psychological Corporation; 2006. [Google Scholar]
- 30.Briggs-Gowan MJCA. Brief Infant-Toddler Social and Emotional Assessment (BITSEA) Manual, version 2. New Haven, CT: Yale University; 2002. [Google Scholar]
- 31.Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Developmental medicine and child neurology. 1997 Apr;39(4):214–223. doi: 10.1111/j.1469-8749.1997.tb07414.x. [DOI] [PubMed] [Google Scholar]
- 32.Krishnan L, Nguyen T, McComb S. From mice to women: the conundrum of immunity to infection during pregnancy. Journal of reproductive immunology. 2013 Mar;97(1):62–73. doi: 10.1016/j.jri.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chaudhry A, Rudra D, Treuting P, et al. CD4+ regulatory T cells control TH17 responses in a Stat3-dependent manner. Science. 2009 Nov 13;326(5955):986–991. doi: 10.1126/science.1172702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lahra MM, Jeffery HE. A fetal response to chorioamnionitis is associated with early survival after preterm birth. American journal of obstetrics and gynecology. 2004 Jan;190(1):147–151. doi: 10.1016/j.ajog.2003.07.012. [DOI] [PubMed] [Google Scholar]
- 35.Wu YW, Escobar GJ, Grether JK, Croen LA, Greene JD, Newman TB. Chorioamnionitis and cerebral palsy in term and near-term infants. JAMA : the journal of the American Medical Association. 2003 Nov 26;290(20):2677–2684. doi: 10.1001/jama.290.20.2677. [DOI] [PubMed] [Google Scholar]
- 36.Dexter SC, Pinar H, Malee MP, Hogan J, Carpenter MW, Vohr BR. Outcome of very low birth weight infants with histopathologic chorioamnionitis. Obstetrics and gynecology. 2000 Aug;96(2):172–177. doi: 10.1016/s0029-7844(00)00886-3. [DOI] [PubMed] [Google Scholar]
- 37.Dempsey E, Chen MF, Kokottis T, Vallerand D, Usher R. Outcome of neonates less than 30 weeks gestation with histologic chorioamnionitis. American journal of perinatology. 2005 Apr;22(3):155–159. doi: 10.1055/s-2005-865020. [DOI] [PubMed] [Google Scholar]
- 38.Strunk T, Doherty D, Jacques A, et al. Histologic chorioamnionitis is associated with reduced risk of late-onset sepsis in preterm infants. Pediatrics. 2012 Jan;129(1):e134–141. doi: 10.1542/peds.2010-3493. [DOI] [PubMed] [Google Scholar]
- 39.O'Shea TM, Allred EN, Kuban KC, et al. Elevated concentrations of inflammation-related proteins in postnatal blood predict severe developmental delay at 2 years of age in extremely preterm infants. The Journal of pediatrics. 2012 Mar;160(3):395–401 e394. doi: 10.1016/j.jpeds.2011.08.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eklind S, Mallard C, Arvidsson P, Hagberg H. Lipopolysaccharide induces both a primary and a secondary phase of sensitization in the developing rat brain. Pediatric research. 2005 Jul;58(1):112–116. doi: 10.1203/01.PDR.0000163513.03619.8D. [DOI] [PubMed] [Google Scholar]
- 41.Furuya K, Zhu L, Kawahara N, Abe O, Kirino T. Differences in infarct evolution between lipopolysaccharide-induced tolerant and nontolerant conditions to focal cerebral ischemia. Journal of neurosurgery. 2005 Oct;103(4):715–723. doi: 10.3171/jns.2005.103.4.0715. [DOI] [PubMed] [Google Scholar]
- 42.Ikeda T, Yang L, Ikenoue T, Mallard C, Hagberg H. Endotoxin-induced hypoxic-ischemic tolerance is mediated by up-regulation of corticosterone in neonatal rat. Pediatric research. 2006 Jan;59(1):56–60. doi: 10.1203/01.pdr.0000191140.87314.ce. [DOI] [PubMed] [Google Scholar]
- 43.Bordet R, Deplanque D, Maboudou P, et al. Increase in endogenous brain superoxide dismutase as a potential mechanism of lipopolysaccharide-induced brain ischemic tolerance. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2000 Aug;20(8):1190–1196. doi: 10.1097/00004647-200008000-00004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


