Abstract
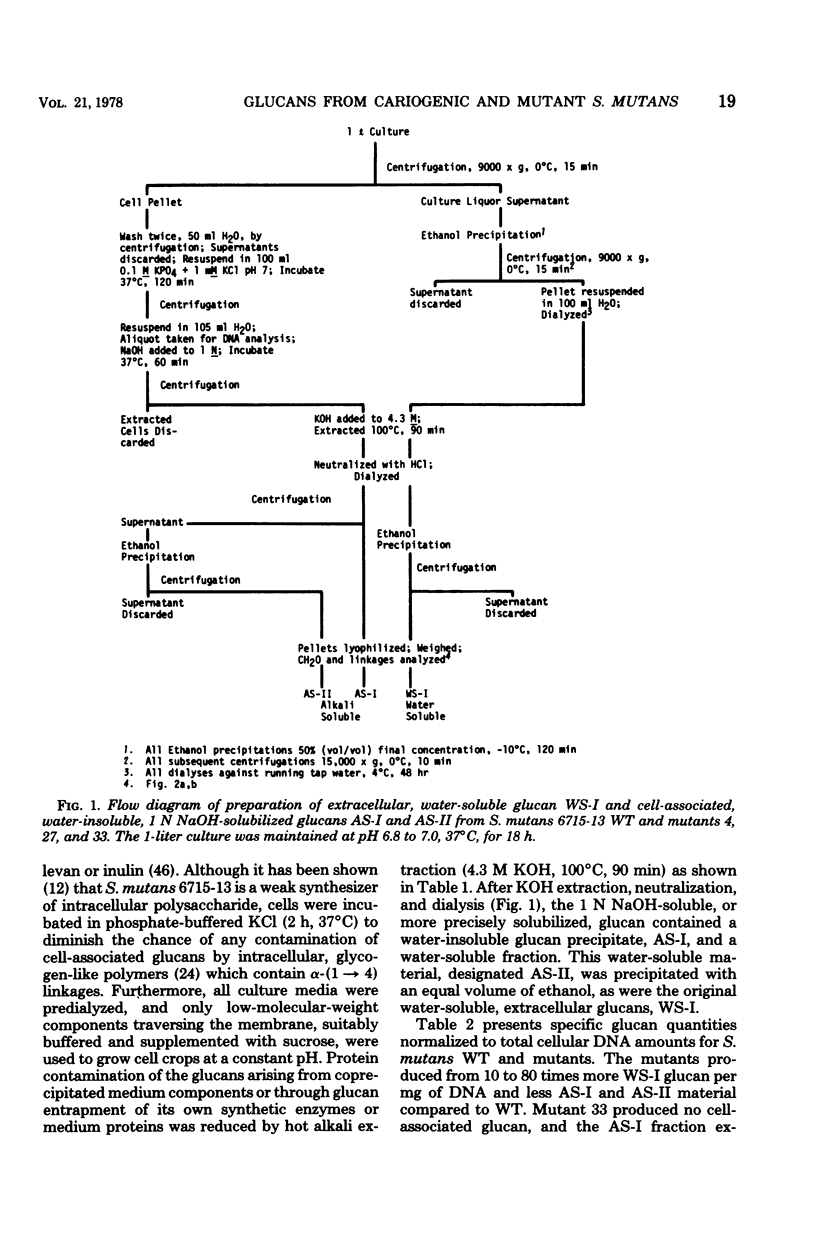
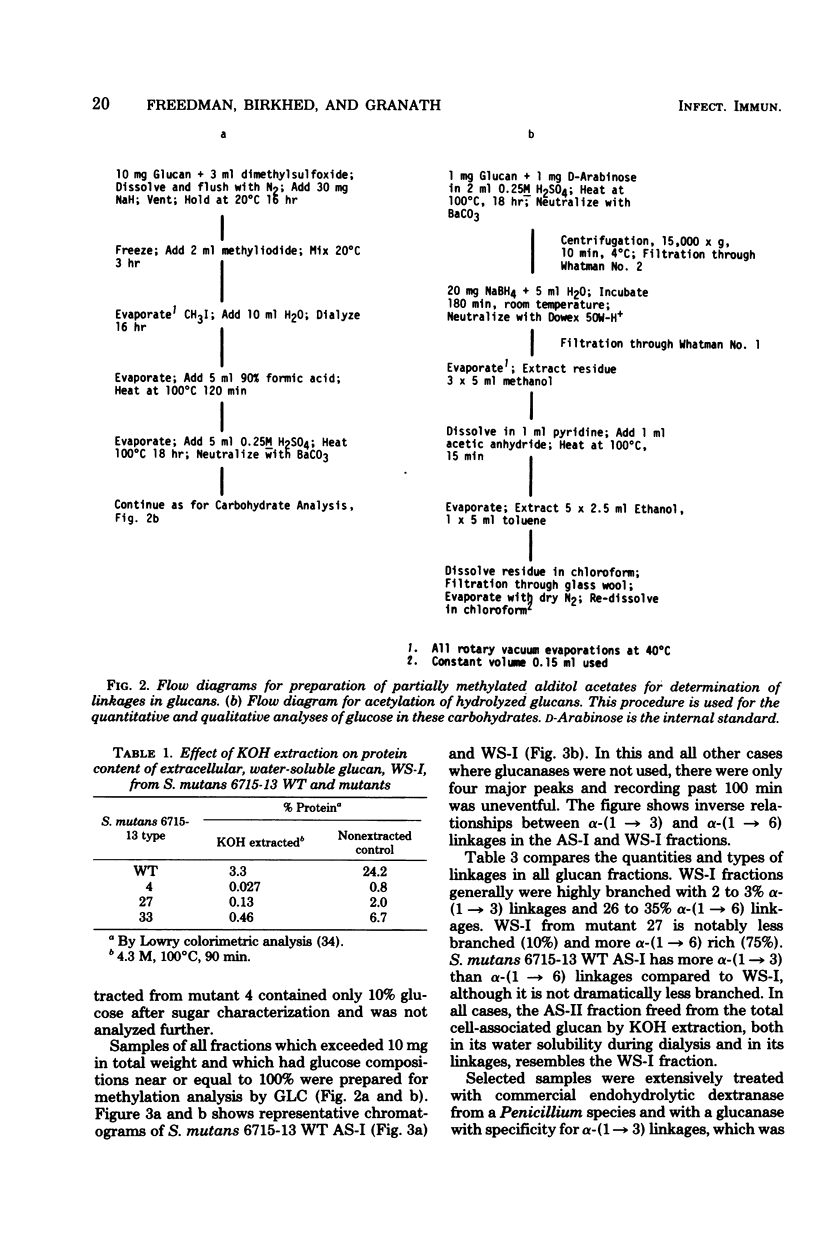
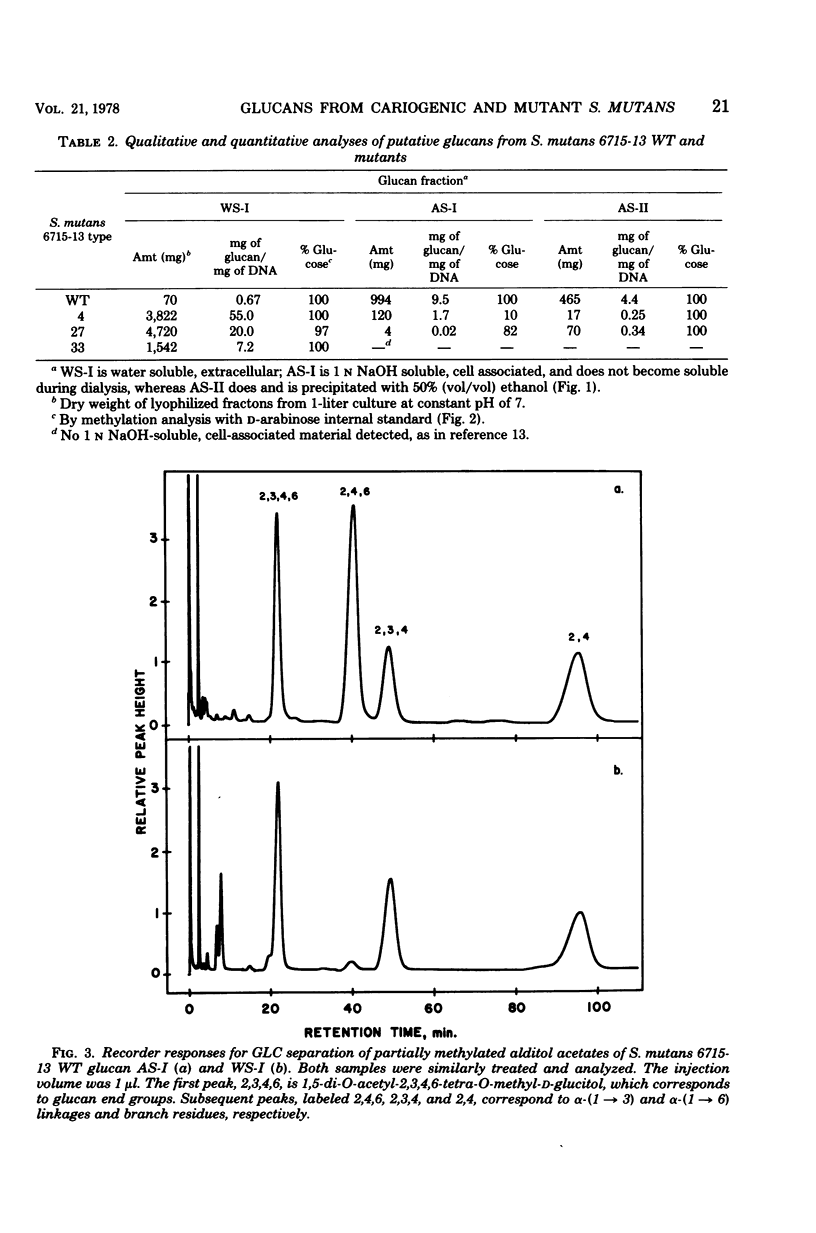
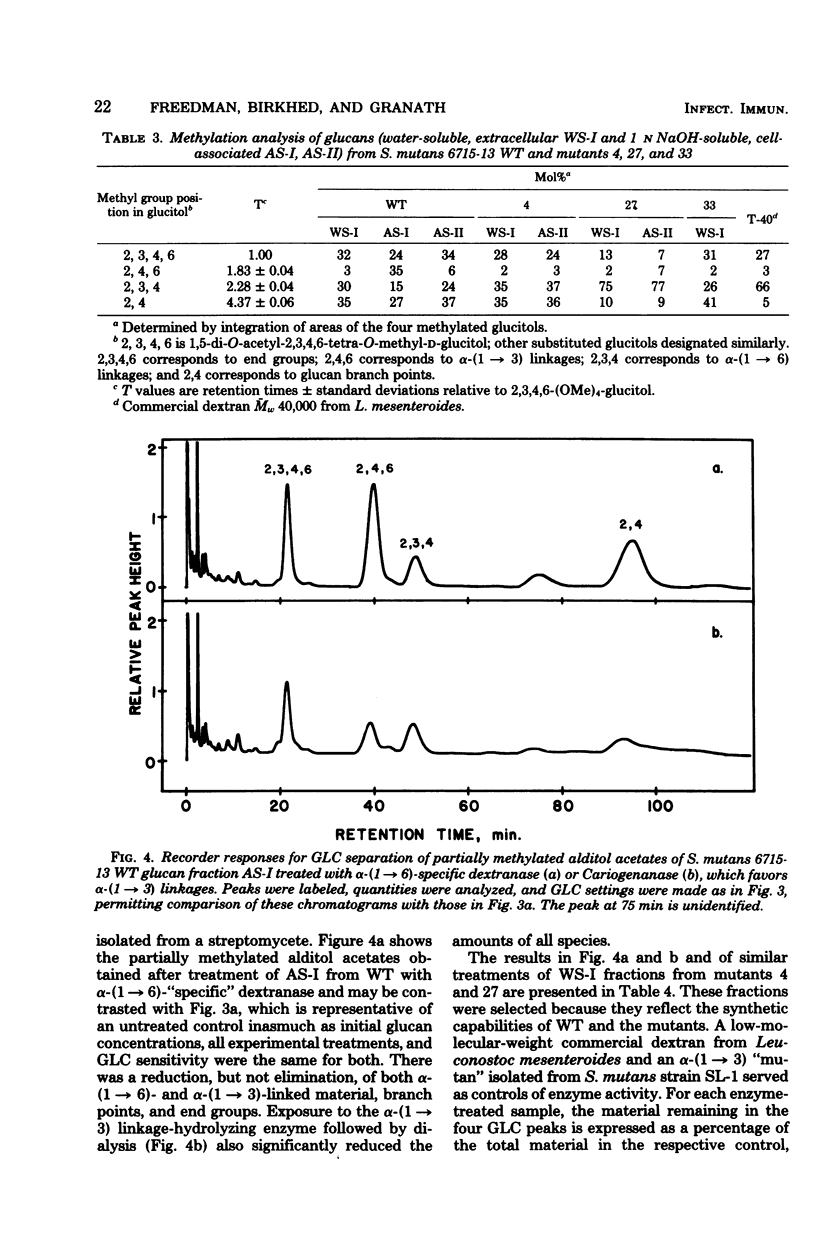
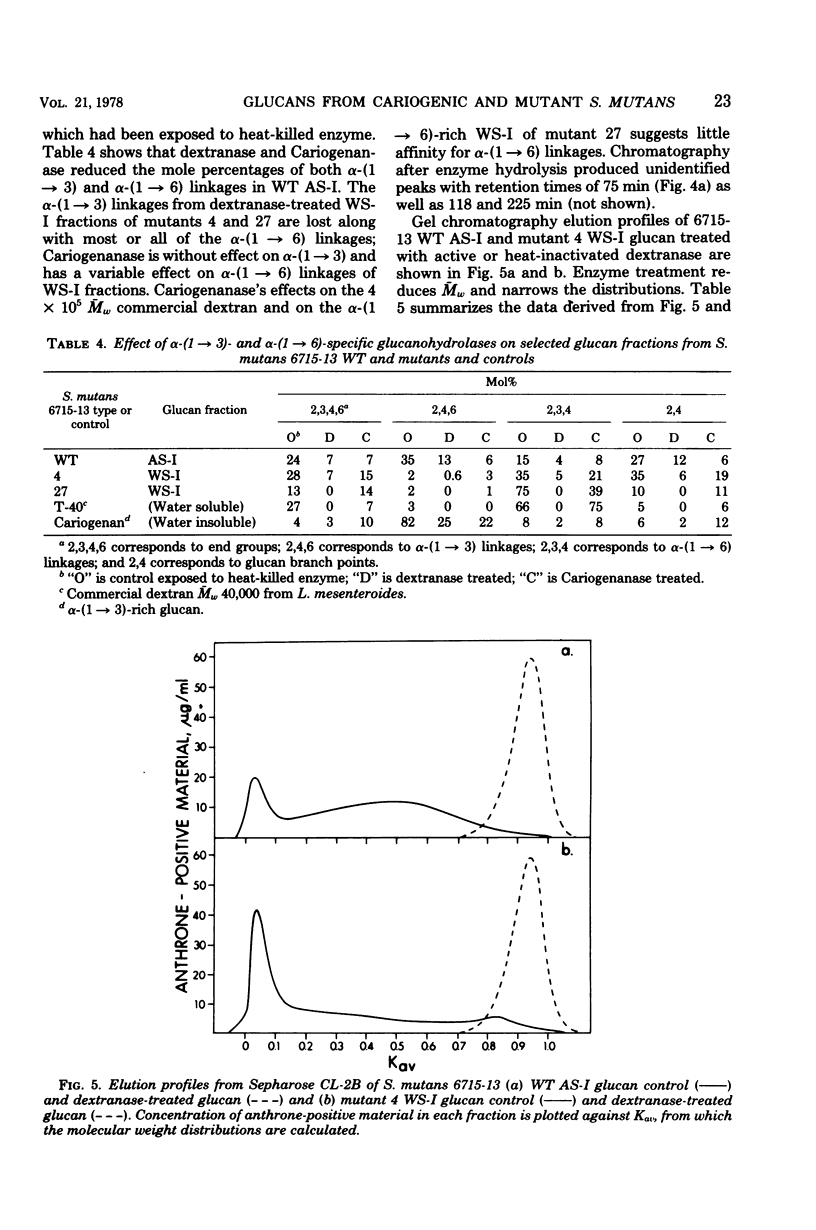
The extracellular, water-soluble and cell-associated, 1 N NaOH-soluble glucans from cariogenic Streptococcus mutans 6715-13 “wild type” (WT) and glucan synthesis-defective mutants with diminished virulence have been quantitatively and qualitatively analyzed by methylation analysis and gel chromatography. The mutants synthesized more of a highly branched α-(1 → 6)-rich extracellular polymer than WT, and some of this glucan was also found to be cell associated in all but one case. WT, in distinction to the mutants, also synthesized a highly branched, α-(1 → 3)-rich, cell-associated polymer. Treatment of these two distinct polymer types with dextranase or an α-(1 → 3)-hydrolyzing enzyme indicated they were composed of both α-(1 → 3) and α-(1 → 6) linkages and of α-(1 → 6) with branches at the 3-position, rather than of separate α-(1 → 3) and α-(1 → 6) homopolymer mixtures. Gel chromatography before enzymatic hydrolysis disclosed a high degree of polydispersity in both glucan classes. After hydrolysis polydispersity was reduced, again without resolution of two glucan populations. These findings suggest that (i) there are two distinct glucan classes, one α-(1 → 3) rich and the other α-(1 → 6) rich in WT, (ii) diminution of virulence in the mutants is probably ascribable to a failure to form the α-(1 → 3)-rich component, (iii) both α-(1 → 6)- and α-(1 → 3)-rich glucans are found in association with the cell, and (iv) both highly branched glucan types are dextranase and α-(1 → 3)-hydrolase sensitive, and methylation analysis and gel chromatography suggest polymers with highly polydisperse molecular weights which contain mixtures of linkage types.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arturson G., Granath K. Dextrans as test molecules in studies of the functional ultrastrucutre of biological membranes. Molecular weight distribution analysis by gel chromatography. Clin Chim Acta. 1972 Mar;37:309–322. doi: 10.1016/0009-8981(72)90450-0. [DOI] [PubMed] [Google Scholar]
- Baird J. K., Longyear V. M., Ellwood D. C. Water insoluble and soluble glucans produced by extracellular glycosyltransferases from Streptococcus mutans. Microbios. 1973 Sep-Oct;8(30):143–150. [PubMed] [Google Scholar]
- Birkhed D., Rosell K. G. Structural studies on polysaccharides synthetised from sucrose by soluble enzymes in human dental plaque material. Odontol Revy. 1975;26(4):281–290. [PubMed] [Google Scholar]
- Ceska M., Granath K., Norrman B., Guggenheim B. Structural and enzymatic studies on glucans synthesized with glucosyltransferases of some strains of oral streptococci. Acta Chem Scand. 1972;26(6):2223–2230. doi: 10.3891/acta.chem.scand.26-2223. [DOI] [PubMed] [Google Scholar]
- Chludzinski A. M., Germaine G. R., Schachtele C. F. Purification and properties of dextransucrase from Streptococcus mutans. J Bacteriol. 1974 Apr;118(1):1–7. doi: 10.1128/jb.118.1.1-7.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chludzinski A. M., Germaine G. R., Schachtele C. F. Streptoccus mutans dextransucrase: purification, properties, and requirement for primer dextran. J Dent Res. 1976 Apr;55(Spec No):C75–C86. doi: 10.1177/002203457605500329011. [DOI] [PubMed] [Google Scholar]
- Ciardi J. E., Beaman A. J., Wittenberger C. L. Purification, resolution, and interaction of the glucosyltransferases of Streptococcus mutans 6715. Infect Immun. 1977 Oct;18(1):237–246. doi: 10.1128/iai.18.1.237-246.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciardi J. E., Hageage G. J., Jr, Wittenberger C. L. Multicomponent nature of the glucosyltransferase system of Streptococcus mutans. J Dent Res. 1976 Apr;55(Spec No):C87–C96. doi: 10.1177/002203457605500330011. [DOI] [PubMed] [Google Scholar]
- Critchley P., Wood J. M., Saxton C. A., Leach S. A. The polymerisation of dietary sugars by dental plaque. Caries Res. 1967;1(2):112–129. doi: 10.1159/000259506. [DOI] [PubMed] [Google Scholar]
- Ebisu S., Misaki A., Kato K., Kotani S. The structure of water-insoluble glucans of cariogenic Streptococcus mutans, formed in the absence and presence of dextranase. Carbohydr Res. 1974 Dec;38:374–381. doi: 10.1016/s0008-6215(00)82375-7. [DOI] [PubMed] [Google Scholar]
- Freedman M. L., Coykendall A. L. Variation in internal polysaccharide synthesis among Streptococcus mutans strains. Infect Immun. 1975 Sep;12(3):475–479. doi: 10.1128/iai.12.3.475-479.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman M. L., Tanzer J. M. Dissociation of plaque formation from glucan-induced agglutination in mutants of Streptococcus mutans. Infect Immun. 1974 Jul;10(1):189–196. doi: 10.1128/iai.10.1.189-196.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui K., Fukui Y., Moriyama T. Purification and properties of dextransucrase and invertase from Streptococcus mutans. J Bacteriol. 1974 Jun;118(3):796–804. doi: 10.1128/jb.118.3.796-804.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Banghart S. B. Synthesis of extracellular dextran by cariogenic bacteria and its presence in human dental plaque. Arch Oral Biol. 1967 Jan;12(1):11–23. doi: 10.1016/0003-9969(67)90137-9. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Houte J. V. Bacterial adherence in oral microbial ecology. Annu Rev Microbiol. 1975;29:19–44. doi: 10.1146/annurev.mi.29.100175.000315. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Nygaard M. Synthesis of insoluble dextran and its significance in the formation of gelatinous deposits by plaque-forming streptococci. Arch Oral Biol. 1968 Oct;13(10):1249–1262. doi: 10.1016/0003-9969(68)90081-2. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., van Houte J. On the formation of dental plaques. J Periodontol. 1973 Jun;44(6):347–360. doi: 10.1902/jop.1973.44.6.347. [DOI] [PubMed] [Google Scholar]
- Guggenheim B., Burckhardt J. J. Isolation and properties of a dextranase from streptococcus mutans OMZ 176. Helv Odontol Acta. 1974 Oct;18(2):101–113. [PubMed] [Google Scholar]
- Guggenheim B. Enzymatic hydrolysis and structure of water-insoluble glucan produced by glucosyltransferases from a strain of streptococcus mutans. Helv Odontol Acta. 1970 Nov;14(Suppl):89+–89+. [PubMed] [Google Scholar]
- Guggenheim B. Extracellular polysaccharides and microbial plaque. Int Dent J. 1970 Dec;20(4):657–678. [PubMed] [Google Scholar]
- Guggenheim B., Newbrun E. Extracellular glucosyltransferase activity of an HS strain of Streptococcus mutans. Helv Odontol Acta. 1969 Oct;13(2):84–97. [PubMed] [Google Scholar]
- Guggenheim B., Schroeder H. E. Biochemical and morphological aspects of extracellular polysaccharides produced by cariogenic streptococci. Helv Odontol Acta. 1967 Oct;11(2):131–152. [PubMed] [Google Scholar]
- Hotz P., Guggenheim B., Schmid R. Carbohydrates in pooled dental plaque. Caries Res. 1972;6(2):103–121. doi: 10.1159/000259783. [DOI] [PubMed] [Google Scholar]
- JORDAN H. V., FITZGERALD R. J., BOWLER A. E. Inhibition of experimental caries by sodium metabisulfite and its effect on the growth and metabolism of selected bacteria. J Dent Res. 1960 Jan-Feb;39:116–123. doi: 10.1177/00220345600390010501. [DOI] [PubMed] [Google Scholar]
- Johnson M. C., Bozzola J. J., Shechmeister I. L., Shklair I. L. Biochemical study of the relationship of extracellular glucan to adherence and cariogenicity in Streptococcus mutans and an extracellular polysaccharide mutant. J Bacteriol. 1977 Jan;129(1):351–357. doi: 10.1128/jb.129.1.351-357.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelstrup J., Funder-Nielsen T. D. Adhesion of dextran to Streptococcus mutans. J Gen Microbiol. 1974 Apr;81(2):485–489. doi: 10.1099/00221287-81-2-485. [DOI] [PubMed] [Google Scholar]
- Kuramitsu H. K. Characterization of cell-associated dextransucrase activity from glucose-grown cells of Streptococcus mutans. Infect Immun. 1974 Jul;10(1):227–235. doi: 10.1128/iai.10.1.227-235.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Long L. W., Edwards J. R. Detailed structure of a dextran from a cariogenic bacterium. Carbohydr Res. 1972 Sep;24(1):216–217. doi: 10.1016/s0008-6215(00)82285-5. [DOI] [PubMed] [Google Scholar]
- McCabe M. M., Smith E. E. Specific method for the purification of Streptococcus mutans dextransucrase. Infect Immun. 1977 Jun;16(3):760–765. doi: 10.1128/iai.16.3.760-765.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalek S. M., Shiota T., Ikeda T., Navia J. M., McGhee J. R. Virulence of Streptococcus mutans: biochemical and pathogenic characteristics of mutant isolates. Proc Soc Exp Biol Med. 1975 Nov;150(2):498–502. doi: 10.3181/00379727-150-39064. [DOI] [PubMed] [Google Scholar]
- Nalbandian J., Freedman M. L., Tanzer J. M., Lovelace S. M. Ultrastructure of Mutants of Streptococcus mutans with Reference to Agglutination, Adhesion, and Extracellular Polysaccharide. Infect Immun. 1974 Nov;10(5):1170–1179. doi: 10.1128/iai.10.5.1170-1179.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbrun E. Extracellular polysaccharides synthesized by glucosyltransferases of oral streptococci. Composition and susceptibility to hydrolysis. Caries Res. 1972;6(2):132–147. doi: 10.1159/000259785. [DOI] [PubMed] [Google Scholar]
- Nilsson G., Nilsson K. Molecular-weight distribution determination of clinical dextran by gel permeation chromatography. J Chromatogr. 1974 Dec 4;101(1):137–153. doi: 10.1016/s0021-9673(01)94739-9. [DOI] [PubMed] [Google Scholar]
- Nisizawa T., Imai S., Akada H., Hinoide M., Araya S. Extracellular glucans produced by oral streptococci. Arch Oral Biol. 1976;21(3):207–213. doi: 10.1016/0003-9969(76)90131-x. [DOI] [PubMed] [Google Scholar]
- Nisizawa T., Imai S., Araya S. Methylation analysis of extracellular glucans produced by Streptococcus mutans strain JC 2. Arch Oral Biol. 1977;22(4):281–285. doi: 10.1016/0003-9969(77)90114-5. [DOI] [PubMed] [Google Scholar]
- Robyt J. F., Corrigan A. J. The mechanism of dextransucrase action. Activation of dextransucrase from Streptococcus mutans OMZ 176 by dextran and modified dextran and the nonexistence of the primar requirement for the synthesis of dextran. Arch Biochem Biophys. 1977 Oct;183(2):726–731. doi: 10.1016/0003-9861(77)90406-4. [DOI] [PubMed] [Google Scholar]
- Robyt J. F., Taniguchi H. The mechanism of dextransucrase action. Biosynthesis of branch linkages by acceptor reactions with dextran. Arch Biochem Biophys. 1976 May;174(1):129–135. doi: 10.1016/0003-9861(76)90331-3. [DOI] [PubMed] [Google Scholar]
- Rosell K. G., Birkhed D. An inulin-like fructan produced by Streptococcus mutans, strain JC2. Acta Chem Scand B. 1974;28(5):589–589. doi: 10.3891/acta.chem.scand.28b-0589. [DOI] [PubMed] [Google Scholar]
- Schachtele C. F., Germaine G. R., Harlander S. K. Production of elevated levels of dextransucrase by a mutant of Streptococcus mutans. Infect Immun. 1975 Oct;12(4):934–937. doi: 10.1128/iai.12.4.934-937.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidebotham R. L. Dextrans. Adv Carbohydr Chem Biochem. 1974;30:371–444. doi: 10.1016/s0065-2318(08)60268-1. [DOI] [PubMed] [Google Scholar]
- Tanzer J. M., Freedman M. L., Fitzgerald R. J., Larson R. H. Diminished virulence of glucan synthesis-defective mutants of Streptococcus mutans. Infect Immun. 1974 Jul;10(1):197–203. doi: 10.1128/iai.10.1.197-203.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]


