Unit Introduction
The ability to store and recall our life experiences defines a person's identity. Consequently, the loss of long-term memory is a particularly devastating part of a variety of cognitive disorders, diseases and injuries. There is a great need to develop therapeutics to treat memory disorders, and thus a variety of animal models and memory paradigms have been developed. Mouse models have been widely used both to study basic disease mechanisms and to evaluate potential drug targets for therapeutic development. The relative ease of genetic manipulation of Mus musculus has led to a wide variety of genetically altered mice that model cognitive disorders ranging from Alzheimer's disease to autism. Rodents, including mice, are particularly adept at encoding and remembering spatial relationships, and these long-term spatial memories are dependent on the medial temporal lobe of the brain. These brain regions are also some of the first and most heavily impacted in disorders of human memory including Alzheimer's disease. Consequently, some of the simplest and most commonly used tests of long-term memory in mice are those that examine memory for objects and spatial relationships. However, many of these tasks, such as Morris water maze and contextual fear conditioning, are dependent upon the encoding and retrieval of emotionally aversive and inherently stressful training events. While these types of memories are important, they do not reflect the typical day-to-day experiences or memories most commonly affected in human disease. In addition, stress hormone release alone can modulate memory and thus obscure or artificially enhance these types of tasks. To avoid these sorts of confounds, we and many others have utilized tasks testing animals’ memory for object location and novel object recognition. These tasks involve exploiting rodents’ innate preference for novelty, and are inherently not stressful. In this protocol we detail how memory for object location and object identity can be used to evaluate a wide variety of mouse models and treatments.
Introduction
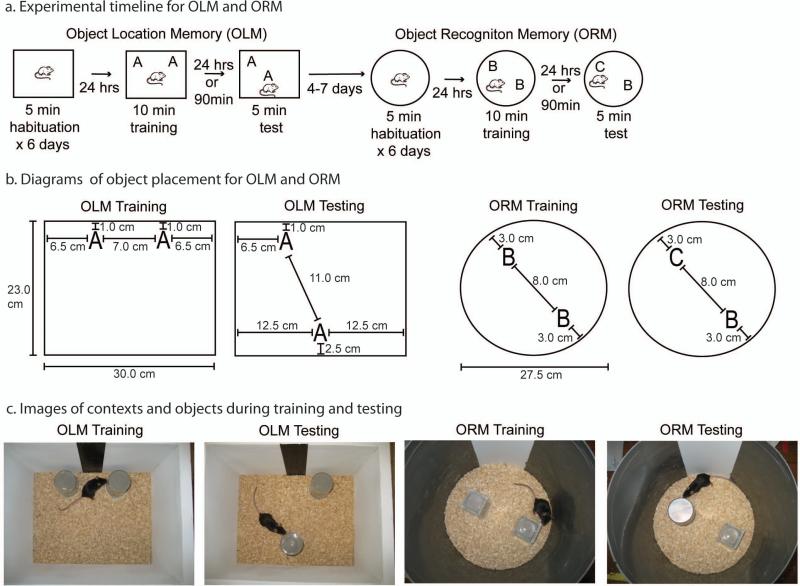
The Object Recognition (ORM) and Object Location Memory (OLM) (see Figure 1) tasks have been widely used in the study of the neurobiological mechanisms underlying long-term memory formation, both by our lab and others (Barrett et al., 2011; McQuown et al., 2011; Haettig et al., 2011; Vogel-Ciernia et al., 2013; McNulty et al., 2012; Stefanko et al., 2009; Balderas et al., 2008; Rossato et al., 2007; Akirav and Maroun, 2006; Murai et al., 2007; Assini et al., 2009; Roozendaal et al., 2010). This unit provides a detailed explanation of the steps involved in conducting both ORM and OLM tasks in adult mice. Both tasks involve handling the animals, habituating them to the empty training arena, training with two objects and then testing with two objects (Figure 1A). The major difference between ORM and OLM occurs on the day of testing, when for OLM one object is moved to a novel location, and for ORM one object is replaced with a novel object. The main measure for both tasks is time spent in exploration of the two objects at test. Both tasks rely on a rodent's innate preference for novelty. Animals that remember the original training experience will preferentially explore the displaced object relative to the non-displaced object (OLM), or the novel object relative to the familiar object (ORM). The exploration times are then used to calculate a discrimination index that is compared across experimental conditions. Both memory impairments and memory enhancements can be examined in these tasks by altering either the training duration or the time of testing (Stefanko et al., 2009). Both tasks can also be performed in the same experimental group of animals by the inclusion of a second, unique training chamber and object set (see Figure 1 and 2). When combined correctly, these two tasks can allow users to address a variety of experimental questions involving manipulations to different brain regions and molecular targets.
FIGURE 1.
Object location and object recognition memory task design. (A) Experimental timeline for Object Location Memory (OLM) followed by Object Recognition Memory (ORM) in the same cohort of animals. (B) Diagrams of context and object placement for OLM and ORM. For OLM the right object is shown as the displaced object during testing. In actual experiments the left object is moved for half the animals and the right object is moved for the other half. Similarly, for ORM either the right or left object can be replaced with the novel object at test. The height of the contexts are 23 cm. (C) Images of the actual experimental setup for OLM and ORM. The mouse shown in the testing apparatus is a C57/Bl6J male mouse age 9 weeks. Note that the objects are all filled with gray cement. For ORM, the training condition uses two identical glass candle holders flipped upside down. In an actual experiment, half of the animals would be trained with two tins. For the ORM test, the animals receive one of each object with the location of the novel object counterbalanced across groups.
FIGURE 2.
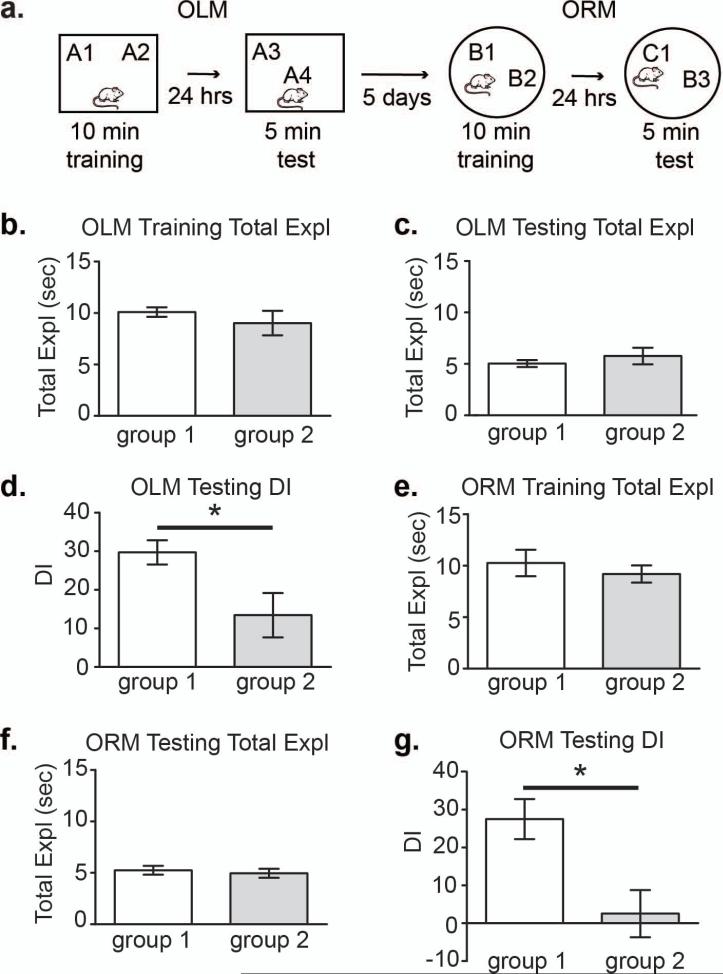
Anticipated results for a typical OLM and ORM sequential experiment. (A) Schematic of behavioral testing. OLM and ORM were then conducted sequentially, as described in the methods, for two independent groups of animals. (B) Total exploration (object 1 and 2) during a 10 min OLM training session (n = 10 animals per group). There was no difference between groups 1 and 2: t(18) = 0.84, p = 0.41. (C) Total exploration (object 1 and 2) duringa5minOLM testing session.Therewas no difference between groups 1 and 2: t(18)= 0.84, p = 0.41. (D) Discrimination Index (DI) for the 5 min OLM testing session. There was a significant difference between groups 1 and 2: t(18) = 2.49, p = 0.02. (E) Total exploration (object 1 and 2) during 10 min ORM training session (n = seven to nine animals per group). There was no difference between groups 1 and 2: t(14) = 0.73, p = 0.48. (F) Total exploration (object 1 and 2) during 5 min ORM testing session. There was no difference between groups 1 and 2: t(14) = 0.47, p = 0.64. (G) Discrimination Index (DI) for the 5 min ORM testing session. There was a significant difference between groups 1 and 2: t(14) = 2.94, p = 0.01. * indicates significant difference at p < 0.05. Portions of this data were previously included in Vogel-Ciernia et al. (2013). Reprinted with the author's permission.
Materials List
Subject mice: e.g., C57BL/6J (B6) age 8 wks to 6 months
10% ethanol (v/v in water) 70% ethanol (v/v in water)
Paper towels
Marking pen (dark)
Test room, with minimal cues visible to the subject
Two (or more) gooseneck desk lamps with incandescent 75-watt light bulbs
Lux meter (Fisher Scientific)
Isolated test room with minimal order and noise (not a colony room)
Holding area: dedicated room or quiet area within the testing room
Stopwatches without beepers or with beepers silenced
Two (or more) gooseneck desk lamps with incandescent 75-watt light bulbs
Lux meter (Fisher Scientific)
Automated video tracking system (e.g., see www.anymaze.com)
Computer (PC with WindowsXP, Pentium II 800GHz or higher, 512MB RAM, SVGA display in 16 bit, 45MB hard disk space if installing ANY-maze) with capture card (ie. Euresys Picolo or Adlink RTV-24)
Camera with adjustable zoom lens (preferably CCTV camera with vari-focal lens; ie. Panasonic WV-PB332 with PLZ27/5 vari-focal lens)
Camera mounting bracket so camera can face straight down
Video cables and adapters as needed (ANY-maze provides a nice wizard to help choose computer a camera, lenses, card, and cables: http://www.anymaze.com/equipwizard.htm)
Video Recording software (ie. mediacruise)
OLM testing chamber: white rectangular open field 30 x 23 x 23 cm with vertical black marking strip (see specifications below)
ORM testing chamber circular bucket: diameter 28.4 cm x height 23 cm with vertical white marking strip
Empty holding cage
Handling sleeve: Ansell (19-120-3177)
Bedding: Sani-Chips (P.J. Murphy Forest Products) and/or standard animal bedding 100 mL Beakers filled with cement (two per chamber)
Tins: Kamenstein Quality Spice Rack item # 31106 6 can rack (Remove the magnets & covers) and fill with cement to the brim (cover the hole in the side/bottom with cement) Two per chamber.
Candle holders: Quick Candles item # 1150 clear 2“x2” square tealight candle holder. Fill with cement. Two per chamber.
NOTE: All protocols using live animals must first be reviewed and approved by an Institutional Animal Care and Use Committee (IACUC) and must follow officially approved procedures for the care and use of laboratory animals.
Steps and Annotations
1. Prepare the room
The experimental room should be a dedicated behavior room that is not used to house animals or surgical equipment, etc. as odors from these and other sources can interfere with exploration. Minimize odor, auditory and illumination cues.
- Set the room illumination using a set of overhead lamps.
- Standard fluorescent ceiling lights are too bright for this task. Adjust the lighting so that a light meter reading taken from the floor of the testing chamber is between 45 and 48 LUX.
- Clean the chambers using 70% ethanol before beginning an experiment.
- No cleaning is required during the experiment (i.e., across days, or between animals). Allow to completely air dry overnight prior to using the chambers.
- Place the chambers so the marking strip (see Figure 2) is always in the same location throughout the experiment.
- Add ~1 cm depth of bedding to the floor of the testing chamber.
- The bedding should be sufficient to completely cover the floor of the arena. Too much bedding will result in increased digging behavior and reduced exploration.
- Set up your recording computer, video camera and software according to manufacture instructions.
- If using ANY-maze, follow their Equipment Wizard guide in choosing your setup configuration. You will need to be able to both live-track the animals during habituation and record videos for later offline analysis.
Gloves should be worn for all steps that involve handling animals, objects, or the testing chamber. Either nitrile or latex gloves can be worn, but should not be interchanged during the experiment.
2. Prepare the animals
If purchasing animals from a vendor, have them arrive at least one week prior to beginning the procedure. This allows animals to recover from the stress of transport and to acclimate to the new colony room. Transfer all animals to standard housing. Either group housing (2 to 5 mice per cage) or single-housed animals can be used. Do not combine male mice from separate shipping containers as they will become aggressive.
We have used animals from 8 weeks to 6 months with similar results.
Prepare an Excel sheet with all of the animal information including animal numbers, tail markings, genotype or treatment, chamber number, training object, testing object and novel object location. Counterbalance the chamber number, training object (ORM) and novel object location (ORM and OLM) across genotype, treatment and sex.
- Mark the tails of the animals in each cage with a dark marking pen to differentiate between the mice.
- It is critical to be able to distinguish between individuals within a cage during habituation, training and testing so that the correct animal is placed in the correct chamber. If the markings fade during the habituation phase, remark after the last day of habituation. DO NOT remark on the training or testing day as marking can be stressful. The experimenter conducting the procedure should be blind to the genotype or treatment condition of the animals tested.
3. Phase I: Handle the animals
- Place the handling sleeve on the arm of your non-dominant hand so that the sleeve covers from the wrist to above the elbow.
- Wear tight fitting glove and clean your gloves and the sleeve with 70% ethanol prior to selecting the first animals and between all animals.
- Prepare the empty holding cage to hold animals after handling.
- The overhead room lights should be on for all handling procedures.
- Transport the animals to the experimental room in their homecage on a cart.
- For higher anxiety animals, allow them to sit for an hour (hr) prior to handling.
Remove the lid of the cage and any water/feeding apparatus so that there is easy access to all the mice.
- Pick up a mouse by grabbing its tail and then immediately transferring it to the handling sleeve.
- Do not chase the mice around the cage with your hand. Practice until you can quickly and easily grab the tail. DO NOT dangle the mouse by its tail at any point. This is stressful for the animal and may result in increased biting behavior. Once the mouse is on the handling sleeve allow it to explore the sleeve while maintaining a gentle hold on the tail with your hand. On the first day of handling the mice may try to jump off the sleeve so make sure your grip on the tail is gentle but secure.
Allow the mouse to explore the sleeve for two minutes and then place the mouse in the holding cage.
Select the next mouse and repeat the handling procedure until all mice in the cage have been handled. Then transfer the mice from the holding cage back to their homecage.
- Repeat handling procedure once a day for five days.
- The mice may urinate and defecate the first few days of handling. This is a normal part of their fear response and should diminish by the third day of handling as the animals become more comfortable with being picked up and transported.
- By the third day of handling, pick up the mice by grabbing the tail and then gently turning them into your hand. You can then release the tail and the mouse should sit in your hand. This form of handling is done without the sleeve and two animals may be handled (one in each hand) at a time. Each mouse should sit and groom or explore your hand.
4. Habituate the animals
- Transport the animals to the experimental room in their homecages on a cart.
- For higher anxiety animals, allow them to sit for an hour prior to habituation.
- Prepare the training chamber as directed above by adding ~1 cm of bedding to the bottom of the chamber. Shake the bedding until it evenly and completely covers the bottom of the chamber.
- For OLM, use the square boxes and for ORM, use the circular chambers.
- Turn off the overhead lights and turn on the lamps above the chambers. Adjust the lighting so that the light meter reads ~47 LUX at the bottom of each chamber and the chamber is uniformly illuminated.
- A single chamber or multiple chambers (up to four) can be run by a single experimenter at the same time. Each mouse goes in a different chamber, but that chamber is held constant across days.
- Start the video recording and live-tracking software.
- Make sure to set the software to record and track for more then the five minute habituation session to account for the time required to transport the mouse to the chamber.
Pick up the mouse to be habituated by the tail and gently turn the mouse into your gloved hand.
- Transport the mouse to the chamber and lower it into the bottom. Turn your hand gently so the mouse can step out of your hand into the chamber.
- This step should be performed quickly and carefully to avoid stressing the animal. Any remaining animals should stay in the homecage on the transport cart.
Start the stopwatch (beep/alarm removed)
- Allow the mouse to explore the chamber for 5 minutes while recording the video and live-tracking the mouse.
- It is important to keep quiet during this time to avoid disturbing the mouse.
- To remove the mouse, grab it by the tail, gently turn it into your hand and transport it back to the homecage if single housed or to an empty holding cage if additional animals in the homecage are waiting for habituation.
- Do not mix habituated and unhabituated animals.
- Remove any feces and shake the bedding to equally distribute any odor cues.
- The bedding is not changed between animals or across days. Make surethat you keep the order of the animals the same across days and that the same mouse always goes into the same chamber.
- Repeat habituation once daily for six days.
- The six-day schedule was determined by tracking the distance travelled during the five-minute daily sessions. Some strains may require more or less habituation. Tracking habituation progress is recommended for all experiments and in particular for lines of genetically modified animals that that have not previously been examined for motor or sensory function.
- The last two days of handling can be overlapped with the first two days of habituation. The animals are first handled and then habituated for the two days.
5. Train the animals
Prepare the training room by turning off the overhead lights and turning on the overhead lamps. Check the illumination with the lightmeter to make sure it reads ~47 LUX at the bottom of the chambers and that illumination is equivalent across the chamber.
- Transport the animals to the room in their homecages on a cart. Leave each cage on the cart until it is used in training.
- The cart can be placed either in a holding room near the training room or in the training room itself as long as it is far enough away from the training setup that the animals in the homecages do not disturb the animals being trained. If using an adjacent room, make sure the light settings are the same as in the training room and that the room is isolated and quiet. The holding room should also be a minimal distance from the training room to reduce effects of transporting the homecage between rooms.
- Clean the training objects with 10% ethanol using a paper towel. Allow the objects to air dry before placing them in the chamber.
- For OLM training, use the 100 mL beakers flipped upside down and facing the same direction. For ORM training, use the candle holders and tins placed upside down. Half the animals should be trained using the candle holders and half using the tins. Counterbalance training objects across conditions as described in the experiment preparation. The objects are filled with cement to prevent the mice from moving them or knocking them over.
Evenly distribute the bedding as previously described for habituation.
Place the objects in the chambers according to your predefined excel sheet. Take care to place the objects directly over the predefined marks on the bottom of the chamber (Figure 1B).
- Open the video recording software and prepare the folder where you are going to store the video files. Include the date, experiment number, and all other identifying information in the file name.
- It is critical to record the full session for both training and testing as this will be used later to score object exploration. Make sure to record more then the actual training duration to allow for extra time to transfer the animal into the chamber. An additional 30 seconds is recommended, but timing can vary depending on the experimenter speed and setup. Two different training times can be used depending on the experimental question. A 10 minute training session is sufficient for long-term memory formation (tested at 24 hrs) in wildtype mice (Stefanko et al., 2009). This training duration is often used to assess potential memory deficits. A 3 minute training session is considered subthreshold and does not result in short-term memory (90 minute) or long-term memory (24 hrs) in wildtype mice. This training duration is used to examine memory enhancements (e.g., HDAC inhibition (Stefanko et al., 2009; McQuown et al., 2011)).
- Check the video feed to make sure the image is in focus and zoomed appropriately to be able to clearly visualize both the animal and objects.
- You will need to be able to clearly see the animal's nose. The clearer the video, the easier the analysis will be later.
- Open the cage and remove any water or food apparatus so you have easy access to the animals.
- If you are transporting the animals from a holding room, wait to open the cage until the animals are in the testing room.
Start the video recording
- Introduce the animals into the training chamber as described for habituation. Place the animal into the chamber as far away from the training objects as possible.
- The placement of the animal in the chamber is designed to avoid introducing any object bias that could occur if the animal is placed directly next to one object or the other.
Animals should explore the training chamber completely and spend time exploring both objects equally.
- Start the stopwatch (beep/alarm removed). Allow the mouse to explore the chamber for the training duration while recording the video.
- It is important to keep quiet during this time to not disturb the mouse.
- It is not necessary to live-track the animal during training or testing.
- To remove the mouse, grab it by the tail and gently turn it into your hand and transport it back to the homecage if single housed or to an empty holding cage if additional animals in the homecage are waiting for training.
- Do not mix trained and untrained animals.
Return the homecage to the cart.
Remove the objects and clean them with 10% ethanol. Allow them to air-dry.
Remove any feces and shake the bedding to equally distribute any odor cues.
Place the objects back in the training chambers in preparation for the next animal.
- Repeat the training procedure for all animals and then transport them back to the colony room.
- Animals can remain in the holding or testing room for an additional few hours (hrs) after training before returning to the colony room.
6. Test the animals
Prepare the testing room exactly as for training. Adjust the lighting and video recording setup, and clean the objects with 10% ethanol as described for training.
Transport the animals to the testing or holding room in their homecage and leave them on the cart until testing
Evenly distribute the bedding as previously described for habituation.
- Place the objects in the chambers according to your predefined Excel sheet. Take care to place the objects directly over the predefined marks on the bottom of the chamber.
- If conducting OLM, place one of the 100 mL beakers in the novel object location (see Figure 1B and C) and one in the familiar object location. The familiar object location should be counterbalanced across all conditions.
- If conducting ORM, place one of the tins and one of the candle holders in the chamber (Figure 1B and C). One of the objects will be novel and one will be familiar depending on which object was used at training. Counterbalance both which object is novel and the location of the novel object across conditions.
- Open the video recording software and prepare the folder where you are going to store the video files. Include the date, experiment number, and all other identifying information in the file name.
- The testing duration is 5 minutes. Set the video recording for additional time to allow for transport of the animal into the testing chamber.
- Animals can be tested either 90 minutes following training (short-term memory) or 24 hrs (long-term memory).
Open the cage and remove any water or food apparatus so you have easy access to the animals.
Start the video recording
Introduce the animals into the training chamber as described for habituation. Place the animal into the chamber as far way from the training objects as possible.
- Start the stopwatch (beep/alarm removed). Allow the mouse to explore the chamber for 5minutes while recording the video.
- It is important to keep quiet during this time to not disturb the mouse.
- To remove the mouse, grab it by the tail and gently turn it into your hand and transport it back to the homecage if single housed or to an empty holding cage if additional animals in the homecage are waiting for training.
- Do not mix tested and untested animals.
Return the homecage to the cart.
Remove the objects and clean them with 10% ethanol. Allow them to air-dry.
Remove any feces and shake the bedding to equally distribute any odor cues.
Place the objects back in the training chambers in preparation for the next animal.
Repeat the testing procedure for all animals and then transport them back to the colony room.
7. Data Collection
- Data analysis is performed offline from a computer that can play your testing videos (VLC player or other media player is required).
- Timing can be recorded using our custom MATLAB code (see supplement) or by hand with two stopwatches (one for each object). Note that most online stopwatches lack sufficient precision to be used for timing.
- If using a computer for scoring, a keyboard with laptop/short keypad is required for accurate scoring. A laptop or Apple keyboard are ideal.
- The following criteria are used to score exploration of each object during the training or testing period.
- Exploration: interaction time of a mouse with the object when the mouse's nose is within 1 cm of the object and is pointing directly at the object so that an elongated line from eyes to nose would hit the object.
- The following do not count as exploration:
- The mouse is not approaching the object (e.g., if the mouse reorientates itself and the nose accidentally comes close to the object)
- The mouse is on top of the object (even if it is looking down at the object)
- The mouse is looking over the object (e.g., mouse rears on the object)
- The mouse is engaged in a repetitive behavior (like digging close to the object or biting the object)
Accurate time requires both patience and practice. We highly recommend practice with the provided videos to match our experienced scorers before proceeding with your own experiments. See Table 1 and supplemental practice videos.
All timing and video analysis should be conducted by an experimenter blind to the experimental conditions.
Score both training and testing. Animals that do not explore more than 3 sec total for both objects during training or testing are excluded from analysis. Animals that have discrimination indexes +/− 20 at training are considered to have a significant location/object bias during training and are also excluded from analysis.
- The discrimination index (DI) is calculated as follows: (time exploring the novel object – time exploring the familiar) / (time exploring novel + familiar) * 100
- A DI of zero indicates equal preference for the two objects. Typical long- and short-term memory DIs range from 25 to 45. A large negative DI could indicate neophobia to the novel object.
- Both total exploration (object 1 + object 2) and DI are then evaluated for each experimental condition of interest (e.g. wildtype vs. mutant). Typical total exploration times range from 4-15 seconds for a 5 minute test.
- If the total exploration differs between the two experimental conditions, conclusions about long- or short-term memory should be made with extreme caution as this task relies on equal exploration between groups both during test and training.
Commentary
Background Information
Object location and object recognition memory paradigms were originally developed for use in rats (Ennaceur and Delacour, 1988; Dix and Aggleton, 1999) and then adapted for use in mice (Murai et al., 2007). Both tasks have become increasingly popular for examining short- and long-term memory in rodents. Recent work has focused on characterizing the brain regions and molecular mechanisms underlying OLM and ORM. We have provided a brief summary of some of the more pertinent background literature as it pertains to conducting and interpreting results from this protocol. We would like to refer readers to several recent and extensive reviews for a more detailed background on ORM and OLM (Winters et al., 2008; Ennaceur, 2010; Mumby, 2001; Dere et al., 2007).
In brief, OLM and ORM appear to rely on unique brain. OLM requires the hippocampus for encoding, consolidation and retrieval (Haettig et al., 2011; Mumby et al., 2002), and is particularly sensitive to manipulations in dorsal CA1 (Barrett et al., 2011; Assini et al., 2009; Vogel-Ciernia et al., 2013). A number of different brain regions appear critical for ORM, including insular cortex (Bermudez-Rattoni et al., 2005; Balderas et al., 2008), perirhineal cortex (Balderas et al., 2008; Winters et al., 2004; Winters and Bussey, 2005), and ventromedial prefrontal cortex (Akirav and Maroun, 2006). However, the role of the hippocampus in object recognition has remained somewhat controversial (Dere et al., 2007; Mumby, 2001) with the necessity for the hippocampus varying based on the timing of the hippocampal manipulation and exact experimental setup (Haettig et al., 2011; Balderas et al., 2008; Rossato et al., 2007). When conducted as described in this protocol, the hippocampus is required for encoding both contextual and object information, such that inactivation of the dorsal hippocampus with muscimol immediately post-training impairs both object recognition and object location memory at 24 hrs. However, muscimol infused into dorsal hippocampus immediately pre-retrieval did not impair 24 hr ORM, indicating additional brain regions are sufficient for ORM retrieval (Haettig et al., 2011).
Recent work with both OLM and ORM tasks has examined a multitude of different durations between training and testing. Typical short-term memory experiments vary in the time separating training and testing from 5 minutes to a few hours. These short-term memory timepoints are thought to occur within the consolidation window and not to require new protein synthesis. Long-term memory in rodents is typically examined at 24 to 48 hrs post-training, and requires de novo transcription and translation. The belief that short- and long-term memory are distinguished by the requirement for new gene expression is largely based on the findings that immediate post-training injections of either transcription or translation inhibitors block long-term memory, without impacting short term memory. For example, infusions of anisomycin, emetine or cycloheximide in the entorhinal cortex immediately after training in an object recognition task impair long-term memory (24 hrs) without affecting short-term memory (3 hrs) (Lima et al., 2009). Similarly, using a 10 minute training procedure like the one described here, Balderas, et al. (2008) demonstrated a requirement for protein synthesis in the perirhinal and insular cortex in object recognition memory. Post-training anisomycin infusions into either perirhinal or insular cortex blocked long-term (24 hr) but not short-term (90 minute) ORM (Balderas et al., 2008). Akirav et al. (2006) found that blocking protein synthesis immediately following a 5 minute training session impaired ORM at 24 hrs but not at 3 hrs (Akirav and Maroun, 2006). Together, this and other work demonstrates a clear distinction between the molecular mechanisms underlying short- and long-term memory. Thus, manipulations that alter transcription or translation mechanisms are predicted to impact long-term memory without altering short-term memory (for an example see Vogel-Ciernia, et al., 2013).
Critical Parameters
1. General behavior guidelines and performance considerations
There are a number of critical parameters that pertain to general animal health and well-being. Since this task relies on rodents’ innate preference for novelty and exploration, any conditions or manipulations that impair exploration are detrimental to this task. These include animal stress, performance confounds, and natural strain or age differences.
Animal Stress: Any conditions in the testing room or colony room that could lead to increased animal stress can impact performance in this task. To minimize animal stress, do not change the cages during the task. Cages can be changed immediately prior to the first day of handling and immediately post-testing. Outside odors and noise should be minimized.NO perfumes or colognes should be worn by anyone who enters either the colony room or testing rooms, and no loud noises made in either room. Animals should be group-housed unless required to be single-housed for experimental reasons. All animals are given free access to food, water and bedding.
Performance Confounds: Prior to conducting OLM or ORM tasks, any experimental manipulation should be assessed to determine whether it alters motor/sensory function or anxiety. Deficits in motor abilities or sensory processing can appear behaviorally similar to memory impairments. To control for potential deficits in performance a series of simple behavioral tests such as open field, elevated plus maze or a light/dark box test can be used. We have successfully conducted these types of tests prior to conducting the OLM/ORM protocol in the same group of animals (Vogel-Ciernia et al., 2013). In addition to measures of anxiety and basic motor/sensory performance, habituation to the novel testing chamber both within a testing session and across days can also be monitored. This protocol uses distance travelled as a measure of habituation, with decreases observed across days of habituation. Animals that are not habituated to the context prior to training fail to show long-term ORM (24 hrs) (Stefanko et al., 2009).
Mouse Strain Variation and Age: Background strain choice and age are important factors to consider when designing OLM/ORM experiments. This protocol has been optimized for 8-12 week old C57/BL6J mice. Strain differences between several common inbred lines have been observed using ORM tasks similar to the one described here. For example, Brooks et al. (2005) examined the performance of six inbred strains (129S2/SvHsd, BALB/cOlaHsd, C3H/HeHNsd, C57BL/6JolaHsd, CBA/CaOlaHsd, DBA/2OlaHsd) in elevated plus maze, visual acuity/discrimination and object recognition memory tasks. There were significant differences in total object exploration among the different strains, with the 129S2/Sv mice exploring the least and the BALB/C and DBA/2 mice exploring the most. When the exploration time for the novel and familiar objects were compared as a percentage of the total exploration time, there was a significant interaction between strain and test time (1 h, 4 h, or 24 h) with what appeared to be a lack of discrimination in the 129S2/Sv and C3H/He strains. These differences are potentially also due to strain effects on anxiety (as seen in differences in elevated plus maze), visual discrimination, and general activity (Brooks et al., 2005). In general, the 129S2/Sv strain appears to be hypoactive, and gene-targeting studies utilizing this strain should consider backcrossing it to another background (e.g., C57BL/6J). Potential strain differences in habituation should also be taken into consideration when planning OLM/ORM experiments. Tracking animals live during habituation sessions allows for real-time assessment of decreases in locomotion. The number of days of habituation can then be adjusted so that the habituation curve across days decreases until all animals have reached a stable level of low locomotion.
In addition to strain variations, age should be taken into consideration when conducting OLM/ORM experiments. For example, using a modified version (3x10 minute training sessions) of the protocol described here, Wimmer et al. (2012) found that aged mice (C56BL/6NIA, 22-24 months) had long-term object location (24 hr) memory deficits compared to younger (2-4 month) controls. Both short-term OLM (tested 3 minutes after the last training session) and long-term ORM (tested 24 hrs after one 10 minute training session) were similar between the young and old animals, indicating an age-dependent deficit in OLM consolidation (Wimmer et al., 2012).
2. Object choice
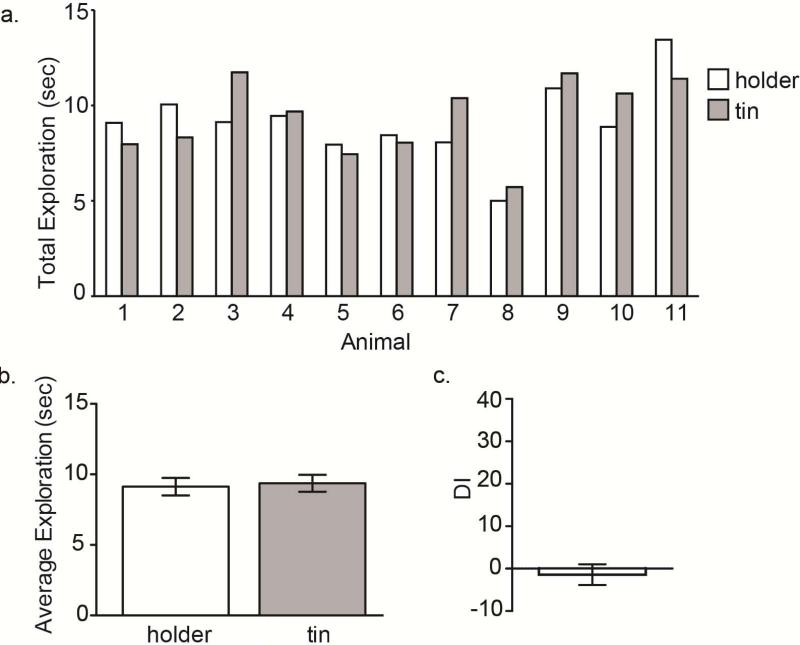
Object choice is potentially one of the most important and underappreciated aspects of conducting a successful ORM/OLM protocol(Ennaceur, 2010). Since this task relies on rodents’ innate preference for novelty, all objects used for this protocol should meet the following criteria: 1) do not produce a fear response, 2) are adequately explored during a testing session (at least 3 sec per object), 3) if used for ORM, the two object have equal innate preference and can be discriminated. We have extensively screened objects in our laboratory to meet these three criteria and highly recommend either using the objects listed in the protocol, or performing your own preference and discrimination testing. Objects were chosen to be matched for size, easily cleanable and made of a non-porous material. To conduct the preference and discrimination testing, perform the ORM protocol as written up until the training day. For object preference testing, on the training day give the test mouse one of each of the two objects to be tested (just as would be done on an ORM test day) (Figure 3). If the mouse innately prefers the two objects equally, the DI will be zero for this session. To test for object discrimination, perform the short-term ORM protocol as written with the two objects (two of object 1 at training and then one of each test object at test). If the animals can discriminate between the two objects, the DI will be positive (~25-40). All objects are filled with cement to prevent animals from moving them during exploration and mounting. The cement should be completely dry and is preferable to other methods for securing objects in place such as Velcro due to its versatility for object placement and lack of odor.
Figure 3.
Object preference testing for ORM. (A) Total exploration for the holder and tin for individual animals during a 10 min test for object preference. Preference testing was performed using the standard ORM paradigm described in the text with one alteration. In place of the typical ORM training session, the animals shown here were given one holder and one tin to explore for 10 min (instead of two identical objects). (B) Average exploration for the holder and tin for a 10 min session. As predicted the two objects are explored equally [paired t-test t(10) = 0.502, p = 0.627]. (C) Discrimination index (DI) for the holder compared to tin for a 10-min session. DI was calculated arbitrarily as if the holder was a novel object. There is no significant difference in the DI from zero [one-sample t-test t(10) = 0.582, p = 0.573].
3. Training duration and testing parameters
As discussed in the Background section, the duration of the training session and the delay between training and testing are both of critical importance when designing OLM and ORM experiments. In order to avoid both floor and ceiling effects, careful consideration should be given as to whether the desired experimental manipulation is predicted to enhance or impair memory formation. A single, 10-minute training session is sufficient to generate robust short- and long-term memory, and can be used to examine impairments in memory formation(Stefanko et al., 2009; Barrett et al., 2011; McNulty et al., 2012; Haettig et al., 2011; Vogel-Ciernia et al., 2013). However, the 10-minute training is not best suited for examining potential long-term memory enhancements at the typical 24 hr testing time point. Stefanko, et al (2009) demonstrated that the memory enhancing effects of HDAC inhibitors could be better revealed using a sub-threshold 3-minute training protocol. This 3-minute training session is not sufficient to result in either short- or long-term memory in normal wildtype mice. However, a post-training injection of a general HDAC inhibitor (Stefanko et al., 2009) or a HDAC3-specific inhibitor(McQuown et al., 2011) transforms this training event into a robust long-term memory (24 hrs). The 10-minute protocol was also used to examine memory enhancements at a 7 day time point, a time by which normal object memory fails (Stefanko et al., 2009; McQuown et al., 2011).
4. Injections, cannulations and surgeries
The OLM and ORM tasks can be used with various pharmacological or viral manipulations. Careful consideration should be made when planning both the dose and timing of any manipulation. Systemic or brain–region-specific infusions via cannulation can be conducted either pre-training (memory encoding), post-training (memory consolidation) or pre-retrieval (memory retrieval). Both pre-training and pre-retrieval delivery can potentially confound interpretations of animal performance because of state-dependent learning, altered sensory-motor function, or altered motivation, arousal or attention. In addition, both the pharmacokinetics and the metabolism of the delivered agent should be taken into consideration. If surgery involving anesthesia is required within a few days of training (e.g., siRNA infusions), animals can undergo surgery following the fifth habituation session, then be habituated the next day and trained two days following surgery (McNulty et al., 2012). For systemic injections, animals should be habituated to restraint for four to five days prior to training. Restraint should be preformed in the same manner as used for drug delivery, and can be performed during the habituation phase of the protocol. The experimenter performing the behavioral testing should not be the experimenter performing the injections or restraint. Ideally, one experimenter runs the entire behavioral experiment and another restrains and performs the injections.
5. Conducting multiple behaviors with the same animals
OLM and ORM can successfully be conducted in the same cohort of animals with a few minor modifications to the above protocol (Vogel-Ciernia et al., 2013). The first behavior test is conducted as described above (e.g., OLM). The animals are then given 4-7 days off, during which they remain in the colony room undisturbed. The animals are then habituated to a novel context (distinct in size, shape, texture and bedding from the context used for the first behavior). Habituation to context 2 is conducted exactly as described in step 4 of the protocol (5 min/day for 6 days). Animals do not need to be handled again unless they appear distressed or anxious, or a new experimenter is conducting the second behavior (in which case the second behavior is preceded by one to two days of handling). Following habituation to context 2, the animals are trained and tested as described in the protocol. However, a new set of objects must be used for the second behavior (e.g., if using beakers for OLM, then the tins/holders are used for ORM). In addition, the location of the novel or moved object should be altered between experiments so that the location of the novel/moved object for the first experiment is the location of the familiar/unmoved object for the second experiment (e.g., if the left object was moved for OLM, then the right object is novel for ORM).
Troubleshooting
1. Failure of animals to habituate
Habituation is a form of non-associative memory that could potentially be impaired following genetic or pharmacological manipulations prior to the habituation phase of the protocol. Failure of the experimental animals to decrease their distance travelled (a measure of habituation) may occur for several reasons. If the experimental manipulation alters basic motor behavior, animals may appear to habituate more quickly (i.e., stop moving), or fail to habituate. If this occurs, extreme caution should be used in interpreting any resulting memory alterations in these animals (see Behavioral Confounds). The habituation portion of the protocol can also be modified to try to mitigate differences in habituation by either decreasing or increasing the number of days of habituation. Additional behavioral measures of habituation, such as increased grooming, decreased rearing and decreased digging behavior, may also be useful.
2. Low Discrimination Index or object exploration in wildtype animals
Failure of the wildtype animals to perform well (DI of >30, total object exploration >3 sec) in either the OLM or ORM tasks can stem from many sources. We highly recommend first testing each individual lab's setup first with C57/Bl6J mice age 8-15 wks before any experiment-specific modification. One of the most common causes of long-term memory failure in control animals is due to anxiety. Check the behavioral testing and colony room for any sources of noxious odors, intrusive noise, fluctuating temperature or other potential sources of stress. Transporting the animals to the testing room 1-2 hrs prior to habituation, training, and testing can also reduce transport stress. In addition, an inexperienced experimenter not trained to properly handle animals can be a source of animal stress. Experimenters should be comfortable picking up, handling and, if required, restraining animals prior to conducting this protocol.
3. Interpreting long-term deficits with short-term memory as a performance control
For experimental manipulations that alter long-term memory processes (e.g., impairments in transcription or translation), performance confounds can be accounted for by demonstrating intact short-term memory on the same task. The long- and short -term memory protocols are performed in different cohorts of animals to prevent carry-over effects. For example, if the experimental animals demonstrate intact short-term OLM but impaired long-term OLM, then the long-term memory deficits are most likely due to a failure in memory consolidation and not to impairments in memory encoding, attention or performance.
Anticipated Results
As shown in Figure 2, during training (Figure 2b and c) and testing (Figure 2e and f) all animals explore both the left and right objects equally and for at least three seconds. During a subsequent test (either short- or long-term), animals that remember the training conditions will demonstrate an increased preference for novelty and will consequently explore the displaced/novel object more than the unmoved/familiar object (Figure 2 d and g).
Time Considerations
The long-term memory protocol takes 11 days, and the short-term memory protocol takes 10 days (Figure 1A). If the same cohort of animals is to be run on both OLM and ORM, the experimenter should plan a 4-7 day separation between the two tasks. The second task then begins with habituation to a second, novel context (6 days) followed by training and testing (one day for short-term and two days for long-term) for a total of 7 to 8 days. Multiple animals can be trained simultaneously if there are multiple training contexts. We routinely run four animals at a time using four training chambers placed side-by-side. Each animal is trained and tested within the same chamber that it was previously habituated in. All animals should be added or removed from the chambers within 10 sec of each other. Allow 3-4 minutes between habituation sessions to remove the animals from the chamber, remove any feces and shake the bedding. On the training and testing days, allow at least 5 minutes between animals to clean the objects, remove feces, shake the bedding and replace objects.
Acknowledgements
Thank you to Richard Dang and Matt Mahavongtrakul for assistance in preparing the practice videos. Thank you to Kasia Bieszczad, Tim Allen and Kourosh Saberi for assistance in writing the MATLAB timing code. Thank you to Jakob Hattig, Ruth Barrett, Daniel Stefanko and all the other members of the Wood lab that worked diligently to refine the protocol described here. Thank you to Elise Kleeman for helpful comments on the manuscript. This work was funded by NIMH and NIDA grants (MH081004, MH101491, DA025922, DA036984) to M.A. Wood and NIMH (F31-MH098565) to A.V.C.
Literature Cited
- Akirav I, Maroun M. Ventromedial prefrontal cortex is obligatory for consolidation and reconsolidation of object recognition memory. Cerebral cortex (New York, N.Y. : 1991) 2006;16:1759–1765. doi: 10.1093/cercor/bhj114. [DOI] [PubMed] [Google Scholar]
- Assini FL, Duzzioni M, Takahashi RN. Object location memory in mice: pharmacological validation and further evidence of hippocampal CA1 participation. Behavioural brain research. 2009;204:206–211. doi: 10.1016/j.bbr.2009.06.005. [DOI] [PubMed] [Google Scholar]
- Balderas I, Rodriguez-Ortiz CJ, Salgado-Tonda P, Chavez-Hurtado J, McGaugh JL, Bermudez-Rattoni F. The consolidation of object and context recognition memory involve different regions of the temporal lobe. Learning & Memory. 2008;15:618–624. doi: 10.1101/lm.1028008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett RM, Malvaez M, Kramar E, Matheos DP, Arrizon A, Cabrera SM, Lynch G, Greene RW, Wood MA. Hippocampal Focal Knockout of CBP Affects Specific Histone Modifications, Long-Term Potentiation, and Long-Term Memory. Neuropsychopharmacology. 2011;36:1545–1556. doi: 10.1038/npp.2011.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bermudez-Rattoni F, Okuda S, Roozendaal B, McGaugh JL. Insular cortex is involved in consolidation of object recognition memory. Learning & memory (Cold Spring Harbor, N.Y.) 2005;12:447–449. doi: 10.1101/lm.97605. [DOI] [PubMed] [Google Scholar]
- Brooks SP, Pask T, Jones L, Dunnett SB. Behavioural profiles of inbred mouse strains used as transgenic backgrounds. II: cognitive tests. Genes, brain, and behavior. 2005;4:307–317. doi: 10.1111/j.1601-183X.2004.00109.x. [DOI] [PubMed] [Google Scholar]
- Dere E, Huston JP, De Souza Silva MA. The pharmacology, neuroanatomy and neurogenetics of one-trial object recognition in rodents. Neuroscience and biobehavioral reviews. 2007;31:673–704. doi: 10.1016/j.neubiorev.2007.01.005. [DOI] [PubMed] [Google Scholar]
- Dix SL, Aggleton JP. Extending the spontaneous preference test of recognition: evidence of object-location and object-context recognition. Behavioural brain research. 1999;99:191–200. doi: 10.1016/s0166-4328(98)00079-5. [DOI] [PubMed] [Google Scholar]
- Ennaceur A. One-trial object recognition in rats and mice: methodological and theoretical issues. Behavioural brain research. 2010;215:244–254. doi: 10.1016/j.bbr.2009.12.036. [DOI] [PubMed] [Google Scholar]
- Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behavioural brain research. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Haettig J, Stefanko DP, Multani ML, Figueroa DX, McQuown SC, Wood MA. HDAC inhibition modulates hippocampus-dependent long-term memory for object location in a CBP-dependent manner. Learning & Memory. 2011;18:71–79. doi: 10.1101/lm.1986911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima RH, Rossato JI, Furini CR, Bevilaqua LR, Izquierdo I, Cammarota M. Infusion of protein synthesis inhibitors in the entorhinal cortex blocks consolidation but not reconsolidation of object recognition memory. Neurobiology of learning and memory. 2009;91:466–472. doi: 10.1016/j.nlm.2008.12.009. [DOI] [PubMed] [Google Scholar]
- McNulty SE, Barrett RM, Vogel-Ciernia A, al E. Differential roles for Nr4a1 and Nr4a2 in object location vs. object recognition long-term memory. Learning &. 2012 doi: 10.1101/lm.026385.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQuown SC, Barrett RM, Matheos DP, Post RJ, Rogge GA, Alenghat T, Mullican SE, Jones S, Rusche JR, Lazar MA, et al. HDAC3 is a critical negative regulator of long-term memory formation. Journal of Neuroscience. 2011;31:764–774. doi: 10.1523/JNEUROSCI.5052-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumby DG. Perspectives on object-recognition memory following hippocampal damage: lessons from studies in rats. Behavioural brain research. 2001;127:159–181. doi: 10.1016/s0166-4328(01)00367-9. [DOI] [PubMed] [Google Scholar]
- Mumby DG, Gaskin S, Glenn MJ, Schramek TE, Lehmann H. Hippocampal damage and exploratory preferences in rats: memory for objects, places, and contexts. Learning & memory (Cold Spring Harbor, N.Y.) 2002;9:49–57. doi: 10.1101/lm.41302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murai T, Okuda S, Tanaka T, Ohta H. Characteristics of object location memory in mice: Behavioral and pharmacological studies. Physiology & behavior. 2007;90:116–124. doi: 10.1016/j.physbeh.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Hernandez A, Cabrera SM, Hagewoud R, Malvaez M, Stefanko DP, Haettig J, Wood MA. Membrane-associated glucocorticoid activity is necessary for modulation of long-term memory via chromatin modification. Journal of Neuroscience. 2010;30:5037–5046. doi: 10.1523/JNEUROSCI.5717-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossato JI, Bevilaqua LRM, Myskiw JC, Medina JH, Izquierdo I, Cammarota M. On the role of hippocampal protein synthesis in the consolidation and reconsolidation of object recognition memory. Learning & Memory. 2007;14:36–46. doi: 10.1101/lm.422607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefanko DP, Barrett RM, Ly AR, Reolon GK, Wood MA. Modulation of long-term memory for object recognition via HDAC inhibition. Proceedings of the National Academy of Sciences. 2009;106:9447–9452. doi: 10.1073/pnas.0903964106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel-Ciernia A, Matheos DP, Barrett RM, Kramár EA, Azzawi S, Chen Y, Magnan CN, Zeller M, Sylvain A, Haettig J, et al. The neuron-specific chromatin regulatory subunit BAF53b is necessary for synaptic plasticity and memory. Nature Neuroscience. 2013;16:552–561. doi: 10.1038/nn.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer ME, Hernandez PJ, Blackwell J, Abel T. Aging impairs hippocampus-dependent long-term memory for object location in mice. Neurobiology of aging. 2012;33:2220–2224. doi: 10.1016/j.neurobiolaging.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BD, Bussey TJ. Transient inactivation of perirhinal cortex disrupts encoding, retrieval, and consolidation of object recognition memory. Journal of Neuroscience. 2005;25:52–61. doi: 10.1523/JNEUROSCI.3827-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BD, Forwood SE, Cowell RA, Saksida LM, Bussey TJ. Double dissociation between the effects of peri-postrhinal cortex and hippocampal lesions on tests of object recognition and spatial memory: heterogeneity of function within the temporal lobe. Journal of Neuroscience. 2004;24:5901–5908. doi: 10.1523/JNEUROSCI.1346-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winters BD, Saksida LM, Bussey TJ. Object recognition memory: neurobiological mechanisms of encoding, consolidation and retrieval. Neuroscience and biobehavioral reviews. 2008;32:1055–1070. doi: 10.1016/j.neubiorev.2008.04.004. [DOI] [PubMed] [Google Scholar]