Abstract
Introduction:
The 2-[18F]-Fluorodeoxyglucose (FDG) positron emission tomography (PET/CT) has become an imaging tool for clinical assessment of tumor, node, metastasis in non–small-cell lung cancer (NSCLC). Primary tumor maximum standardized uptake value (SUVmax) on 18F-FDG PET/CT before and after radiation therapy (RT) has been studied as a potential prognostic factor for NSCLC patients receiving radiotherapy. However, the sample sizes of most studies were small, and the results of the prediction value of SUVmax remained undetermined, which lead us to perform a meta-analysis to improve the precision in estimating its effect.
Methods:
We performed a meta-analysis of published literature for primary tumor SUVmax-based biomarkers of the outcome of NSCLC receiving radiotherapy. The required data for estimation of individual hazard ratios (HRs) to compare patients with a low and a high SUVmax were extracted from each publication. A combined HR was calculated by Stata statistical software (Version 11). All of the results were verified by two persons to ensure its accuracy.
Results:
Thirteen studies were finally included into this meta-analysis; data are available in 13 studies for pre-RT primary tumor SUVmax and in five studies for post-RT. For overall survival, the combined HR estimate was 1.05 (95% confidence interval [CI], 1.02–1.08) and 1.32 (95% CI, 1.15–1.51) for pre-RT SUVmax and post-RT SUVmax, respectively; 1.26 (95% CI, 1.05–1.52) and 2.01 (95% CI, 1.16–3.46) for local control (LC). In stereotactic body radiotherapy (SBRT) group, HR for LC was 1.11 (95% CI, 1.06–1.18) and 2.19 (95% CI, 1.34–3.60) for pre-SBRT SUVmax and post-SBRT SUVmax, respectively.
Conclusion:
Both pre-RT and post-RT primary tumor SUVmax can predict the outcome of patients with NSCLC treated with radiotherapy. Patients with high levels of pre-RT SUVmax seemed to have poorer overall survival and LC.
Keywords: Primary tumor maximum standardized uptake value, Prognosis, Non–small-cell lung cancer, Meta-analysis, Radiotherapy
Lung cancer is the leading cause of cancer-related death worldwide.1 Non–small-cell lung cancer (NSCLC) accounts for 85% of all lung cancers. NSCLC is often treated with a combination of multiple types of therapies, including radiation therapy (RT). The dose limitation and therefore therapeutic efficacy of RT have been addressed by recent progresses, including stereotactic body radiotherapy (SBRT) and image-guided and intensity-modulated radiotherapy.2 For inoperable patients with early stage NSCLC, SBRT presents as a new standard treatment. Despite these improvements, the 5-year survival rate is generally approximately 15%,3 and somewhat higher at 17% to 47%4 in patients with early stage NSCLC.
Information about reliable prognostic factors for RT responses (and prognosis in general) is essential in identifying subjects suitable for aggressive RT treatment. The most commonly used factor for predicting RT responses is the tumor-node-metastasis (TNM) stage.5 Weight loss,6 performance status,6 and molecular markers7,8 were reported to predict the outcome and could be used to stratify patients for the most optimal strategy for RT but require further validation.9 Morphologic changes as reflected by computed tomography (CT) have also been identified as prognostic factors, but technical issues remain (e.g., it is also difficult to differentiate residual tumor from necrosis or fibrosis).10,11
A variety of imaging methods has been developed to examine tumor metabolism.12–14 For example, 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT is becoming the standard practice for staging and is widely used in post-treatment evaluation of various types of cancer, including breast cancer, lymphoma, head and neck cancer, and NSCLC.15–18 For NSCLC, 18F-FDG PET/CT has been found useful for diagnosing, restaging at recurrence,19 delineating radiotherapeutic targets,20 and evaluating radiotherapeutic or chemotherapeutic effects.19–21 Maximum standardized uptake value (SUVmax) derived from 18F-FDG PET/CT has been reported by some,22–24 but not all,25,26 to predict the responses to RT in patients with NSCLC.
In the current study, we systematically reviewed available information of all published studies of primary tumor SUVmax of NSCLC. Potential prognostic value of primary tumor SUVmax in predicting RT responses was examined using meta-analysis.
MATERIALS AND METHODS
Search Strategy and Eligible Criteria
The PubMed database was searched (updated on April 3, 2013) used the following terms: (non–small cell lung cancer OR NSCLC) AND (PET imaging tomography OR positron emission tomography OR PET OR 18F-FDG OR fluorodeoxyglucose) AND (prognostic OR survival OR prognostic factor OR outcome OR predict) AND (“radiotherapy”[Mesh] OR radiotherapy OR irradiation). References cited by the articles identified in the electronic search were also reviewed. Conference Abstracts were not included in the search because of a lack of meta-analysis. When the same patient population was reported in more than one article, only the most recent or complete report was included in the final analysis.
This meta-analysis was limited to the studies on prognostic implications (for either overall survival [OS] or local control [LC]) of primary tumor SUVmax, as examined using 18F-FDG PET/CT, in NSCLC patients receiving RT. Case studies and review articles were not included. Studies using prognostic indexes other than OS or LC were also not included.
Data Extraction
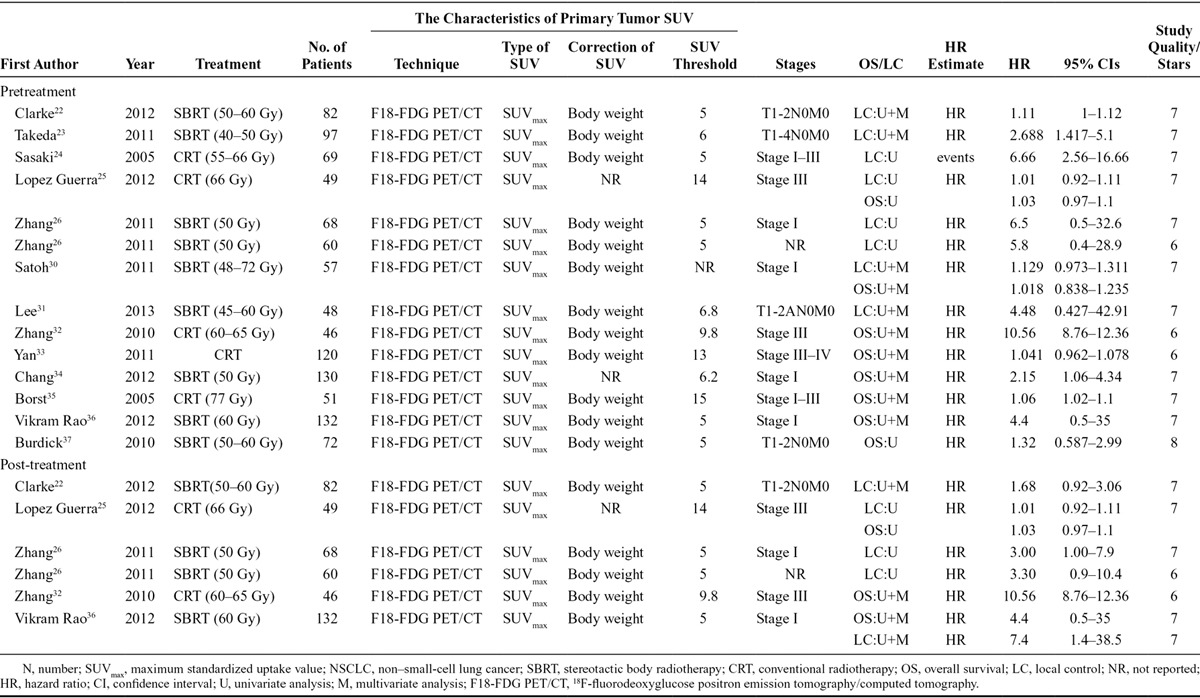
Information was independently extracted by two investigators (FFN and CL), and it included authors, publication year, source of patients, sample size, main primary tumor SUVmax characteristics, tumor stage, treatment strategy, and survival information (Table 1). OS was defined as the period from the date of enrollment to the date of death. LC was defined as the time between diagnosis and the first local-regional failure.
TABLE 1.
Characteristic and Results of Eligible Prognostic Studies Evaluating NSCLC Surviving
Study Quality Control
The Newcastle–Ottawa Quality Assessment Scale27 was used to assess the methodological quality of the meta-analysis; each study was reviewed by two independent reviewers (FFN and CL). A “star system” is used to obtain the score. Briefly, each study was judged on three broad perspectives: the selection of the study groups, the comparability of the groups, and the ascertainment of either the exposure or outcome of interest. The number of total stars was used to reflect the quality of the included studies. The evaluation was performed by two investigators independently (FFN and CL).
Statistical Analysis
Stata statistical software Version 11.0 software (Stata Corporation, College Station, TX) was used to perform this meta-analysis. For OS and LC, hazard ratios (HRs) with 95% confidence interval (95% CI) were calculated. The interstudy heterogeneity was evaluated with the Cochrane’s Q test (α = 0.05) as well as I2.28 A fixed effects model (Mantel–Haenszel method) was used to analyze the data as to whether there was no significant heterogeneity; otherwise, a random effects model was used according to the DerSimonian–Laird method.29 Publishing bias was tested with the Begg’s and Egger’s bias indicator test.
HR and 95% CI were directly extracted from the studies that used a multivariate survival analysis22,23,30–35 or univariate analysis.25,26,36,37 If the HR was not given explicitly,24 p value and total events were used to calculate the HR based on a method reported by Tierney et al.38 The final combination of HR was the effect value to show the prognostic significance.
A sensitivity analysis was performed by including only studies of the highest quality (with seven or more stars) or with similar cutoffs.
RESULTS
Study Identification and Quality
The literature search identified 234 relevant studies, among which 170 studies were eliminated from further analysis by the screening based on title/abstract review and supplemental author searches. The full texts of the remaining 64 articles were retrieved. Careful review of the full text eliminated 51 articles because of a lack of sufficient data (Fig. 1). The final analysis included 13 articles, and, according to the sequence of 18F-FDG PET/CT and RT performing, they were divided into two groups: pre-RT primary tumor SUVmax and post-RT primary tumor SUVmax. Five of the 13 studies had available data for both pre-RT SUVmax and post-RT SUVmax; the remaining eight studies only had pre-RT SUVmax data. The quality of the included studies is shown in Table 1. The quality score ranged from 6 to 8 with a median of 7.38; all 13 studies satisfied most of the items and reported all of the assay method and confounders. Assessment of outcome was the worst described item.
FIGURE 1.
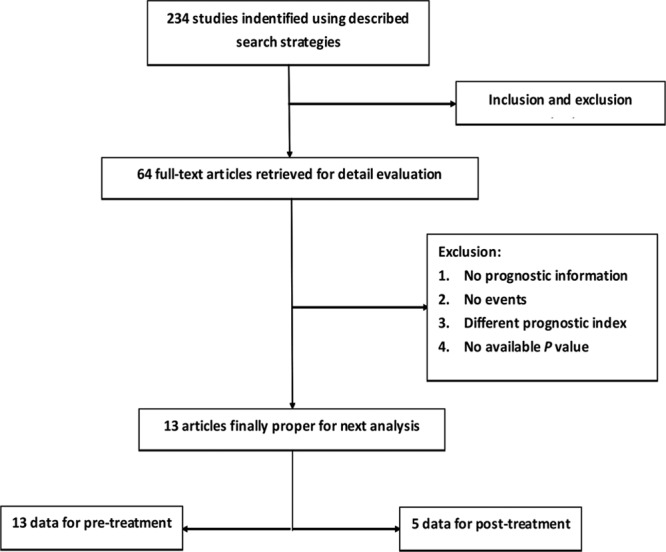
Flow diagram of the literature search strategy and assessment of studies identified for meta-analysis.
Characteristics of the Included Studies
The basic characteristics of the included studies are summarized in Table 1. Studies are listed twice if they provided survival data for both pre-RT and post-RT. All the studies were published during a period from 2005 to 2013. The sample size ranged from 46 to 132.
All 13 studies reported pre-RT SUVmax. In the eight studies (657 patients) that reported OS, three claimed significant positive predict value of OS for RT. In the seven studies (530 patients) that reported LC, three claimed predict effect of LC for RT.
Post-RT SUVmax was described in five studies (all five also described Pre-RT SUVmax). In the three studies (227 patients) that reported OS, two claimed that primary tumor SUVmax could predict OS for RT. In the four studies (391 patients) that reported LC, three claimed significant positive predict value on LC for RT.
Tumor stage and RT regimen were described in most studies (Table 1). In the analysis of pre-RT SUVmax, seven studies (54.5% of the total patients) focused on patients with stage I tumor; all these patients were treated with SBRT, for a total dose of 40 to 70 Gy. In the six remaining studies, the tumor stage was variable: stage I to III (n = 3), stage III (n = 2), stage III to IV (n = 1), and not given (n = 1), and the patients received conventional radiotherapy (CRT) with a total dose of 55 to 77 Gy. In post-RT primary tumor SUVmax, two studies included patients with stage III tumor, whereas the remaining three reports focused on stage I tumor and SBRT.
In all 13 studies included in the meta-analysis, all patients had fasted for at least 6 hours before PET/CT scanning and had a measured blood sugar level approximately 130 to 2000 mg/dl at the time of injection. The threshold level was 150 mg/dl24–26,30,34 in five studies, 200 mg/dl in three,23,33,36 130 mg/dl31 in one, 175 mg/dl22 in one, and not given in the other three publications.32,35,37 All the studies obtained emission and transmission scans 60 minutes after the injection of 18F-FDG; however, the dose of 18F-FDG varied across the studies: 5 MBq/kg in three studies,22,35,36 10 to 20 mCi in four,26,33,34,37 10 to 15 mCi in three,25,31,32 and 3,30 3.5,23 and 7.4 MBq/kg32 in the remaining three studies, respectively. Primary tumor SUVmax was normalized by body weight in all studies to minimize the partial volume effect.
“High” primary tumor SUVmax correlated with the outcome. The cutoff for primary tumor SUVmax ranged from 5 to 15 and was chosen with varying methods across the studies: four using the median SUVmax of the study sample, four using receiver-operating characteristic curve analysis, one referring to the validation results from another article, and one determined by log-rank test, and not described in the remaining two articles. Optimal timing of 18F-FDG PET/CT after the treatment also varied considerably: 12 weeks (n = 2), 10 weeks (n = 1), 8 to 24 weeks (n = 1), and not described in the remaining one study.32
Meta-Analysis
The analysis was performed separately for pre-RT SUVmax and post-RT SUVmax. The OS and LC data of both groups were analyzed. Eight studies reported pre-RT SUVmax and OS25,30,32,33–37; seven reported pre-RT SUVmax and LC22,23,25,26,30–32; three reported post-RT SUVmax and OS25,32,36; and four studies reported post-RT SUVmax and LC.22,25,26,36 Pooled HRs was then calculated for all groups.
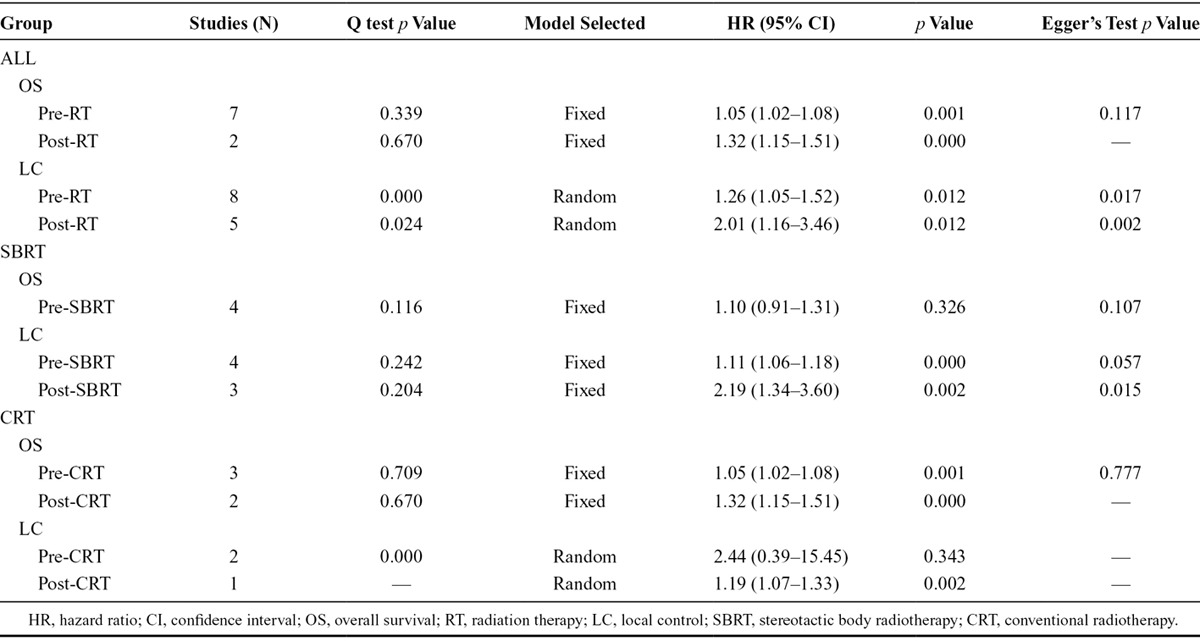
Higher pre-RT SUVmax was correlated with shorter OS (pooled HR, 1.69; 95% CI, 1.82–2.42). The heterogeneity was significant (I2 statistic = 99%, Pheterogeneity = 0.000). A forest plot attributed most of the heterogeneity to one study.32 Reanalyzing the data after exclusion of this article (I2 statistic = 11.9%, Pheterogeneity = 0.339) using a fixed effects model reduced the HR (fixed model) to 1.05 (95% CI, 1.02–1.08) (Fig. 2A). Sensitivity analysis (by excluding studies with or below six stars) revealed a combined HR at 1.05 (95% CI, 1.02–1.09) without heterogeneity (I2 = statistic 25.2%, Pheterogeneity = 0.245), indicating that the sensitivity is low and the result is more robust and credible. The pooled HR estimate for LC of the six studies using a random-effects model (I2 statistic = 77.8%, Pheterogeneity = 0.000) was 1.26 (95% CI, 1.05–1.52) (Fig. 2B). The combined HR was 1.24 (95% CI, 1.04–1.49) n a sensitivity analysis (I2 = statistic 79.5%, Pheterogeneity = 0.0255).
FIGURE 2.
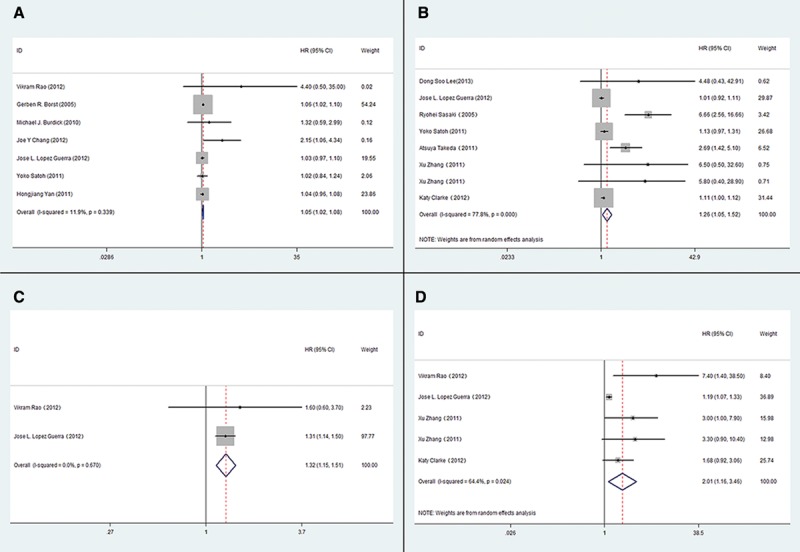
The association between primary tumor SUVmax and OS and LC of NSCLC treated by radiotherapy. A, The pooled HR estimate for OS of pre-RT primary tumor SUVmax; B, The pooled HR estimate for LC of pre-RT primary tumor SUVmax; C, The pooled HR estimate for OS of post-RT primary tumor SUVmax; D, The pooled HR estimate for LC of post-RT primary tumor SUVmax. (OS, overall survival; LC, local control; HR, hazard ratio; CI, confidence interval.)
Post-RT SUVmax also correlated with OS and LC. For OS, the pooled HR was 2.47 (95% CI, 0.58–13.03; I2 statistic = 99.3%, Pheterogeneity = 0.000). Excluding the same study excluded in the post-RT/OS association,32 the HR was reduced to 1.32 (95% CI, 1.15–1.51) with well homogeneity (I2 statistic = 0.0%, Pheterogeneity = 0.670) (Fig. 2C). The pooled HR estimate using a random effects model for LC was 2.01 (95% CI, 1.16–3.46) (Fig. 2D). Again, there was considerable heterogeneity (I2 statistic = 70.6%, Pheterogeneity = 0.017). A sensitivity analysis was assessed after excluding studies with six stars33 and excessive cutoff value (cutoff = 14).25 The combined HR (HR, 2.19; 95% CI, 1.34–3.60 and HR, 2.32; 95% CI, 1.47–3.68) indicates that the sensitivity is low and the result is robust and credible.
The studies were stratified into two groups: SBRT and CRT. In pre-SBRT primary tumor SUVmax group, the subjects were stage I patients, and high pre-SBRT SUVmax was not significantly associated with poor OS (HR, 1.10; 95% CI, 0.91–1.31) with modest homogeneity across the four included studies (I2 statistic = 49.3%, Pheterogeneity = 0.245) (Fig. 3A). High pre-SBRT SUVmax also correlated to unfavorable LC (HR, 1.28; 95% CI, 1.02–1.60). Eliminating heterogeneity (I2 statistic = 63.4%, Pheterogeneity = 0.018) by excluding a study that included T1-4N0M0 tumors23 reduced the HR estimate for LC to 1.11 (95% CI, 1.06–1.18; I2 statistic = 28.3%, Pheterogeneity = 0.242) (Fig. 3B). The post-SBRT SUVmax correlated to LC (HR, 2.19; 95% CI, 1.34–3.60; I2 statistic = 37.1%, Pheterogeneity = 0.204) (Fig. 3C), with limited data that prevented analysis of OS. For CRT, both high pre-CRT and post-CRT SUVmax correlated to poor OS (Table 2).
FIGURE 3.
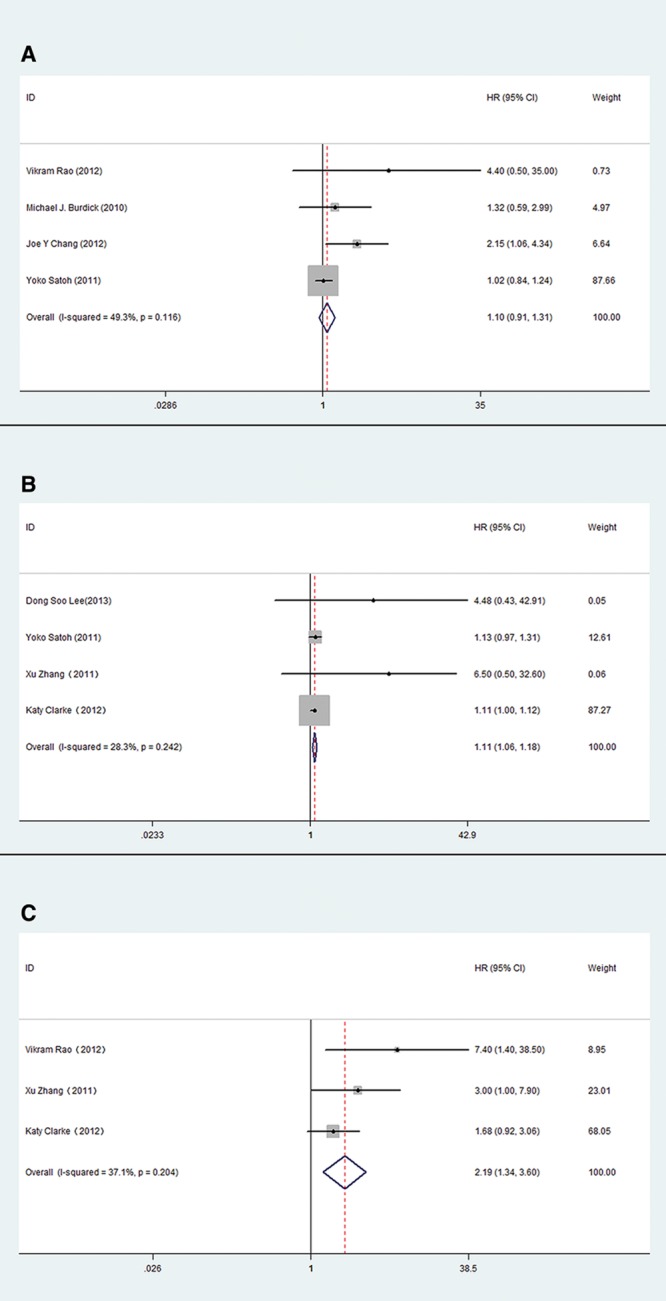
The association between primary tumor SUVmax and OS and LC of NSCLC treated by SBRT. A, The pooled HR estimate for OS of pre-SBRT primary tumor SUVmax; B, The pooled HR estimate for LC of pre-SBRT primary tumor SUVmax; C, The pooled HR estimate for LC of post-SBRT primary tumor SUVmax.
TABLE 2.
Meta-Analysis after Correcting Heterogeneity
Publication Bias
Publication bias was assessed by Begg’s and Egger’s test. Begg’s test did not find overt publication bias. Formal evaluation using Egger’s test also failed to identify significant publication bias in the analysis of pre-RT SUVmax versus OS (p = 0.317), and post-RT SUVmax versus LC (p = 0.916). Similarly, there was no evidence for significant publication bias in pre-SBRT SUVmax versus OS (p = 0.107) and in pre-CRT SUVmax versus OS (p = 0.139). The results with heterogeneity adjusted are listed in Table 2.
DISCUSSION
During the past decade, the role of 18F-FDG PET/CT has become increasingly important to the patients staging and the radiation treatment planning process in NSCLCs. It has been widely reported that SUVmax of the primary tumor in the 18F-FDG PET/CT analysis has potential prognostic use among differently staged and treated populations.22,37,39 For patients with resectable NSCLC, a meta-analysis of 13 studies showed that primary tumor SUVmax has significant prognostic value on patient survival.39 Some previous studies suggested an association of high SUVmax before RT with poor LC22 and OS,34 but such findings were not replicated by others.30
The results of the correct study confirmed a significant correlation of high level of both pre-RT and post-RT SUVmax with the increased risk of death and local recurrence. Stratified analysis (SBRT versus conventional radiation) also confirmed the prognostic value of primary tumor SUVmax regardless of the type of the treatment. These results are generally consistent with a previous study,24 and particularly so for stage I NSCLC patients treated with SBRT. Whether SUVmax is a prognostic factor of the outcome in NSCLC patients receiving RT regardless of stage and performance status requires further evaluation.
In the analysis of pre-RT versus OS, most of the heterogeneity could be attributed to one study performed in 46 Chinese patients with stage III NSCLC.32 This heterogeneity may be explained by the different methods used to estimate the results and the different threshold used. For example, the method used by the excluded study32 to estimate the results was logistic regression model. The analysis of post-RT with OS also had significant heterogeneity (because of the same study). Excluding the prediction value of primary tumor SUVmax for OS turned into significant (HR, 1.32; 95% CI, 1.15–1.51) from insignificant (HR, 2.47; 95% CI, 0.58–13.03) and demonstrated a poorer OS with high SUVmax. It is noteworthy, however, that only two studies with 181 patients were included in meta-analysis with acceptable heterogeneity. The results, therefore, must be validated by additional studies. Varying cutoff levels, different scanning equipment, and fractionated dose schemes also contributed to the heterogeneity.
Patients in all studies, including the SBRT subgroup analysis, had stage I NSCLC. As a result, the association of high pre-SBRT SUVmax with unfavorable OS and LC is not clear in patients with more advanced NSCLC. Also, the prognostic value of post-SBRT SUVmax on OS could not be confirmed in this meta-analysis because of the insufficient available data. A similar situation seems to be true of CRT as well: analysis of all five CRT studies using a random-effects model failed to confirm an association between the pre-CRT or post-CRT SUVmax and OS/LC; but reducing heterogeneity by excluding the study from China32 revealed significant association.
In addition to the absolute value of pre-RT or post-RT SUVmax, the change of duel time primary tumor SUVmax also correlated with patient outcome after RT. A study by Clarke et al.22 found significantly higher rate of distant failure in patients with a post-SBRT SUVmax of 2 or more and a reduction of less than 2.55. Another study25 also reported the greater decrease in primary tumor SUVmax that had the highest SUVmax at diagnosis, the longer OS and disease-free survival for RT. The Satoh study30 also suggested that, in comparison with pre-SBRT SUVmax, high retention index and reduced ratio of SUVmax had better prognostic value (HR, 47.546; p = 0.026). The value of the change in dual time points FDG-PET/CT as a prognostic factor for outcome in NSCLC patients receiving RT needs to be further studied, with attention to the method of calculating this change and the cutoff value.
The number of the studies, as well as the number of the patients, included in this meta-analysis is relatively small. Also, the data in some of the included studies were analyzed using univariate analysis. As a result, the nature of the observed association is obscure. Another fact is that most of the data were derived from patients with stage I NSCLC. As a result, whether the observed association could be extrapolated to patients with more advanced NSCLC is not certain. Therefore, more high-quality studies with sufficient information needs to be performed, and it should lead to a more significant meta-analysis.
In summary, the current meta-analysis confirmed an association of high pre-RT and post-RT SUVmax of primary tumor with poor outcome in NSCLC patients receiving RT. Such an association seems to be particularly strong for patients with stage I NSCLC receiving SBRT. And it supports further and high-quality investigations of SUVmax on 18F-FDG PET/CT for predicting poor outcome in NSCLC patients receiving RT.
TABLE 3.
Newcastle–Ottawa Quality Assessment Scalea

Footnotes
Disclosure: The authors declare no conflict of interest.
References
- 1.Rebecca S, Deepa N, Ahmedin J. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. [Google Scholar]
- 2.Verellen D, De Ridder M, Linthout N, Tournel K, Soete G, Storme G. Innovations in image-guided radiotherapy. Nat Rev Cancer. 2007;7:949–960. doi: 10.1038/nrc2288. [DOI] [PubMed] [Google Scholar]
- 3.Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest. 1997;111:1710–1717. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
- 4.Beitler JJ, Badine EA, El-Sayah D, et al. Stereotactic body radiation therapy for nonmetastatic lung cancer: an analysis of 75 patients treated over 5 years. Int J Radiat Oncol Biol Phys. 2006;65:100–106. doi: 10.1016/j.ijrobp.2005.11.028. [DOI] [PubMed] [Google Scholar]
- 5.Chansky K, Sculier JP, Crowley JJ, Giroux D, Van Meerbeeck J, Goldstraw P International Staging Committee and Participating Institutions. The International Association for the Study of Lung Cancer Staging Project: prognostic factors and pathologic TNM stage in surgically managed non-small cell lung cancer. J Thorac Oncol. 2009;4:792–801. doi: 10.1097/JTO.0b013e3181a7716e. [DOI] [PubMed] [Google Scholar]
- 6.Jeremić B, Milicić B, Dagović A, Acimović L, Milisavljević S. Pretreatment prognostic factors in patients with early-stage (I/II) non-small-cell lung cancer treated with hyperfractionated radiation therapy alone. Int J Radiat Oncol Biol Phys. 2006;65:1112–1119. doi: 10.1016/j.ijrobp.2006.01.044. [DOI] [PubMed] [Google Scholar]
- 7.Rades D, Setter C, Dahl O, Schild SE, Noack F. Fibroblast growth factor 2—a predictor of outcome for patients irradiated for stage II-III non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2012;82:442–447. doi: 10.1016/j.ijrobp.2010.08.048. [DOI] [PubMed] [Google Scholar]
- 8.Langendijk H, Thunnissen E, Arends JW, et al. Cell proliferation and apoptosis in stage III inoperable non-small cell lung carcinoma treated by radiotherapy. Radiother Oncol. 2000;56:197–207. doi: 10.1016/s0167-8140(00)00218-8. [DOI] [PubMed] [Google Scholar]
- 9.Choi N, Baumann M, Flentjie M, et al. Predictive factors in radiotherapy for non-small cell lung cancer: present status. Lung Cancer. 2001;31:43–56. doi: 10.1016/s0169-5002(00)00156-2. [DOI] [PubMed] [Google Scholar]
- 10.Mac Manus MP, Hicks RJ, Matthews JP, et al. Positron emission tomography is superior to computed tomography scanning for response-assessment after radical radiotherapy or chemoradiotherapy in patients with non-small-cell lung cancer. J Clin Oncol. 2003;21:1285–1292. doi: 10.1200/JCO.2003.07.054. [DOI] [PubMed] [Google Scholar]
- 11.Yu JM, Chen SQ. [Advances in research on biological target volume and conformal intensity-modulated radiotherapy]. Zhonghua Zhong Liu Za Zhi. 2006;28:801–803. [PubMed] [Google Scholar]
- 12.Massaccesi M, Calcagni ML, Spitilli MG, et al. 18F-FDG PET-CT during chemo-radiotherapy in patients with non-small cell lung cancer: the early metabolic response correlates with the delivered radiation dose. Radiat Oncol. 2012;7:106. doi: 10.1186/1748-717X-7-106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aerts HJ, van Baardwijk AA, Petit SF, et al. Identification of residual metabolic-active areas within individual NSCLC tumours using a pre-radiotherapy (18)Fluorodeoxyglucose-PET-CT scan. Radiother Oncol. 2009;91:386–392. doi: 10.1016/j.radonc.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mankoff DA, Bellon JR. Positron-emission tomographic imaging of cancer: glucose metabolism and beyond. Semin Radiat Oncol. 2001;11:16–27. doi: 10.1053/srao.2001.18100. [DOI] [PubMed] [Google Scholar]
- 15.Erasmus JJ, Rohren E, Swisher SG. Prognosis and reevaluation of lung cancer by positron emission tomography imaging. Proc Am Thorac Soc. 2009;6:171–179. doi: 10.1513/pats.200806-059LC. [DOI] [PubMed] [Google Scholar]
- 16.Malone JP, Gerberi MA, Vasireddy S, et al. Early prediction of response to chemoradiotherapy for head and neck cancer: reliability of restaging with combined positron emission tomography and computed tomography. Arch Otolaryngol Head Neck Surg. 2009;135:1119–1125. doi: 10.1001/archoto.2009.152. [DOI] [PubMed] [Google Scholar]
- 17.Meignan M, Itti E, Gallamini A, Haioun C, et al. Interim 18F-fluorodeoxyglucose positron emission tomography in diffuse large B-cell lymphoma: qualitative or quantitative interpretation—where do we stand? Leuk Lymphoma. 2009;50:1753–1756. doi: 10.3109/10428190903308056. [DOI] [PubMed] [Google Scholar]
- 18.Specht JM, Kurland BF, Montgomery SK, et al. Tumor metabolism and blood flow as assessed by positron emission tomography varies by tumor subtype in locally advanced breast cancer. Clin Cancer Res. 2010;16:2803–2810. doi: 10.1158/1078-0432.CCR-10-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ryu JS, Choi NC, Fischman AJ, Lynch TJ, Mathisen DJ. FDG-PET in staging and restaging non-small cell lung cancer after neoadjuvant chemoradiotherapy: correlation with histopathology. Lung Cancer. 2002;35:179–187. doi: 10.1016/s0169-5002(01)00332-4. [DOI] [PubMed] [Google Scholar]
- 20.Vanuytsel LJ, Vansteenkiste JF, Stroobants SG, et al. The impact of (18)F-fluoro-2-deoxy-D-glucose positron emission tomography (FDG-PET) lymph node staging on the radiation treatment volumes in patients with non-small cell lung cancer. Radiother Oncol. 2000;55:317–324. doi: 10.1016/s0167-8140(00)00138-9. [DOI] [PubMed] [Google Scholar]
- 21.Mac Manus MP, Hicks RJ, Matthews JP, et al. Positron emission tomography is superior to computed tomography scanning for response-assessment after radical radiotherapy or chemoradiotherapy in patients with non-small-cell lung cancer. J Clin Oncol. 2003;21:1285–1292. doi: 10.1200/JCO.2003.07.054. [DOI] [PubMed] [Google Scholar]
- 22.Clarke K, Taremi M, Dahele M, et al. Stereotactic body radiotherapy (SBRT) for non-small cell lung cancer (NSCLC): is FDG-PET a predictor of outcome? Radiother Oncol. 2012;104:62–66. doi: 10.1016/j.radonc.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 23.Takeda A, Yokosuka N, Ohashi T, et al. The maximum standardized uptake value (SUVmax) on FDG-PET is a strong predictor of local recurrence for localized non-small-cell lung cancer after stereotactic body radiotherapy (SBRT). Radiother Oncol. 2011;101:291–7. doi: 10.1016/j.radonc.2011.08.008. [DOI] [PubMed] [Google Scholar]
- 24.Sasaki R, Komaki R, Macapinlac H, et al. [18F]fluorodeoxyglucose uptake by positron emission tomography predicts outcome of non-small-cell lung cancer. J Clin Oncol. 2005;23:1136–1143. doi: 10.1200/JCO.2005.06.129. [DOI] [PubMed] [Google Scholar]
- 25.Lopez Guerra JL, Gladish G, Komaki R, Gomez D, Zhuang Y, Liao Z. Large decreases in standardized uptake values after definitive radiation are associated with better survival of patients with locally advanced non-small cell lung cancer. J Nucl Med. 2012;53:225–233. doi: 10.2967/jnumed.111.096305. [DOI] [PubMed] [Google Scholar]
- 26.Zhang X, Liu H, Balter P, et al. Positron emission tomography for assessing local failure after stereotactic body radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2012;83:1558–1565. doi: 10.1016/j.ijrobp.2011.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wells GA, Brodsky L, O’Connell D, et al. An Evaluation of the Newcastle Ottawa Scale: An Assessment Tool for Evaluating the Quality of on-Randomized Studies. In XI Cochrane Colloquium. Vol. O-63. Barcelona: XI International Cochrane Colloquium Book of Abstracts; 2003. p. p 26. [Google Scholar]
- 28.Dinnes J, Deeks J, Kirby J, Roderick P. A methodological review of how heterogeneity has been examined in systematic reviews of diagnostic test accuracy. Health Technol Assess. 2005;9:1–113, iii. doi: 10.3310/hta9120. [DOI] [PubMed] [Google Scholar]
- 29.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: an update. Contemp Clin Trials. 2007;28:105–114. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 30.Satoh Y, Nambu A, Onishi H, et al. Value of dual time point F-18 FDG-PET/CT imaging for the evaluation of prognosis and risk factors for recurrence in patients with stage I non-small cell lung cancer treated with stereotactic body radiation therapy. Eur J Radiol. 2012;81:3530–3534. doi: 10.1016/j.ejrad.2011.11.047. [DOI] [PubMed] [Google Scholar]
- 31.Lee DS, Kim YS, Yoo IeR, et al. Long-term clinical experience of high-dose ablative lung radiotherapy: high pre-treatment [18F]fluorodeoxyglucose-positron emission tomography maximal standardized uptake value of the primary tumor adversely affects treatment outcome. Lung Cancer. 2013;80:172–178. doi: 10.1016/j.lungcan.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 32.Zhang HQ, Yu JM, Meng X, Yue JB, Feng R, Ma L. [Prognostic value of (18)F-fluorodeoxyglucose uptake in patients with non-small cell lung cancer treated by concurrent chemoradiotherapy]. Zhonghua Zhong Liu Za Zhi. 2010;32:603–606. [PubMed] [Google Scholar]
- 33.Yan H, Wang R, Zhao F, et al. Measurement of tumor volume by PET to evaluate prognosis in patients with advanced non-small cell lung cancer treated by non-surgical therapy. Acta Radiol. 2011;52:646–650. doi: 10.1258/ar.2011.100462. [DOI] [PubMed] [Google Scholar]
- 34.Chang JY, Liu H, Balter P, et al. Clinical outcome and predictors of survival and pneumonitis after stereotactic ablative radiotherapy for stage I non-small cell lung cancer. Radiat Oncol. 2012;7:152. doi: 10.1186/1748-717X-7-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Borst GR, Belderbos JS, Boellaard R, et al. Standardised FDG uptake: a prognostic factor for inoperable non-small cell lung cancer. Eur J Cancer. 2005;41:1533–1541. doi: 10.1016/j.ejca.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 36.Bollineni VR, Widder J, Pruim J, Langendijk JA, Wiegman EM. Residual ¹8F-FDG-PET uptake 12 weeks after stereotactic ablative radiotherapy for stage I non-small-cell lung cancer predicts local control. Int J Radiat Oncol Biol Phys. 2012;83:e551–e555. doi: 10.1016/j.ijrobp.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 37.Burdick MJ, Stephans KL, Reddy CA, Djemil T, Srinivas SM, Videtic GM. Maximum standardized uptake value from staging FDG-PET/CT does not predict treatment outcome for early-stage non-small-cell lung cancer treated with stereotactic body radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78:1033–1039. doi: 10.1016/j.ijrobp.2009.09.081. [DOI] [PubMed] [Google Scholar]
- 38.Tierney JF, Stewart LA, Ghersi D. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;7:8–16. doi: 10.1186/1745-6215-8-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Berghmans T, Dusart M, Paesmans M, et al. European Lung Cancer Working Party for the IASLC Lung Cancer Staging Project. Primary tumor standardized uptake value (SUVmax) measured on fluorodeoxyglucose positron emission tomography (FDG-PET) is of prognostic value for survival in non-small cell lung cancer (NSCLC): a systematic review and meta-analysis (MA) by the European Lung Cancer Working Party for the IASLC Lung Cancer Staging Project. J Thorac Oncol. 2008;3:6–12. doi: 10.1097/JTO.0b013e31815e6d6b. [DOI] [PubMed] [Google Scholar]


