Abstract
The purpose of this study was to determine the pharmacokinetics of hydrocodone and its active metabolite hydromorphone in six healthy Greyhound dogs. Hydrocodone bitartrate was administered at a targeted dose of 0.5 mg/kg PO. Plasma concentrations of hydrocodone and hydromorphone were determined by liquid chromatography triple quadrupole mass spectrometry. The mean hydrocodone CMAX was 11.73 ng/mL at 0.74 h with a terminal half-life of 1.60 h. The mean hydromorphone CMAX was 5.2 ng/mL at 1.37 h with a terminal half-life of 3.07 h. Mean plasma hydromorphone concentrations exceeded 2 ng/mL from 0.5-8 h after hydrocodone administration. Further studies assessing the antinociceptive effects of oral hydrocodone are needed.
Keywords: Hydrocodone, Hydromorphone, Opioid, Analgesic, Dog, Greyhound
Hydrocodone is a mu opioid agonist that is partially metabolized to the active metabolite hydromorphone (Tomkins et al., 1997). The pharmacokinetics of oral hydrocodone and its metabolism to hydromorphone have not been well described in dogs. Previous studies of the oral morphine, oxycodone, and methadone resulted in low drug concentrations suggesting these drugs will produce minimal opioid effects in dogs when administered orally (Weinstein and Gaylord, 1979; KuKanich et al., 2005a; KuKanich et al., 2005b). In contrast, the mean oral bioavailability of chemical grade hydrocodone bitartrate administered to two male Beagle dogs (1.85 mg/kg hydrocodone) was 39%, and hydromorphone was detected as a metabolite (Findlay et al., 1979). Limitations of the study included the low number of dogs, the high dose administered, and the use of a radioimmunoassay which may have cross-reactivity between drug and metabolites.
The purpose of the present study was to assess the plasma concentrations of hydrocodone and its metabolite hydromorphone after oral administration of a clinically relevant dose of hydrocodone tablets to dogs. Hydrocodone bitartrate 10 mg tablets (equivalent to 6.054 mg hydrocodone) with acetaminophen 325 mg (Qualitest Pharmaceuticals) were administered per os (PO) to each dog at a targeted dose of 0.5 mg/kg hydrocodone bitartrate to the nearest ½ tablet. Any remaining portions of ½ tablet doses were analyzed by liquid chromatography coupled with triple quadrapole mass spectrometry for content to confirm the actual amount drug administered. The content of quartered tablets (5 tablets, 20 quarters) was also assessed to determine content uniformity.
Plasma concentrations of hydrocodone and hydromorphone were determined with liquid chromatography (Shimadzu Prominence, Shimadzu Scientific Instruments) and triple quadrupole mass spectrometry (API 2000, Applied Biosystems). The ions monitored were: hydrocodone (m/z 300→199.3), hydromorphone (m/z 286.14→185.2), and the internal standard hydrocodone d6 (m/z 306.21→202.30). The mobile phase consisted of A: acetonitrile and B: 0.1% formic acid. The mobile phase gradient was 100% B to 95% B from 0-2 min, 95% B to 60% B from 2-3.5 min, 60% B to 100% B from 5-7.2 min and a phenyl column (Zorbax-SB, 150×3 mm, 5μM, Agilent Technologies) achieved separation. Sample processing used solid phase extraction cartridges (SPE).
Plasma, 1 mL, was mixed with internal standard, 100 μL (hydrocodone d6, 500 ng/mL), and 1 mL 0.1 M borate buffer and vortexed for 5 s. The SPE were conditioned with 1 mL methanol and 1 mL deionized water. The plasma mixture was added, the SPE were rinsed with 1 mL deionized water and the drug eluted with 1 mL methanol. The eluate was evaporated to dryness under a stream of air at 40 °C and reconstituted with 200 μL of 15% methanol in 0.1% formic acid. The injection volume was 50 μL. The standard curves were linear from 1 to 500 ng/mL for hydromorphone and hydrocodone. Standard curves were accepted if the correlation coefficient was at least 0.99 and the predicted values were within 15% of the actual values. The accuracy of the assay was 101.3 ± 6.3% and 102.4 ± 8.2% for hydrocodone and hydromorphone, respectively.
The hydrocodone content in all the remaining ½ tablet fractions were within 10% of the expected drug content (geometric mean = 105.2%, range = 93-109%). These results suggest the appropriate dose was administered to the dogs despite administration of ½ tablet fractions to some dogs. The mean content of the quartered tablet was 96.3%, range 79 - 122%.
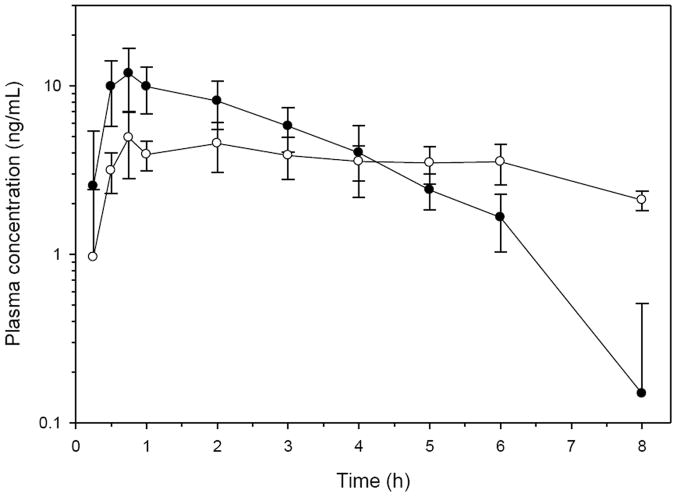
The pharmacokinetic parameters were determined by non-compartmental methods (WinNonlin 5.2, Pharsight) using default methods for hydrocodone and hydromorphone and are presented in Tables 1 and 2, and the plasma profiles are presented in Fig. 1. The geometric mean CMAX of hydrocodone was 11.73 ng/mL with a range of 7.64-20.6 ng/mL at a mean TMAX of 0.74 hours (range 0.5-1.0 h). The terminal half-life of hydrocodone was 1.60 hours (range 1.37-2.18 h).
Table 1.
Hydrocodone pharmacokinetic parameters after a mean dose of 0.5 mg/kg hydrocodone bitartrate (equivalent to 0.3 mg/kg hydrocodone base) PO to six healthy Greyhounds.
| Parameter | Units | Geometric Mean | Min | Median | Max |
|---|---|---|---|---|---|
| Dose (hydrocodone bitartrate) | mg/kg | 0.50 | 0.45 | 0.48 | 0.58 |
| AUC extrapolated | % | 8.38 | 3.86 | 8.33 | 16.72 |
| AUC inf | h*ng/mL | 36.36 | 28.67 | 32.98 | 53.57 |
| Cl/F | mL/min/kg | 139.38 | 85.25 | 147.12 | 205.80 |
| CMAX | ng/mL | 11.73 | 7.64 | 12.20 | 20.60 |
| T ½ λz | h | 1.60 | 1.37 | 1.54 | 2.18 |
| λz | 1/h | 0.433 | 0.318 | 0.450 | 0.506 |
| MRT inf | h | 2.89 | 2.54 | 2.83 | 3.55 |
| TMAX | h | 0.74 | 0.50 | 0.75 | 1.00 |
| Vz/F | L/kg | 19.33 | 12.50 | 18.93 | 31.42 |
| Actual Dose | mg/kg | 0.50 | 0.045 | 0.49 | 0.61 |
Table 2.
Hydromorphone pharmacokinetic parameters after a mean dose of 0.5 mg/kg hydrocodone bitartrate (equivalent to 0.3 mg/kg hydrocodone base) PO to six healthy Greyhounds.
| Parameter | Units | Geometric Mean | Min | Median | Max |
|---|---|---|---|---|---|
| AUC extrapolated | % | 24.77 | 17.77 | 22.64 | 38.13 |
| AUC inf | h*ng/mL | 37.17 | 30.29 | 37.49 | 41.72 |
| CMAX | ng/mL | 5.20 | 3.54 | 5.01 | 8.61 |
| T ½ λz | h | 3.07 | 2.11 | 3.08 | 4.68 |
| λz | 1/h | 0.226 | 0.148 | 0.242 | 0.329 |
| MRT inf | h | 6.11 | 5.08 | 5.69 | 8.04 |
| TMAX | h | 1.37 | 0.75 | 1.50 | 3.00 |
| Vz/F | L/kg | 36.21 | 20.68 | 39.63 | 55.61 |
Figure 1.
Plasma concentrations (mean and SD) of hydrocodone (solid circles) and hydromorphone (open circles) after a targeted dose of 0.5 mg/kg hydrocodone bitartrate (equivalent to 0.3 mg/kg hydrocodone base) PO to six healthy Greyhounds. Concentrations below the LOQ of the assay were entered as 0 for the determination of the mean and SD plasma concentrations, however values below the LOQ were excluded from the pharmacokinetic analysis.
The CMAX of hydrocodone after oral administration hydrocodone in a previous study (Findlay, et al., 1979) was ~120 ng/mL after 1.85 mg/kg of hydrocodone base (equivalent to 3.1 mg/kg hydrocodone bitartrate). If the CMAX of hydrocodone is proportional to the dose, than the expected CMAX normalized to 0.5 mg/kg hydrocodone bitartrate would be 19.4 ng/mL for the previous study, which is in the range of the current study.
The geometric mean CMAX of hydromorphone was 5.20 ng/mL with a range of 3.54-8.61 ng/mL at a mean TMAX of 1.37 h (range 0.75-3.0 h). The terminal half-life of hydromorphone was 3.07 h (range 2.11-4.68 h).
The dose normalized (0.5 mg/kg) AUCs of hydrocodone and hydromorphone from the previous study (Findlay et al., 1979) were 35 and 17.9 h*ng/mL for hydrocodone and hydromorphone, respectively. The dose normalized AUC of hydrocodone from the previous study is within the range of this study (28.67 – 53.57 h*ng/mL), but the dose normalized AUC of hydromorphone from the previous study is less than the range in this study (30.29 – 41.72 h*ng/mL). The potential differences in the hydromorphone AUCs could be due to different drug formulations, random variability, differences in assay methods, differences in dose, or breed differences in metabolism. The previous study used Beagles, whereas this study used Greyhounds and the metabolism pathway of hydrocodone to hydromorphone has not been described in dogs, therefore it is not known if there are inter-dog or inter-breed differences in hydrocodone metabolism to hydromorphone.
It is unknown if the plasma concentrations of hydrocodone and hydromorphone achieved from oral administration of hydrocodone bitartrate would produce an analgesic effect in dogs. Previous studies of 0.2 mg/kg IV hydromorphone suggest antinociceptive effects for 4 h using an electrical stimulus (Wegner et al., 2008). Pharmacokinetic studies suggest plasma hydromorphone concentrations are approximately 1.6 ng/mL at 4 h after dosing (Guedes et al., 2008). The plasma concentrations of hydromorphone after oral hydrocodone exceeded 1.6 ng/mL through 8 h in this study.
In conclusion, administration of oral hydrocodone produced both hydrocodone and hydromorphone in the plasma. Further studies should assess the antinociceptive effects of oral hydrocodone in dogs. Hydrocodone content within ½ fractions was closer to the expected content than in ¼ tablet fractions, but both were within an acceptable range.
Acknowledgments
The authors would like to thank Michelle Meyer for her technical assistance with the study. Funding was provided by the Department of Anatomy and Physiology and the Analytical Pharmacology Laboratory at Kansas State University and the Veterinary Research Scholars Program at Kansas State University (funded by NIH NCRR 5T35RR007064-10).
Footnotes
Conflict of interest statement
None of the authors of this paper has a financial or personal relationship with other people or organizations that could inappropriately influence or bias the content of the paper
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Findlay JW, Jones EC, Welch RM. Radioimmunoassay determination of the absolute oral bioavailabilities and O-demethylation of codeine and hydrocodone in the dog. Drug Metabolism and Disposition. 1979;7:310–314. [PubMed] [Google Scholar]
- Guedes AG, Papich MG, Rude EP, Rider MA. Pharmacokinetics and physiological effects of intravenous hydromorphone in conscious dogs. Journal of Veterinary Pharmacology and Therapeutics. 2008;31:334–343. doi: 10.1111/j.1365-2885.2008.00966.x. [DOI] [PubMed] [Google Scholar]
- Kukanich B, Lascelles BD, Aman AM, Mealey KL, Papich MG. The effects of inhibiting cytochrome P450 3A, p-glycoprotein, and gastric acid secretion on the oral bioavailability of methadone in dogs. Journal of Veterinary Pharmacology and Therapeutics. 2005a;28:461–466. doi: 10.1111/j.1365-2885.2005.00681.x. [DOI] [PubMed] [Google Scholar]
- Kukanich B, Lascelles BD, Papich MG. Pharmacokinetics of morphine and plasma concentrations of morphine-6-glucuronide following morphine administration to dogs. Journal of Veterinary Pharmacology and Therapeutics. 2005b;28:371–376. doi: 10.1111/j.1365-2885.2005.00661.x. [DOI] [PubMed] [Google Scholar]
- Tomkins DM, Otton SV, Joharchi N, Li NY, Balster RF, Tyndale RF, Sellers EM. Effect of cytochrome P450 2D1 inhibition on hydrocodone metabolism and its behavioral consequences in rats. Journal of Pharmacology and Experimental Therapeutics. 1997;280:1374–1382. [PubMed] [Google Scholar]
- Wegner K, Horais KA, Tozier NA, Rathbun ML, Shtaerman Y, Yaksh TL. Development of a canine nociceptive thermal escape model. Journal of Neuroscience Methods. 2008;168:88–97. doi: 10.1016/j.jneumeth.2007.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein SH, Gaylord JC. Determination of oxycodone in plasma and identification of a major metabolite. Journal of Pharmaceutical Sciences. 1979;68:527–528. doi: 10.1002/jps.2600680441. [DOI] [PubMed] [Google Scholar]