Abstract
Background
The use of testosterone among aging men has been increasing, but results from studies addressing the effectiveness of testosterone replacement therapy (TRT) have been equivocal.
Objective
Given our prior pre-clinical studies that reported a major influence of oxidative stress (OS) on testosterone’s neuroprotective effects, we investigated whether the negative effects of testosterone on brain function were predicted by oxidative load.
Methods
In order to test our hypothesis, we determined whether circulating total testosterone and luteinizing hormone (LH) correlated with cognition in a subset of the Texas Alzheimer’s Research & Care Consortium (TARCC) cohort, consisting of Caucasian (n=116) and Mexican-American (n=117) men. We also assessed whether OS (as indexed by homocysteine levels) modified this relationship between sex hormones and cognition, and whether the levels of two antioxidants, superoxide dismutase-1 (SOD), and glutathione S-transferase (GST) varied as a function of circulating testosterone.
Results
In a low OS environment, testosterone was positively associated with the level of the antioxidant, GST, while no deleterious effects on cognitive function were noted. In contrast, under conditions of high OS (homocysteine levels >12 μM), testosterone and LH were associated with cognitive impairment, but only among Caucasians. The ethnic difference was attributed to significantly higher GST levels among Mexican-Americans.
Conclusion
While testosterone may be beneficial under conditions of low OS, testosterone appears to have negative consequences under conditions of elevated OS, but only in Caucasians. Mexican-Americans, however, were protected from any deleterious effects of testosterone, potentially due to higher levels of endogenous antioxidant defenses such as GST.
Key terms: androgens, homocysteine, Mexican American, antioxidants, luteinizing hormone
INTRODUCTION
It is estimated that between 20–50% of men over the age of 60 are hypogonadal on the basis of their circulating total testosterone levels in the Baltimore Longitudinal Study of Aging (BLSA). Similarly, approximately 38% of men over 45 years of age are estimated to be hypogonadal in the Hypogonadism in Males (HIM) study [1]. In our study of cognitively intact, mild cognitive impairment (MCI), and Alzheimer’s disease (AD) male participants over 50 years of age, 25% and 58% of the Caucasian men and 5% and 50% of the Mexican-American men were hypogonadal or borderline hypogonadal, respectively. These estimates indicate that a significant number of men may be candidates for testosterone replacement therapy (TRT).
In aging men, low testosterone levels are predictive of future mobility limitations and frailty [2, 3]. Symptoms of testosterone deficiency in aging men include low muscle mass and strength, decreased ratio of lean body mass to adipose tissue, osteoporosis, decreased libido, decreased hematocrit, impaired cognition, and mood disorders [4]. TRT has been considered as a restorative/protective treatment in men against neurodegenerative disease [5, 6]. TRT has become widespread [1], as evidenced by the three fold increase in TRT prescription sales for men over 40 years of age this past decade [7]. However, in contrast to findings in younger hypogonadal men, the protective effects of TRT in older men have been equivocal [8–13]. For example, 20–28% of aging men experience either no response or a negative adverse event in response to TRT [10, 14]. Importantly, these adverse effects of TRT appear to be concentration dependent, with serious adverse effects observed in 28% of aging men with testosterone levels >10 ng/ml, 23% of aging men with 5–10 ng/ml total testosterone, and 15% of aging men with testosterone <5 ng/ml [15].
Concerns about the efficacy and safety of TRT in aging men have been raised by the FDA, and the value of TRT trials was questioned by the NIH. In order to address these concerns, the Institute of Medicine reviewed epidemiologic data on the levels of testosterone across the lifespan and ascertained the risks and benefits of TRT towards gauging the potential public health impact of TRT in the United States. Among their conclusions were that there was insufficient data to support a clear recommendation and more research was warranted [16]. Current contraindications consist of obstructive benign prostatic hyperplasia, obstructive pulmonary disease, heavy smoking, clinical prostatic carcinoma, polycythemia, mammary carcinoma, prolactinoma, and dyslipidemia [17]. Interestingly, TRT effects on the central nervous system have not been thoroughly examined; even though cognition is one of the purported benefits of TRT, and advanced age along with male gender are two of the clinical risk factors for many neurodegenerative disorders [18, 19].
A possible explanation for the variable efficacy of TRT may be that the level of oxidative stress (OS) determines whether TRT has positive or negative therapeutic effects. OS has been shown to contribute to neurodegeneration [18–20]. Our recent publications and those from other laboratories have found that androgens, such as testosterone can either be neuroprotective under conditions of low OS [21–23] or further exacerbate OS damage in a high OS environment [23, 24].
In addition to the clinical risk factors of age and gender 16,17, ethnicity has also been found to play a significant role in influencing the risk for neurodegenerative diseases, such as Alzheimer’s disease (AD) and Parkinson’s disease (PD). For example, our recent studies show increased cognitive dysfunction in Mexican-Americans diagnosed with AD and MCI compared to Caucasians [25, 26]. Further, studies from other laboratories have found differences in the incidence of PD between Caucasians and Hispanics [27, 28]. These studies indicate that ethnicity is an important variable in conditions associated with OS and should be included in clinical cohorts that are examining OS interactions.
Therefore, the current study was undertaken to determine how OS impacts the link between testosterone and cognitive/dementia status among aging Caucasian and Mexican-American men. It was hypothesized that normal testosterone levels in a high OS condition would lead to detrimental effects on cognitive/dementia status. This hypothesis was based on our previous publication showing that the extent of OS determines testosterone’s effects on neuronal function [23].
METHODS
Participants
To achieve a range of OS values, blood was drawn from individuals over 50 years of age enrolled in the longitudinal research cohort of the Texas Alzheimer’s Research Care and Consortium (TARCC). AD patients met consensus-based diagnosis for probable AD based on NINCDS-ADRDA criteria [29], MCI was defined using Petersen’s criteria [25], and normal controls performed within normal limits on cognitive testing. Participants for the current study were selected from the TARCC cohort based upon having measured levels of homocysteine, total testosterone, luteinizing hormone (LH), sex hormone binding globulin (SHBG), glutathione S-transferase, superoxide dismutase, and a CDR sum of boxes score. The final sample of 233 participants consisted of 116 Caucasian men with a mean age of 74.5 (SD = 8.4) and 117 Mexican-American men with a mean age of 68.9 (SD = 9.3). Table 1 presents the characteristics of the sample. Institutional Review Board approval was obtained at each TARCC site and written informed consent was obtained from participants and/or caregivers.
Table 1.
Demographic and Clinical Characteristics
| Caucasian Men | Mexican American Men | |||||
|---|---|---|---|---|---|---|
| Variable | N | Mean | St. Dev. | N | Mean | St. Dev. |
| Age (years) | 116 | 74.5 | 8.4 | 117 | 68.9 | 9.3 |
| min. age 54 |
max. age 94 |
min. age 51 |
max. age 94 |
|||
| N | % | N | % | |||
| hypogonadal testosterone (< 2.5 ng/ml) | 29 | 25.0 | 6 | 5.1 | ||
| borderline testosterone (2.6–3.5 ng/ml) | 67 | 57.8 | 59 | 50.4 | ||
| normal testosterone (> 3.6 ng/ml) | 20 | 17.2 | 52 | 44.4 | ||
| low oxidative stress (≤ 12 umol/L homocysteine) | 49 | 42.2 | 47 | 40.2 | ||
| high oxidative stress (> 12 umol/L homocysteine) | 67 | 57.8 | 70 | 59.8 | ||
| hyperlipidemia | 70 | 60.3 | 75 | 64.1 | ||
| hypertension | 74 | 63.8 | 77 | 65.8 | ||
| obese | 21 | 18.1 | 51 | 43.6 | ||
| diabetes mellitus | 20 | 17.2 | 45 | 38.5 | ||
| Alzheimer’s disease | 27 | 23.3 | 10 | 8.5 | ||
| Mild cognitive impairment | 22 | 19 | 26 | 22 | ||
| Cognitively intact | 67 | 57.8 | 81 | 69.2 | ||
| < high school diploma | 4 | 34.5 | 32 | 27.4 | ||
| high school diploma | 16 | 13.8 | 21 | 17.9 | ||
| ≤ 4 yrs college | 49 | 42.2 | 46 | 39.3 | ||
| > college | 47 | 40.5 | 18 | 15.4 | ||
Assays
Non-fasting peripheral blood samples were collected with 10mL serum-separating (tiger-top) vacutainers tubes at the time of interview. Samples were allowed to clot at room temperature for 30 minutes in a vertical position before being centrifuged at 1300 × g for 10 minutes. Next, 1mL aliquots were pipetted into polypropylene cryovial tubes and placed in −80° C freezers until shipment to TARCC Biobank (typically less than one week). Total processing time (blood draw to freezer) was two hours or less. Samples were shipped on dry ice to Myriad Rules Based Medicine (Myriad RBM; Austin, TX) for assessment of serum biomarkers using the Multi-Analyte Profile (humanMAP). In an effort to relate circulating total testosterone to markers of oxidative stress, the serum protein biomarkers selected for analysis in this study included total testosterone, LH, SHBG, superoxide dismutase 1 (SOD), and glutathione S-transferase alpha (GST). Information regarding the least detectable dose (LDD), inter-run coefficient of variation, dynamic range, overall spiked standard recovery, and cross-reactivity with other human MAP analytes can be obtained from Myriad Rules Based Medicine (http://www.myriadrbm.com/). Measurement of serum total homocysteine was performed in the Atherosclerosis Clinical Research Laboratory at Baylor College of Medicine. Aliquots of serum were collected as described above and stored at −80°C awaiting shipment on dry ice to Baylor. Serum homocysteine concentrations were measured using the recombinant enzymatic cycling assay (Roche Hitachi 911)[30].
Methods
Total testosterone levels were separated into three groups: hypogonadal (≤ 2.5 ng/ml), borderline (2.6–3.5 ng/ml), and normal (≥ 3.6 ng/ml), using the widely published definition of hypogonadism that ranges from <2.3–3.5 ng/ml total testosterone [31, 32]. LH levels were separated into two groups: normal (≤ 8 ng/ml) and high (≥ 8.1 ng/ml)[33, 34]. Homocysteine is an indicator of low folate and B-12 status [35], and has been used as a marker for OS in the brain [36]. Homocysteine was separated into two groups: low OS (≤ 12 umol/L) and high OS (> 12.1 umol/L) [35, 37]. The present study investigated the relationship between testosterone, OS and a measure of cognitive functioning, the Clinical Dementia Rating Sum of Boxes (CDRSUM). The CDR is one of the most commonly utilized clinical measures of clinical dementia severity in clinical trials. The CDR is a semistructured interview of patients and informants, in which the patient’s dysfunction due to cognitive decline was rated in six domains that include memory, orientation, judgment and problem solving, community affairs, home and hobbies and personal care. A global score is calculated via algorithm (scores of 0 [normal], 0.5 [questionable cognitive impairment], 1 [mild dementia], 2 [moderate dementia] and 3 [severe dementia]). The sum of boxes score (CDRSUM) is calculated via summation of the individual domain scores and reflects a more refined measure that can be utilized to track changes both within and between stages of dementia. Our group has generated interpretive guidelines for CDRSUB scores29,30. The CDRSUM has been widely used to stage severity of cognitive impairment across time [38] and has been found to be able to differentiate MCI from Alzheimer’s [39]. The relationship between testosterone, OS and the levels of two antioxidants (SOD & GST) were also investigated.
Statistical Analysis
Data is presented as mean ± SEM, and significance was designated at p ≤ 0.05. Univariate ANOVA analyses by ethnicity, total testosterone, and OS were conducted on demographic variables, irrespective of age or clinical diagnosis. Univariate ANCOVA analyses were conducted on clinical variables, wherein age and education were used as covariates. Clinical diagnosis was not included given that the CDRSUM was utilized as a linear outcome variable, and therefore, severity of cognitive impairment is a direct outcome of this study. Tukey’s HSD was used for secondary post hoc analyses. Correlations between 1) total testosterone /LH/ SHBG and age and 2) OS and age were analyzed by Pearson correlation with correlation significant at the 0.01 level (two-tailed). Due to the low number of subjects among Mexican-American men in the high OS condition, a post hoc power analysis of the association between testosterone and CDRSUM was conducted. Estimated power was at 0.701. Post hoc power calculations were made with G*Power 3.1.3 using an effect size of 0.339, α = 0.05, and a total sample size of 70 Mexican-American men in the three testosterone groups. Effect size was calculated using group means of 4.375, 0.614, and 0.323 for hypogonadal, borderline, and normal testosterone groups, respectively. All analyses were performed using SPSS version 20.
RESULTS
Participant characteristics and assessments
A total of 233 participants were assessed. Participants were separated based on ethnicity. Demographic and clinical characteristics are summarized in Table 1. While the Mexican-Americans subjects within the TARCC cohort studied were significantly younger than Caucasians (F1,232 = 22.813, p<0.001), the age range was similar. Mexican-Americans had higher rates of diabetes mellitus compared to Caucasians (F1,232 = 13.694, p<0.001). Caucasians achieved significantly more years of education than Mexican-Americans (F1,232 = 47.820, p<0.001). No differences in the prevalence of hypertension, hyperlipidemia, obesity, or OS were found between ethnic groups.
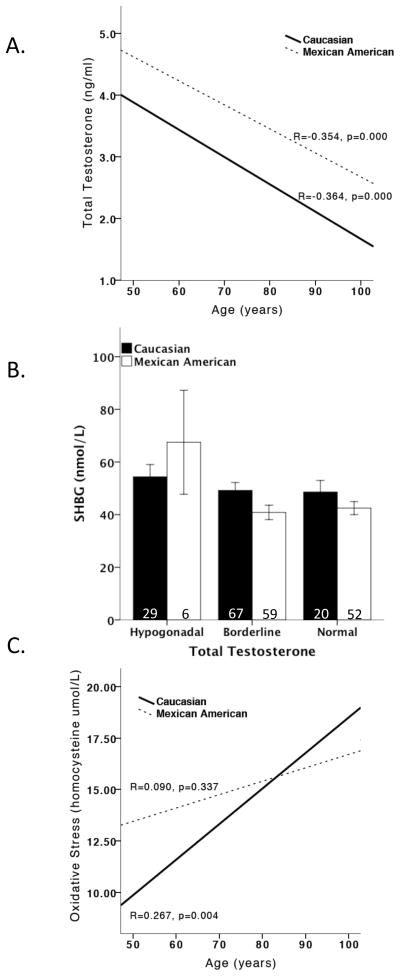
Male participants, regardless of ethnicity, had decreased total testosterone levels as a function of age. However, total testosterone levels were different between the two ethnic groups. Mexican-American men had significantly higher testosterone levels than Caucasian men (F1,232 = 65.855, p<0.001). While we did not measure free testosterone levels, analysis of sex hormone binding globulin (SHBG) revealed that SHBG levels did not vary as a function of testosterone levels, but rather varied similarly as a function of age in both Caucasian (r2=0.059, p<0.010) and Mexican American (r2=0.045, p<0.05) men. These results support the use of total testosterone in the analyses presented. Homocysteine levels, a surrogate marker for OS, were not significantly different between ethnic groups. As expected, OS increased with age in Caucasian men (r2 = 0.071, p < 0.010) but surprisingly, not in Mexican-American men (Figure 1).
Figure 1. Total testosterone and oxidative stress levels by ethnicity.
Mexican-American men had significantly higher total testosterone levels than Caucasian Men. Total testosterone levels significantly decreased as a function of age, regardless of ethnicity (A). SHBG levels were not altered by total testosterone, regardless of ethnicity (B). Oxidative stress increased as a function of age in only Caucasian men not Mexican-American men. This effect of oxidative stress in Mexican-American men may be due to testosterone’s preconditioning effects in younger Mexican-American men (C).
CDR-Sum of Boxes (CDRSUM), a measure of cognitive dysfunction, and the influence of oxidative stress and testosterone
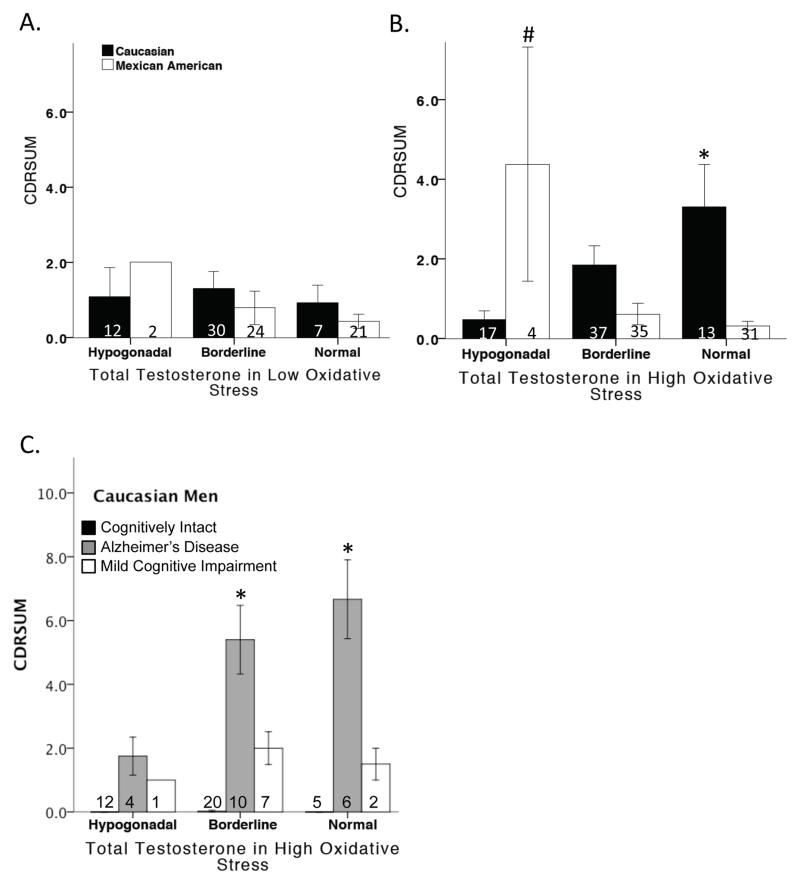
Of the 96 male participants that met the criteria for having low OS, ANCOVA revealed no deleterious effects of testosterone on CDRSUM (Figure 2A).
Figure 2. CDRSUM, a measure of cognitive dysfunction, and the influence of oxidative stress and testosterone.
In the low oxidative stress condition, testosterone levels did not alter CDRSUM scores in either Caucasian or Mexican-American men (A). In the high oxidative stress condition, testosterone significantly increased CDRSUM scores in Caucasian men, but significantly decreased CDRSUM scores in Mexican-American men (B). Testosterone significantly increased CDRSUM in Caucasians diagnosed with Alzheimer’s disease (C). * vs. hypogonadal Caucasian men and # vs. borderline and hypogonadal Mexican-American men.
Of the 137 male participants with elevated homocysteine levels (defining the high OS condition), ANCOVA analysis adjusted for age and education revealed a significant overall effect of testosterone and ethnicity (F7,137 = 6.375, p<0.001) on CDRSUM. Among Caucasian men classified as having high OS, normal total testosterone levels were associated with significantly higher CDRSUM values (i.e., poorer cognitive function) relative to hypogonadal Caucasian men (F4,67 = 3.578, p=0.011). Interestingly, testosterone increased cognitive impairment in only Caucasian men diagnosed with AD (F10,67 = 13.671, p<0.001) and had no effect on cognitively intact or men diagnosed with MCI. In contrast, hypogonadal Mexican-American men had significantly higher CDRSUM scores than Mexican-American men with either borderline and normal total testosterone levels (F4,70 = 8.022, p<0.001). Unfortunately, the sample size for Mexican American men was too low to determine the effects of testosterone as a function of clinical diagnosis (Figure 2B–C).
Superoxide dismutase 1 (SOD) and the influence of oxidative stress and testosterone
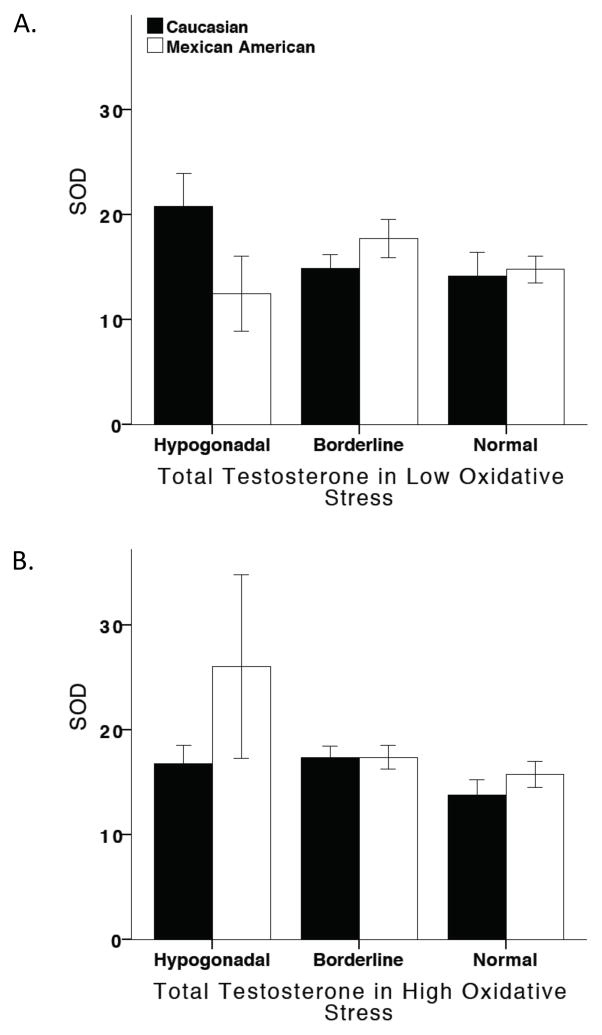
No correlation existed between testosterone levels and SOD expression, regardless of OS or ethnicity (Figure 3).
Figure 3. SOD and the influence of oxidative stress and testosterone.
Testosterone did not alter SOD levels in either Caucasians or Mexican-Americans, regardless of oxidative stress.
Glutathione S-transferase alpha (GST) and the influence of oxidative stress and testosterone
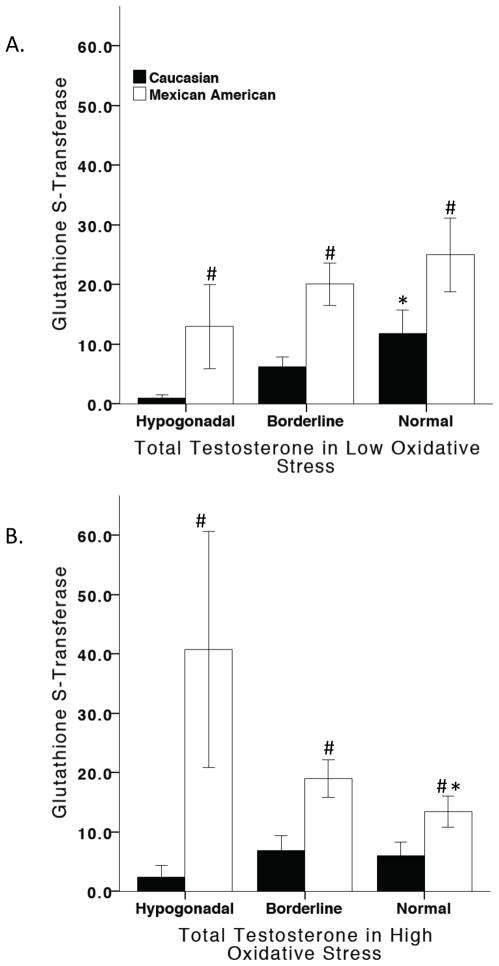
In male subjects with low OS, ANCOVA analysis adjusted for age and education showed a significant overall effect of testosterone and ethnicity on GST (F7,96 = 3.961, p=0.001). Mexican-American men in the low OS condition had higher GST levels than Caucasian men. Further analysis revealed that levels of testosterone were positively associated with GST levels in Caucasian men (F4,49 = 3.560, p=0.013), while no effects of testosterone on GST levels in Mexican-American were found in the low OS condition (Figure 4A). In subjects with high OS, ANCOVA analysis adjusted for age and education revealed a significant effect of testosterone and ethnicity on GST (F7,137 = 5.009, p<0.001). Mexican-American men in the high OS condition had higher GST levels than Caucasian men. Statistical analysis by ethnicity showed that testosterone was negatively associated with GST levels in Mexican-American men (F4,70 = 3.767, p=0.008) whereas no effects of testosterone on GST levels in a high OS condition were found in Caucasian men (Figure 4B).
Figure 4. GST and the influence of oxidative stress and testosterone.
In low oxidative stress, Mexican-American men had significantly higher GST levels than Caucasian men. Testosterone increased GST levels in Caucasian men (A). In high oxidative stress, Mexican-American men had significantly higher GST levels than Caucasian men. Testosterone significantly decreased GST levels in Mexican-American men (B). # vs. Caucasian men, * vs. hypogonadal men.
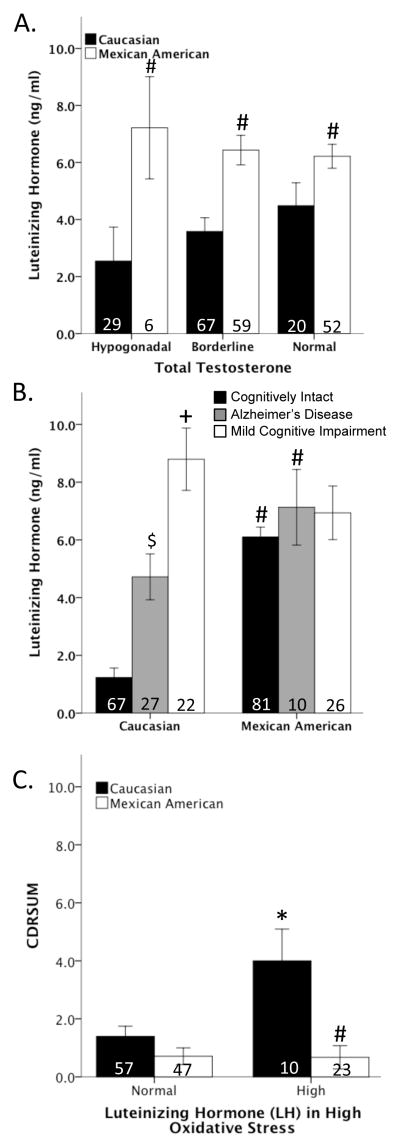
Luteinizing hormone (LH) and cognition
LH regulates the secretion of testosterone from the Leydig cells of the testis. ANCOVA analysis adjusted for age and education revealed an overall effect of ethnicity on LH (F1,233 = 10.822, p < 0.001), in which Mexican American men had greater LH levels than Caucasian men, but LH did not vary as a function of testosterone levels (Figure 5A). Analysis of LH revealed that LH levels increased as a function of age in only Caucasian (r2=0.097, p<0.001) and not Mexican American men. Consistent with prior literature in which LH associated with cognitive impairment [40–42], ANCOVA showed a significant effect of clinical diagnoses and ethnicity on LH (F7,233 = 18.377, p < 0.001). Caucasian men diagnosed with either AD or MCI had significantly higher LH levels than cognitively intact men, and no effect of clinical diagnosis was observed in Mexican American men. Statistical analyses by ethnicity revealed that Mexican American men had higher LH levels than Caucasian men (F1,233 = 6.043, p < 0.05) (Figure 5B). In subject with high OS, ANCOVA analysis adjusted for age and education revealed a significant overall effect of LH and ethnicity (F3,137 = 5.710, p<0.001) on CDRSUM. Among men classified as having high OS, LH was associated with significantly higher CDRSUM values (i.e., poorer cognitive function) in only Caucasian men (F1,137 = 6.318, p<0.05) (Figure 5C).
Figure 5. Luteinizing Hormone (LH) and cognition.
Testosterone did not alter LH levels in either Caucasians or Mexican-American men. However, Mexican-Americans have significantly higher LH levels than Caucasians (A). In Caucasians, LH is higher in men diagnosed with Alzheimer’s disease and Mild Cognitive Impairment compared to cognitively intact men. No association between LH and disease status in Mexican-Americans (B). In a high oxidative stress condition, increased LH significantly increased CDRSUM (cognitive dysfunction) only in Caucasians (C). # vs. Caucasian men, + vs. Alzheimer’s disease and cognitively intact Caucasian men, $ v. cognitively intact Caucasian men, * vs. Caucasian men with normal LH.
DISCUSSION
TRT use in older men has been increasing [5, 6], as supported by a recent three-fold increase in TRT prescriptions for men over 40 years of age [43]. Results from studies addressing TRT efficacy and safety in older men have been equivocal, resulting in concerns by the FDA and NIH [16, 44, 45]. Further, TRT effects on the CNS have not been thoroughly examined, even though aging and male gender are risk factors for certain neurodegenerative disorders [18, 19]. Given our prior pre-clinical studies that reported a major influence of OS on testosterone’s neuroprotective/neurotoxic effects, such that under conditions of elevated OS, testosterone exerted deleterious effects [23, 24], we examined whether a correlation existed between levels of testosterone and cognitive function in aged men, and whether the relationship differed as a function of their OS status (as defined by their levels of serum homocysteine). Further, given emerging evidence on ethnic differences in the neurobiology of neurodegenerative disorders 26,27, we also determined if the relationship between testosterone and cognitive function differed between Caucasians and Mexican-Americans. The current study is the first to investigate the impact of testosterone and ethnicity on these relationships.
This study supports the conclusion that in older Caucasians, testosterone may be beneficial under conditions of low OS, but has deleterious effects under the conditions of elevated OS, especially among Caucasian men diagnosed with AD. Interestingly, this relationship was not apparent among Mexican-Americans such that testosterone levels were not associated with negative effects on cognitive function in either subjects with low OS or those with high OS. One explanation underlying this ethnic difference is the relative abundance of antioxidant enzymes, as supported by the fact that the Mexican-Americans in our study had higher levels of GST. As such, Mexican-Americans may have been “protected” from the OS-exacerbating effects of androgens. Further supporting the “protected” nature of Mexican-Americans was the fact that OS levels did not vary with age in this ethnic group, in contrast to the expected increase in OS as a function of age in Caucasians.
In low OS, testosterone was positively associated with the antioxidant enzyme, GST, and no deleterious effects of testosterone on cognition were noted. This increase in antioxidants may be one mechanism underlying the lack of cognitive dysfunction in Caucasians with low OS. However, testosterone’s effects on antioxidants were lost in a high OS condition, in which testosterone was associated with cognitive dysfunction in Caucasians.
Interestingly, this study showed that 20% of Caucasians exhibited cognitive dysfunction, which is consistent with prior studies reporting 20–28% of aging men experienced adverse events in response to TRT 11,12. This suggests that a negative interaction between testosterone and OS may underlie some of the adverse events associated with TRT.
Our prior pre-clinical studies showed that under conditions of elevated OS, testosterone switches from exerting cytoprotective effects to exacerbating OS-induced neuronal damage [23]. We suggest that the data presented herein are consistent with our prior cellular studies; where only under conditions of high OS did testosterone compromise neuronal health and viability [23].
In addition to testosterone association with deleterious effects under conditions of oxidative stress, our studies found that elevated LH, a regulator of testosterone secretion, is also associated with cognitive impairment under condition of elevated oxidative stress. Generally, a negative feedback relationship exists between testosterone and LH. However, this relationship may not be accurate in aging men. Several studies, including this study, consistently show LH increasing as a function of age in elderly men [46–49]. Furthermore, increasing evidence indicates that LH may not be correlated with testosterone in aging men [46, 50], which is consistent with our findings. Associations between elevated LH levels and AD have been reported by a number of groups, including our study [40–42, 51, 52]. In both rodent and human studies, LH increased cognitive dysfunction and amyloid-β deposition [51–54], and an increase in LH levels has been postulated as an initial event in AD pathology [51]. Our observations of increased levels of LH in Caucasian men diagnosed with MCI relative to cognitively intact men tends to support this hypothesis.
One of the more interesting findings in the present study is the ethnicity difference between Caucasian and Mexican American men with regard to their sex steroid profile. There is a dearth of literature on ethnic differences in sex steroid profiles, and this lack of knowledge is particularly acute with respect to the impact of aging on such differences. In our study, Mexican American men consistently had higher serum LH and testosterone levels than Caucasian men. This increase in serum testosterone levels in Mexican American men is consistent with previous findings in Mexican American men [55, 56]. This is the first study to compare LH across different ethnic male populations.
Since our data support the conclusion that OS influences the effect of testosterone, assessment of the OS “burden” by measuring serum homocysteine levels may be a way to pre-screen individuals for whom TRT may be indicated. In fact, the current findings, along with our prior studies, strongly suggest that OS status, and perhaps ethnicity, should be considered in the decision to treat men with testosterone [17]. Specifically, we propose that TRT could be beneficial as a neuroprotectant for individuals with low OS. However, under conditions of high OS (i.e. AD, PD, ischemic stroke), TRT should be avoided, as there appears to be a high risk for testosterone to exacerbate OS damage. Further, given that OS did not increase with age in Mexican-Americans, TRT may be an option for young OR older Mexican-American men.
Although we used homocysteine as a marker for OS, homocysteine is also an indicator of low folate and B-12 status [35]. Folate deficiency can induce OS in numerous cell types [57–59], including OS-mediated degeneration in neuronal cells [60–62]. Prior studies indicate folate deficiency as a risk factor for AD and dementia [63–66]. However, randomized clinical trials of B-12 and folate supplements have been unable to show cognitive benefits [67].
This study was subject to several limitations, primarily a low participant number in the Mexican-American hypogonadal group (n=2 for low OS and n=4 for high OS). Other concerns include the fact that our findings have only been observed in a single cross-sectional cohort and that only a single marker for OS was measured. With respect to limitations concerning antioxidant enzymes, this study only examined two antioxidant enzymes, SOD and GST. Clinical studies examining AD and MCI have found differences in other antioxidant enzymes, such as catalase, glutathione peroxidase, and glutathione reductase [68–71]. Previous studies have also shown that testosterone can affect catalase activity in cells [22, 72, 73]. Although the current study only evaluated the relationship between testosterone and GST, we feel that it is probable that testosterone alters the other two enzymes involved in glutathione metabolism, glutathione peroxidase and glutathione reductase. Ongoing and future experiments are aimed at addressing these issues.
Acknowledgments
Funding/Support: This study was supported by the Texas Alzheimer’s Research and Care Consortium (TARCC) funded by the state of Texas through the Texas Council on Alzheimer’s Disease and Related Disorders. Research reported in this publication was supported by the National Institute on Aging (NIA) under award numbers R01AG039389 and P30AG12300 to SO, Garvey Texas Foundation, UNT Health Science Center Young Investigator Intramural Funding, and American Heart Association BGIA to RLC, and NIH/NIA awards numbers AG022550 and AG027956 to MS.
Footnotes
Author contributions: Dr. Cunningham has full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Conflict of Interest Disclosures: The authors have no conflicts of interest.
References
- 1.Mulligan T, Frick MF, Zuraw QC, Stemhagen A, McWhirter C. Prevalence of hypogonadism in males aged at least 45 years: the HIM study. International journal of clinical practice. 2006;60:762–769. doi: 10.1111/j.1742-1241.2006.00992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Krasnoff JB, Basaria S, Pencina MJ, Jasuja GK, Vasan RS, Ulloor J, Zhang A, Coviello A, Kelly-Hayes M, D’Agostino RB, Wolf PA, Bhasin S, Murabito JM. Free testosterone levels are associated with mobility limitation and physical performance in community-dwelling men: the Framingham Offspring Study. J Clin Endocrinol Metab. 2010;95:2790–2799. doi: 10.1210/jc.2009-2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hyde Z, Flicker L, Almeida OP, Hankey GJ, McCaul KA, Chubb SA, Yeap BB. Low free testosterone predicts frailty in older men: the health in men study. J Clin Endocrinol Metab. 2010;95:3165–3172. doi: 10.1210/jc.2009-2754. [DOI] [PubMed] [Google Scholar]
- 4.Tenover JL. Testosterone replacement therapy in older adult men. Int J Androl. 1999;22:300–306. doi: 10.1046/j.1365-2605.1999.00184.x. [DOI] [PubMed] [Google Scholar]
- 5.Morales A, Johnston B, Heaton JP, Lundie M. Testosterone supplementation for hypogonadal impotence: assessment of biochemical measures and therapeutic outcomes. J Urol. 1997;157:849–854. doi: 10.1016/s0022-5347(01)65062-6. [DOI] [PubMed] [Google Scholar]
- 6.Tenover JS. Effects of testosterone supplementation in the aging male. J Clin Endocrinol Metab. 1992;75:1092–1098. doi: 10.1210/jcem.75.4.1400877. [DOI] [PubMed] [Google Scholar]
- 7.Baillargeon J, Urban RJ, Ottenbacher KJ, Pierson KS, Goodwin JS. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Internal Medicine. 2013;173:1465–1466. doi: 10.1001/jamainternmed.2013.6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Okun MS, Fernandez HH, Rodriguez RL, Romrell J, Suelter M, Munson S, Louis ED, Mulligan T, Foster PS, Shenal BV, Armaghani SJ, Jacobson C, Wu S, Crucian G. Testosterone therapy in men with Parkinson disease: results of the TEST-PD Study. Arch Neurol. 2006;63:729–735. doi: 10.1001/archneur.63.5.729. [DOI] [PubMed] [Google Scholar]
- 9.Cunningham GR, Toma SM. Clinical review: Why is androgen replacement in males controversial? The Journal of clinical endocrinology and metabolism. 2011;96:38–52. doi: 10.1210/jc.2010-0266. [DOI] [PubMed] [Google Scholar]
- 10.Hildreth KL, Barry DW, Moreau KL, Vande Griend J, Meacham RB, Nakamura T, Wolfe P, Kohrt WM, Ruscin JM, Kittelson J, Cress ME, Ballard R, Schwartz RS. Effects of testosterone and progressive resistance exercise in healthy, highly functioning older men with low-normal testosterone levels. The Journal of clinical endocrinology and metabolism. 2013;98:1891–1900. doi: 10.1210/jc.2012-3695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cherrier MM, Matsumoto AM, Amory JK, Asthana S, Bremner W, Peskind ER, Raskind MA, Craft S. Testosterone improves spatial memory in men with Alzheimer disease and mild cognitive impairment. Neurology. 2005;64:2063–2068. doi: 10.1212/01.WNL.0000165995.98986.F1. [DOI] [PubMed] [Google Scholar]
- 12.Cherrier MM. Testosterone effects on cognition in health and disease. Frontiers of hormone research. 2009;37:150–162. doi: 10.1159/000176051. [DOI] [PubMed] [Google Scholar]
- 13.Tan RS, Pu SJ. A pilot study on the effects of testosterone in hypogonadal aging male patients with Alzheimer’s disease. The aging male : the official journal of the International Society for the Study of the Aging Male. 2003;6:13–17. [PubMed] [Google Scholar]
- 14.Grober ED, Khera M, Soni SD, Espinoza MG, Lipshultz LI. Efficacy of changing testosterone gel preparations (Androgel or Testim) among suboptimally responsive hypogonadal men. International journal of impotence research. 2008;20:213–217. doi: 10.1038/sj.ijir.3901618. [DOI] [PubMed] [Google Scholar]
- 15.Basaria S, Coviello AD, Travison TG, Storer TW, Farwell WR, Jette AM, Eder R, Tennstedt S, Ulloor J, Zhang A, Choong K, Lakshman KM, Mazer NA, Miciek R, Krasnoff J, Elmi A, Knapp PE, Brooks B, Appleman E, Aggarwal S, Bhasin G, Hede-Brierley L, Bhatia A, Collins L, LeBrasseur N, Fiore LD, Bhasin S. Adverse events associated with testosterone administration. The New England journal of medicine. 2010;363:109–122. doi: 10.1056/NEJMoa1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Committee on Assessing the Need for Clinical Trials of Testosterone Replacement Therapy. Testosterone and Aging: Clinical Research Directions. The National Academies Press; 2004. [PubMed] [Google Scholar]
- 17.Vermeulen A. Androgen replacement therapy in the aging male--a critical evaluation. J Clin Endocrinol Metab. 2001;86:2380–2390. doi: 10.1210/jcem.86.6.7630. [DOI] [PubMed] [Google Scholar]
- 18.Schrag A, Ben-Shlomo Y, Quinn NP. Cross sectional prevalence survey of idiopathic Parkinson’s disease and Parkinsonism in London. BMJ. 2000;321:21–22. doi: 10.1136/bmj.321.7252.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gillies GE, Murray HE, Dexter D, McArthur S. Sex dimorphisms in the neuroprotective effects of estrogen in an animal model of Parkinson’s disease. Pharmacol Biochem Behav. 2004;78:513–522. doi: 10.1016/j.pbb.2004.04.022. [DOI] [PubMed] [Google Scholar]
- 20.Farooqui T, Farooqui AA. Aging: an important factor for the pathogenesis of neurodegenerative diseases. Mech Ageing Dev. 2009;130:203–215. doi: 10.1016/j.mad.2008.11.006. [DOI] [PubMed] [Google Scholar]
- 21.Cunningham RL, Giuffrida A, Roberts JL. Androgens induce dopaminergic neurotoxicity via caspase-3-dependent activation of protein kinase C delta. Endocrinology. 2009;150:5539–5548. doi: 10.1210/en.2009-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahlbom E, Prins GS, Ceccatelli S. Testosterone protects cerebellar granule cells from oxidative stress-induced cell death through a receptor mediated mechanism. Brain research. 2001;892:255–262. doi: 10.1016/s0006-8993(00)03155-3. [DOI] [PubMed] [Google Scholar]
- 23.Holmes S, Abbassi B, Su C, Singh M, Cunningham RL. Oxidative stress defines the neuroprotective or neurotoxic properties of androgens in immortalized female rat dopaminergic neuronal cells. Endocrinology. 2013;154:4281–4292. doi: 10.1210/en.2013-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cunningham RL, Macheda T, Watts LT, Poteet E, Singh M, Roberts JL, Giuffrida A. Androgens exacerbate motor asymmetry in male rats with unilateral 6-hydroxydopamine lesion. Hormones and behavior. 2011;60:617–624. doi: 10.1016/j.yhbeh.2011.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Bryant SE, Johnson L, Balldin V, Edwards M, Barber R, Williams B, Devous M, Cushings B, Knebl J, Hall J. Characterization of Mexican Americans with mild cognitive impairment and Alzheimer’s disease. Journal of Alzheimer’s disease : JAD. 2013;33:373–379. doi: 10.3233/JAD-2012-121420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Bryant SE, Xiao G, Edwards M, Devous M, Gupta VB, Martins R, Zhang F, Barber R. Biomarkers of Alzheimer’s disease among Mexican Americans. Journal of Alzheimer’s disease : JAD. 2013;34:841–849. doi: 10.3233/JAD-122074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Marder K, Tang MX, Mejia H, Alfaro B, Cote L, Louis E, Groves J, Mayeux R. Risk of Parkinson’s disease among first-degree relatives: A community-based study. Neurology. 1996;47:155–160. doi: 10.1212/wnl.47.1.155. [DOI] [PubMed] [Google Scholar]
- 28.Van Den Eeden S, Tanner C, Bernstein A, Fross R, Leimpeter A, Bloch D, Nelson L. Incidence of Parkinson’s disease: variation by age, gender, and race/ethnicity. Am J Epidemiol. 2003;157:1015–1022. doi: 10.1093/aje/kwg068. [DOI] [PubMed] [Google Scholar]
- 29.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 30.Hafner G, Fickenscher K, Friesen HJ, Rupprecht HJ, Konheiser U, Ehrenthal W, Lotz J, Prellwitz W. Evaluation of an automated chromogenic substrate assay for the rapid determination of hirudin in plasma. Thrombosis research. 1995;77:165–173. doi: 10.1016/0049-3848(95)91622-r. [DOI] [PubMed] [Google Scholar]
- 31.Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, Montori VM. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. The Journal of clinical endocrinology and metabolism. 2010;95:2536–2559. doi: 10.1210/jc.2009-2354. [DOI] [PubMed] [Google Scholar]
- 32.Wang C, Nieschlag E, Swerdloff RS, Behre H, Hellstrom WJ, Gooren LJ, Kaufman JM, Legros JJ, Lunenfeld B, Morales A, Morley JE, Schulman C, Thompson IM, Weidner W, Wu FC. ISA, ISSAM, EAU, EAA and ASA recommendations: investigation, treatment and monitoring of late-onset hypogonadism in males. The aging male : the official journal of the International Society for the Study of the Aging Male. 2009;12:5–12. doi: 10.1080/13685530802389628. [DOI] [PubMed] [Google Scholar]
- 33.Kaplan LA, Pesce AJ. The Gonads. In: Kazmierczak SC, editor. Clinical Chemestry: Theory, Analysis, and Correlation. Mosby-Year Book; St. Louis, MO: 1996. p. 894. [Google Scholar]
- 34.Mayo Clinic Mayo Medical Laboratories. Test ID: LH Luteinizing Hormone (LH) Serum: Mayo Foundation for Medical Education and Research; [Accessed 12-2-2013]. http://www.mayomedicallaboratories.com/test-catalog/Clinical+and+Interpretive/8663. [Google Scholar]
- 35.Refsum H, Smith AD, Ueland PM, Nexo E, Clarke R, McPartlin J, Johnston C, Engbaek F, Schneede J, McPartlin C, Scott JM. Facts and recommendations about total homocysteine determinations: an expert opinion. Clinical chemistry. 2004;50:3–32. doi: 10.1373/clinchem.2003.021634. [DOI] [PubMed] [Google Scholar]
- 36.Marlatt MW, Lucassen PJ, Perry G, Smith MA, Zhu X. Alzheimer’s disease: cerebrovascular dysfunction, oxidative stress, and advanced clinical therapies. Journal of Alzheimer’s disease : JAD. 2008;15:199–210. doi: 10.3233/jad-2008-15206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hortin GL. Homocysteine: Clinical significance and laboratory measurement. Lab Med. 2006;37:551–553. [Google Scholar]
- 38.O’Bryant SE, Waring SC, Cullum CM, Hall J, Lacritz L, Massman PJ, Lupo PJ, Reisch JS, Doody R. Staging dementia using Clinical Dementia Rating Scale Sum of Boxes scores: a Texas Alzheimer’s research consortium study. Archives of neurology. 2008;65:1091–1095. doi: 10.1001/archneur.65.8.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Bryant SE, Xiao G, Barber R, Reisch J, Doody R, Fairchild T, Adams P, Waring S, Diaz-Arrastia R. A serum protein-based algorithm for the detection of Alzheimer disease. Archives of neurology. 2010;67:1077–1081. doi: 10.1001/archneurol.2010.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bowen RL, Isley JP, Atkinson RL. An association of elevated serum gonadotropin concentrations and Alzheimer disease? Journal of neuroendocrinology. 2000;12:351–354. doi: 10.1046/j.1365-2826.2000.00461.x. [DOI] [PubMed] [Google Scholar]
- 41.Short RA, Bowen RL, O’Brien PC, Graff-Radford NR. Elevated gonadotropin levels in patients with Alzheimer disease. Mayo Clinic proceedings. Mayo Clinic. 2001;76:906–909. doi: 10.4065/76.9.906. [DOI] [PubMed] [Google Scholar]
- 42.Bowen RL, Smith MA, Harris PL, Kubat Z, Martins RN, Castellani RJ, Perry G, Atwood CS. Elevated luteinizing hormone expression colocalizes with neurons vulnerable to Alzheimer’s disease pathology. Journal of neuroscience research. 2002;70:514–518. doi: 10.1002/jnr.10452. [DOI] [PubMed] [Google Scholar]
- 43.Baillargeon JURJOKJPKSGJS. Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Internal Medicine. 2013:1–2. doi: 10.1001/jamainternmed.2013.6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pittman D. MedPage Today. MedPage Today; 2013. [Google Scholar]
- 45.Clarke T. Reuters. Reuters; 2013. [Google Scholar]
- 46.Morley JE, Kaiser FE, Perry HM, 3rd, Patrick P, Morley PM, Stauber PM, Vellas B, Baumgartner RN, Garry PJ. Longitudinal changes in testosterone, luteinizing hormone, and follicle-stimulating hormone in healthy older men. Metabolism: clinical and experimental. 1997;46:410–413. doi: 10.1016/s0026-0495(97)90057-3. [DOI] [PubMed] [Google Scholar]
- 47.van den Beld A, Huhtaniemi IT, Pettersson KS, Pols HA, Grobbee DE, de Jong FH, Lamberts SW. Luteinizing hormone and different genetic variants, as indicators of frailty in healthy elderly men. The Journal of clinical endocrinology and metabolism. 1999;84:1334–1339. doi: 10.1210/jcem.84.4.5616. [DOI] [PubMed] [Google Scholar]
- 48.Campbell B, Leslie P, Campbell K. Age-related patterns of urinary gonadotropins (FSH and LH) and E-3-G as measures of reproductive function among Turkana males of northern Kenya. Social biology. 2006;53:30–45. doi: 10.1080/19485565.2006.9989115. [DOI] [PubMed] [Google Scholar]
- 49.Gray A, Feldman HA, McKinlay JB, Longcope C. Age, disease, and changing sex hormone levels in middle-aged men: results of the Massachusetts Male Aging Study. J Clin Endocrinol Metab. 1991;73:1016–1025. doi: 10.1210/jcem-73-5-1016. [DOI] [PubMed] [Google Scholar]
- 50.Pincus SM, Mulligan T, Iranmanesh A, Gheorghiu S, Godschalk M, Veldhuis JD. Older males secrete luteinizing hormone and testosterone more irregularly, and jointly more asynchronously, than younger males. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:14100–14105. doi: 10.1073/pnas.93.24.14100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Webber KM, Casadesus G, Bowen RL, Perry G, Smith MA. Evidence for the role of luteinizing hormone in Alzheimer disease. Endocrine, metabolic & immune disorders drug targets. 2007;7:300–303. doi: 10.2174/187153007782794326. [DOI] [PubMed] [Google Scholar]
- 52.Bowen RL, Verdile G, Liu T, Parlow AF, Perry G, Smith MA, Martins RN, Atwood CS. Luteinizing hormone, a reproductive regulator that modulates the processing of amyloid-beta precursor protein and amyloid-beta deposition. The Journal of biological chemistry. 2004;279:20539–20545. doi: 10.1074/jbc.M311993200. [DOI] [PubMed] [Google Scholar]
- 53.Lalonde R. The neurobiological basis of spontaneous alternation. Neuroscience and biobehavioral reviews. 2002;26:91–104. doi: 10.1016/s0149-7634(01)00041-0. [DOI] [PubMed] [Google Scholar]
- 54.Casadesus G, Webber KM, Atwood CS, Pappolla MA, Perry G, Bowen RL, Smith MA. Luteinizing hormone modulates cognition and amyloid-beta deposition in Alzheimer APP transgenic mice. Biochimica et biophysica acta. 2006;1762:447–452. doi: 10.1016/j.bbadis.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 55.Rohrmann S, Nelson WG, Rifai N, Brown TR, Dobs A, Kanarek N, Yager JD, Platz EA. Serum estrogen, but not testosterone, levels differ between black and white men in a nationally representative sample of Americans. The Journal of clinical endocrinology and metabolism. 2007;92:2519–2525. doi: 10.1210/jc.2007-0028. [DOI] [PubMed] [Google Scholar]
- 56.Nyante SJ, Graubard BI, Li Y, McQuillan GM, Platz EA, Rohrmann S, Bradwin G, McGlynn KA. Trends in sex hormone concentrations in US males: 1988–1991 to 1999–2004. International journal of andrology. 2012;35:456–466. doi: 10.1111/j.1365-2605.2011.01230.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chern CL, Huang RF, Chen YH, Cheng JT, Liu TZ. Folate deficiency-induced oxidative stress and apoptosis are mediated via homocysteine-dependent overproduction of hydrogen peroxide and enhanced activation of NF-kappaB in human Hep G2 cells. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2001;55:434–442. doi: 10.1016/s0753-3322(01)00095-6. [DOI] [PubMed] [Google Scholar]
- 58.Huang RF, Hsu YC, Lin HL, Yang FL. Folate depletion and elevated plasma homocysteine promote oxidative stress in rat livers. The Journal of nutrition. 2001;131:33–38. doi: 10.1093/jn/131.1.33. [DOI] [PubMed] [Google Scholar]
- 59.Ho PI, Ortiz D, Rogers E, Shea TB. Multiple aspects of homocysteine neurotoxicity: glutamate excitotoxicity, kinase hyperactivation and DNA damage. Journal of neuroscience research. 2002;70:694–702. doi: 10.1002/jnr.10416. [DOI] [PubMed] [Google Scholar]
- 60.Ho PI, Ashline D, Dhitavat S, Ortiz D, Collins SC, Shea TB, Rogers E. Folate deprivation induces neurodegeneration: roles of oxidative stress and increased homocysteine. Neurobiology of disease. 2003;14:32–42. doi: 10.1016/s0969-9961(03)00070-6. [DOI] [PubMed] [Google Scholar]
- 61.Kruman II, Culmsee C, Chan SL, Kruman Y, Guo Z, Penix L, Mattson MP. Homocysteine elicits a DNA damage response in neurons that promotes apoptosis and hypersensitivity to excitotoxicity. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2000;20:6920–6926. doi: 10.1523/JNEUROSCI.20-18-06920.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mattson MP, Shea TB. Folate and homocysteine metabolism in neural plasticity and neurodegenerative disorders. Trends in neurosciences. 2003;26:137–146. doi: 10.1016/S0166-2236(03)00032-8. [DOI] [PubMed] [Google Scholar]
- 63.Ravaglia G, Forti P, Maioli F, Martelli M, Servadei L, Brunetti N, Porcellini E, Licastro F. Homocysteine and folate as risk factors for dementia and Alzheimer disease. The American journal of clinical nutrition. 2005;82:636–643. doi: 10.1093/ajcn.82.3.636. [DOI] [PubMed] [Google Scholar]
- 64.Faux NG, Ellis KA, Porter L, Fowler CJ, Laws SM, Martins RN, Pertile KK, Rembach A, Rowe CC, Rumble RL, Szoeke C, Taddei K, Taddei T, Trounson BO, Villemagne VL, Ward V, Ames D, Masters CL, Bush AI. Homocysteine, vitamin B12, and folic acid levels in Alzheimer’s disease, mild cognitive impairment, and healthy elderly: baseline characteristics in subjects of the Australian Imaging Biomarker Lifestyle study. Journal of Alzheimer’s disease : JAD. 2011;27:909–922. doi: 10.3233/JAD-2011-110752. [DOI] [PubMed] [Google Scholar]
- 65.Wang HX, Wahlin A, Basun H, Fastbom J, Winblad B, Fratiglioni L. Vitamin B(12) and folate in relation to the development of Alzheimer’s disease. Neurology. 2001;56:1188–1194. doi: 10.1212/wnl.56.9.1188. [DOI] [PubMed] [Google Scholar]
- 66.Seshadri S, Beiser A, Selhub J, Jacques PF, Rosenberg IH, D’Agostino RB, Wilson PW, Wolf PA. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. The New England journal of medicine. 2002;346:476–483. doi: 10.1056/NEJMoa011613. [DOI] [PubMed] [Google Scholar]
- 67.Luchsinger JA, Noble JM, Scarmeas N. Diet and Alzheimer’s disease. Current neurology and neuroscience reports. 2007;7:366–372. doi: 10.1007/s11910-007-0057-8. [DOI] [PubMed] [Google Scholar]
- 68.Lovell MA, Ehmann WD, Butler SM, Markesbery WR. Elevated thiobarbituric acid-reactive substances and antioxidant enzyme activity in the brain in Alzheimer’s disease. Neurology. 1995;45:1594–1601. doi: 10.1212/wnl.45.8.1594. [DOI] [PubMed] [Google Scholar]
- 69.Marcus DL, Thomas C, Rodriguez C, Simberkoff K, Tsai JS, Strafaci JA, Freedman ML. Increased peroxidation and reduced antioxidant enzyme activity in Alzheimer’s disease. Experimental neurology. 1998;150:40–44. doi: 10.1006/exnr.1997.6750. [DOI] [PubMed] [Google Scholar]
- 70.Rinaldi P, Polidori MC, Metastasio A, Mariani E, Mattioli P, Cherubini A, Catani M, Cecchetti R, Senin U, Mecocci P. Plasma antioxidants are similarly depleted in mild cognitive impairment and in Alzheimer’s disease. Neurobiology of aging. 2003;24:915–919. doi: 10.1016/s0197-4580(03)00031-9. [DOI] [PubMed] [Google Scholar]
- 71.Omar RA, Chyan YJ, Andorn AC, Poeggeler B, Robakis NK, Pappolla MA. Increased Expression but Reduced Activity of Antioxidant Enzymes in Alzheimer’s Disease. Journal of Alzheimer’s disease : JAD. 1999;1:139–145. doi: 10.3233/jad-1999-1301. [DOI] [PubMed] [Google Scholar]
- 72.Chisu V, Manca P, Lepore G, Gadau S, Zedda M, Farina V. Testosterone induces neuroprotection from oxidative stress. Effects on catalase activity and 3-nitro-L-tyrosine incorporation into alpha-tubulin in a mouse neuroblastoma cell line. Archives italiennes de biologie. 2006;144:63–73. [PubMed] [Google Scholar]
- 73.Siddiqui IA, Raisuddin S, Shukla Y. Protective effects of black tea extract on testosterone induced oxidative damage in prostate. Cancer letters. 2005;227:125–132. doi: 10.1016/j.canlet.2004.10.046. [DOI] [PubMed] [Google Scholar]