Abstract
Objectives
To examine the relationship between depression and glycemic control in the Strong Heart Study (SHS), a longitudinal study of cardiovascular disease in American Indians.
Methods
This cross sectional analysis focused on the relationship between depression, diabetes, and glycemic control among 2,832 individuals ≥age 15. Depression was measured by the CES-D scale and diabetes by American Diabetes Association criteria. An ordered logit regression model was used to assess whether diabetes was related to level of depression (none, mild, moderate, severe). Multiple logistic regression was used to explore the relationship between A1c and severe depression in participants with diabetes.
Results
Rates of depression were higher in men and women with diabetes when compared to those without diabetes, respectively (p<.05). For every 1-unit increase in A1c, the odds of severe depression increased by 22% (OR 1.22, 95% CI: 1.05–1.42). Female gender (OR 2.97, 95% CI: 1.32–6.69) and BMI (OR 1.04, 95% CI: 1.00–1.08) also were significantly associated with increased risk for severe depression. Although BMI appears to be significantly associated with increased risk for severe depression, the magnitude of this effect was small.
Conclusions
Individuals with diabetes have higher rates of depression than those without diabetes, consistent with other populations. There is a positive relationship between severity of depression and A1c levels; men and women with severe depression have higher A1c levels than those with moderate-to-no depression.
Keywords: diabetes complications, depression, Strong Heart Study
INTRODUCTION
Diabetes is a chronic disease that becomes increasingly difficult to manage when it is accompanied by depression (Anderson et al., 2001). Those with diabetes and depression are at increased risk of hyperglycemia and long-term diabetes complications. Additionally, improvements in depression have been shown to lead to improvements in glycemic control in several separate trials (Lustman et al., 1997; Lustman et al., 1998; Lustman et al., 2000). Because these two conditions may exacerbate one another, simultaneous treatment of both may become standard care in management of diabetes (Lustman & Clouse, 2002).
American Indian populations have been shown to have a diabetes prevalence of more than three times that of the general U.S. population, with mortality rates approximately four times those of whites and twice those of African Americans (Gohdes & Bennet, 1993). The findings regarding depression are more mixed, with indications of the importance of cultural considerations in the assessment of major depression (Beals et al., 2005; Manson & Kleinman, 1998). Nevertheless, depression is also a significant problem in many American Indian communities (Finkbonner, 2002).
The examination of psychosocial factors, which play a major role in diabetes management, has been of recent interest; however, published articles in this area are limited. Depression has been associated with an increased risk for diabetes in specific American Indian communities (Jian et al., 2007). Additional preliminary support has been found for an inverse relationship between glycemic control and depression among those with diabetes in an American Indian community (Sahota et al., in press). A small study suggested that co-morbid depression may have a similar impact on the treatment and management of diabetes among American Indian populations as among non-American Indian populations (Singh et al., 2004). However, to date there has been no large-scale investigation of the relationship between glycemic control and depression in a broad group of American Indians with or without diabetes. The Strong Heart Study (SHS) provides an opportunity to examine the relationship between depression and glycemic control in a large geographically and age-diverse population of American Indians.
METHODS
The SHS is a longitudinal study of cardiovascular disease and its risk factors in American Indians in three geographic areas. During the fourth exam (2001–2003) of the SHS, data were collected on a range of psychosocial characteristics. This cross sectional analysis focused on the relationship between depression and glycemic control among men and women ages 15 years and older.
Participants
SHS included 13 American Indian communities in three distinct geographic areas. The study has recruited and examined members of 95 families ranging in size from 5 to 110 examined individuals. These include 32 families from Arizona with 9 to 66 members, 27 families from the Dakotas with 5 to 110 members, and 36 from Oklahoma with 20 to 61 members. For the fourth exam, 3665 participants completed a personal interview, physical examination, and laboratory tests. Of these, 819 were missing data for depression and 14 were missing data for diabetes. The final sample with complete data included 2,832 participants (Table I).
Table I.
Participant characteristics, N = 2832
| Total (N=2832) |
Men (n=1081) |
Women (n=1751) |
p-value | |
|---|---|---|---|---|
| Age (years) | 39.55 (16.73) | 38.42 (16.58) | 40.26 (16.79) | 0.0045 |
| BMI (kg/m2) | 32.24 (7.83) | 31.37 (7.48) | 32.78 (7.99) | <0.0001 |
| A1c (%) | 6.73 (2.06) | 6.59 (2.01) | 6.81 (2.08) | 0.0594 |
| Education (years) | 11.9 (2.3) | 11.9 (2.3) | 11.9 (2.4) | 0.6074 |
| Weight* | ||||
| Underweight | 37 (1.3%) | 20 (1.9%) | 17 (1.0%) | <0.0001 |
| Normal | 451 (15.9%) | 188 (17.4%) | 263 (15.0%) | |
| Overweight | 738 (26.1%) | 321 (29.7%) | 417 (23.8%) | |
| Obese | 1606 (56.7%) | 552 (51.1%) | 1054 (60.2%) | |
| Current alcohol use | 1639 (58.0%) | 717 (66.6%) | 922 (52.7%) | <0.0001 |
| Diabetes | 672 (23.7%) | 244 (22.6%) | 428 (24.4%) | 0.2554 |
| Mean fasting glucose | ||||
| Diabetes | 178.7 (76.1) | 174.1 (73.5) | 181.4 (77.5) | 0.2358 |
| IFG | 115.7 (4.4) | 115.6 (4.3) | 115.9 (4.5) | 0.6128 |
| NFG | 91.8 (8.4) | 93.5 (8.0) | 90.8 (8.5) | <0.0001 |
| Duration of diabetes** | ||||
| Newly diagnosed | 91 (13.5%) | 43 (17.6%) | 48 (11.2%) | 0.0071 |
| < 5 years | 257 (38.2%) | 97 (39.8%) | 160 (37.4%) | |
| 5–10 years | 95 (14.1%) | 39 (16.0%) | 56 (13.1%) | |
| 10 + years | 229 (34.1%) | 65 (26.6%) | 164 (38.3%) | |
| Diabetes Treatmentˆ | ||||
| Lifestyle/No meds | 112 (19.3%) | 45 (22.4%) | 67 (17.6%) | 0.0123 |
| Oral agents | 300 (51.6%) | 114 (56.7%) | 186 (49.0%) | |
| Insulin | 83 (14.3%) | 18 (9.0%) | 65 (17.1%) | |
| Oral agents + insulin | 86 (14.8%) | 24 (11.9%) | 62 (16.3%) | |
Abbreviations: BMI = body mass index; IFG = impaired fasting glucose; NFG = normal fasting glucose.
Underweight = BMI < 18.5; Normal = BMI 18.5–25; Overweight = BMI 25–30; Obese = BMI >30.
Out of 244 men and 428 women (672 total) with diabetes.
Out of 201 men and 380 women (581 total) with an established diagnosis of diabetes.
Assessment of Depression
The Center for Epidemiologic Studies of Depression Scale (CES-D) scale is a screening tool for depression (Radloff, 1977). It is composed of 20-items, administered as a self-report instrument (Radloff, 1977). The CES-D has been used in several published studies with American Indian participants (Baron et al., 1990; Manson et al., 1990; Somervell et al., 1993; Beals et al., 1991; Dick et al., 1994). The CES-D is described in previous SHS publications as a “non clinical measure of depressive symptomatology” (Plaud et al., 1997). The CES-D is designed to measure current level of depressive symptoms and depressive affect. The items were chosen (from five previously used depression scales) to represent all major components of depressive symptoms, including depressed mood, feelings of guilt and worthlessness, feelings of helplessness and hopelessness, loss of appetite, sleep disturbance, and psychomotor retardation. Items are rated on a 4-point scale indicating the degree of their occurrence during the last week. The ratings range from “rarely or not at all” to “most of the time.” The CES-D has been used as a standard measure of depression for many large-scale studies, including the Honolulu Heart Program, the Inter-Tribal Heart Project (Menominee, Red Lake, and White Earth), CARDIA, and the Stanford Coronary Prevention Project (SHS Family Study Phase IV Operations Manual).
Reliability and Validity
The CES-D has been found to be adequate in both test-retest reliability and internal consistency, with a Cronbach’s alpha of .89 (Radloff, 1977). Its internal consistency also has been tested in American Indians and is reported as .86 (Somervell et al., 1993). The scale has been shown to distinguish between clinically depressed and general community groups (Radloff, 1977). Although it is usually scored continuously, there are various cut-off scores, with reasonable associations between cut-off scores and severity of depression (Radloff, 1977).
Administration
The CES-D is designed for self-administration or to be given in an interview format.
Scoring
Twenty items are rated on a 4-point Likert scale, ranging from “rarely, or not at all,” scored as 0, to “most of the time,” scored as 3. Four positively worded items are reversed when scored. Item scores are then summed for a total depression score (the higher the score, the greater the depressive symptoms). Four categories of depressive symptoms were created based on the following cut-off points of the CES-D score: none = < 10; mild = 10–15; moderate = 16–24; severe > 24 (Radloff, 1977; Greden & Schwenk, 1997; Weissman et al., 1977).
Diagnosis of Diabetes
Given the high prevalence rates of diabetes in American Indians, all participants were asked to provide fasting blood samples for measurement of glucose and insulin. Diabetes was diagnosed using fasting glucose measures in accordance with American Diabetes Association criteria (Lee et al., 1990). In addition, participants were included as having diabetes if they had ever been told they had diabetes by their doctor, were currently taking diabetes medications, or had a fasting glucose level of > 126mg/dL.
Statistical Methods
Chi-square and Student’s t tests were used to examine differences in proportions and means between categorical and continuous variables, respectively. First, the differences between male and female participants were explored. Because the purpose of this report was to understand the relationship between diabetes and symptoms of depression in a cross-sectional setting, further comparisons focused on differences between participants with diagnosed diabetes who did and did not have depressive symptoms. An ordered logit regression model was used to assess whether diabetes was related to categories of depressive symptoms (none, mild, moderate, severe) after adjusting for potential confounders. Multiple logistic regression was then used to explore the relationship between A1c and severe depressive symptoms in participants with diabetes. The Hosmer-Lemeshow goodness-of-fit test was used to determine whether the model assumptions were violated. All analyses were performed using SAS version 9.1.3.
RESULTS
Table I describes the demographic and health related characteristics of the sample. Comparisons by gender indicated statistically significant differences in age, body mass index (BMI), weight, alcohol use, normal fasting glucose, duration of diabetes, and form of diabetes treatment. Differences in A1c levels also showed a trend that approached significance.
Women with diabetes showed higher occurrence of depressive symptoms than those without diabetes (p < 0.05) (Figure 1). Among male participants, the pattern was similar as those with diabetes showed higher rates of depressive symptoms than those without diabetes (p < 0.05). Results from the ordered logit regression model showed that diabetes was significantly related to odds of occurrence of depressive symptoms after adjusting for age, gender, BMI, alcohol use, and education (p < 0.05).
Figure 1.
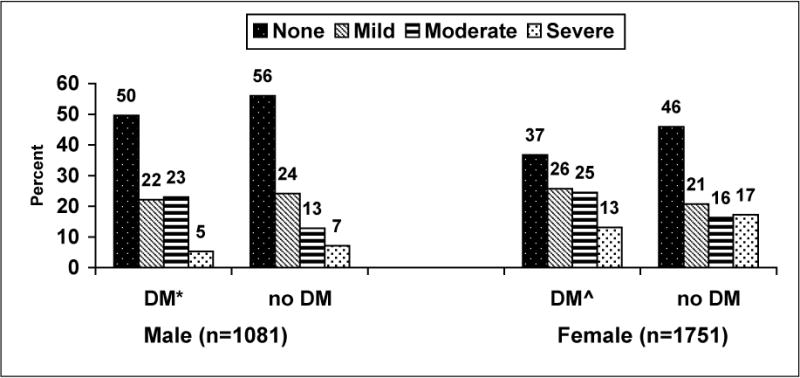
Occurrence of depressive symptoms, by gender and diabetes status, Strong Heart Study, n = 2832
DM (diabetes mellitus) includes newly diagnosed and diagnosed cases.
None = Center for Epidemiologic Studies of Depression Scale (CES-D) score < 10; Mild = CES-D score 10–15; Moderate = CES-D 16–24; Severe = CES-D score > 24.
*p-value for differences in depressive symptoms in men = 0.0014.
ˆp-value for differences in depressive symptoms in women < 0.0001.
The relationship between severity of depressive symptoms and other variables in participants with diagnosed diabetes is shown in Table II. Individuals who reported severe depressive symptoms were younger (p < 0.05), more likely to be female (p < 0.05), and had significantly higher BMI scores (p < 0.05). In addition, participants with a severe level of depressive symptoms also had significantly higher A1c levels. There were no differences in types of diabetes treatment used between those with severe depressive symptoms and those without severe depressive symptoms.
Table II.
Relationship of severity of depression to other variables in participants with diagnosed diabetes, n = 581
| Depression Level | |||
|---|---|---|---|
| None/Mild/Moderate CES-D ≤ 24 (n=520) |
Severe CES-D > 24 (n=61) |
p-value | |
| Age | 52.09 (14.23) | 47.01 (13.33) | *0.0082 |
| Female (%) | 63.65% | 80.33% | *0.0096 |
| Weight (kg) | 95.40 | 98.72 | 0.2980 |
| BMI (kd/m2) | 34.64 | 37.34 | *0.0087 |
| Diabetes duration | 11.42 | 12.28 | 0.5575 |
| A1c | 8.32 | 9.27 | *0.0026 |
| Education | 11.68 | 11.44 | 0.4622 |
| Current Alcohol use | 37.38% | 45.90% | 0.1952 |
| Treatment Type | |||
| Oral agents | 52.31% | 45.90% | 0.4833 |
| Insulin | 14.42% | 13.11% | |
| Oral agents + insulin | 14.04% | 21.31% | |
Abbreviations: BMI = body mass index; CES-D = Center for Epidemiologic Studies of Depression Scale.
p < 0.05.
A multiple logistic regression model in individuals with diagnosed diabetes is presented in Table III. The model, comparing 48 participants with severe depressive symptoms as measured by the CES-D and 445 without severe depressive symptoms who had complete data for all the covariates, was statistically significant (likelihood ratio χ2 = 25.36, p = 0.0003). For every 1-unit increase in A1c, the odds of severe depression increased by 22% (OR 1.22, 95% CI: 1.05–1.42), after adjusting for the other covariates in the model. Female gender (OR 2.97, 95% CI: 1.32–6.69) and BMI (OR 1.04, 95% CI: 1.00–1.08) were also significantly associated with increased risk for severe depression.
Table III.
Logistic regression analysis of factors associated with severe depressive symptoms among participants with diagnosed diabetes
| B-coefficient (SE) | OR (95% CI) | p-value | |
|---|---|---|---|
| A1c | 0.199 (0.078) | 1.22 (1.05–1.42) | 0.0101 |
| Age (years) | −0.015 (0.014) | 0.99 (0.96–1.01) | 0.2863 |
| Gender (F v. M) | 0.545 (0.207) | 2.97 (1.32–6.69) | 0.0085 |
| BMI (kg/m2) | 0.040 (0.020) | 1.04 (1.00–1.08) | 0.0403 |
| Alcohol use (N v. Y) | −1.389 (0.175) | 0.76 (0.38–1.51) | 0.4289 |
| Education (years) | 0.026 (0.069) | 1.03 (0.90–1.17) | 0.7075 |
Abbreviations: BMI = body mass index.
Hosmer-Lemeshow goodness of fit test: χ2 = 7.16, p = 0.5194.
DISCUSSION
These findings present the first analyses of depression and its relation to glycemic control among diabetic individuals in a large geographically and age-diverse group of American Indians. The data showed higher rates of depressive symptoms among both men and women with diabetes compared to those without diabetes. A positive relationship was observed between depression severity and level of glycemic control among those with diabetes. Those with severe depressive symptoms had A1c levels almost a full point higher than those with moderate-to-no depression.
This finding is consistent with a large body of previous cross sectional studies within the general population demonstrating a relationship between depression and glycemic control. In a meta-analysis, support was found for a relationship between depression and hyperglycemia (Lustman et al., 2000). In a review of the 2006 Behavioral Risk Factor Surveillance System, Li et al. (2008) concluded that many of those with diabetes are at increased risk of depression and recommended screening patients with diabetes for depression. Lustman and Clouse (2005) found depression to be common in those with diabetes and that those with depression and diabetes have decreased adherence to treatment regimens, increased difficulty with glycemic control, and increased risk for retinopathy (Lustman & Clouse, 2005). The overall effect of comorbid diabetes and depression appears to increase the likelihood of negative outcomes in diabetes care.
In this sample of American Indians, we observed a positive relationship between glycemic control and depression. This relationship suggests that the interaction between glycemic control and depression may create additional challenges in managing each respective condition. Several factors probably contribute to the impact of comorbid diabetes and depression, including the length of illness, rate of complications, and degree of disability.
The relationship between A1c and depressive symptoms, in addition to being a statistically significant relationship, is a clinically relevant finding. A decrease in A1c of 1.0 has been reported to be associated with nearly a 33% reduction in the progression of retinopathy (Morisaki et al., 1994). For every 1 unit increase in A1c, the odds of severe depressive symptoms rise at an estimated rate of 22%; thus as A1c levels increase, the risk for severe depression increases and the treatment and management of both conditions become more difficult and complicated.
There may be several mechanisms for the relation between diabetes and symptoms of depression. Support for a bidirectional relationship between depression and diabetes was recently discovered in the Multi-Ethnic Study of Atherosclerosis (Golden et al., 2008). Depression could result as a reaction to a diagnosis of diabetes, although other studies have suggested that the temporal relationship does not support this premise (Talbot & Nouwen, 2000). The co-morbidities and care requirements of diabetes may enhance depression, but Talbot and Nouwen concluded that evidence of major depression resulting from biochemical changes directly due to type 2 diabetes or the treatment of type 2 diabetes (psychosocial demands) do not appear to be supported by the literature (Talbot & Nouwen, 2000). Alternatively, an interaction of factors, both biological and psychosocial, may increase the likelihood of development of type 2 diabetes in those who are depressed; also, incidence of diabetes has been shown in one study to be higher in those on antidepressant medications (Rubin et al., 2008).
This study has a number of strengths. The SHS is the largest epidemiological survey of American Indians, with participants from several culturally and geographically diverse tribes. Data were systematically collected using standardized methods and rigorous quality control. In addition, the instrument used to assess symptoms of depression has been shown to be useful in American Indian participants. The CES-D is a measure of depressive symptoms but does not equate with a clinical diagnosis of major depressive disorder. Such self-report instruments are widely used to quantify depressive symptoms in research and to identify patients in clinical setting who are in need of further mental health evaluation and treatment; however, there is not complete concordance between this instrument and the clinical diagnosis of depression (Beals et al., 2005). This study is limited because although it includes 13 communities, it may not be representative of the larger American Indian population. Finally, this study is cross sectional and, therefore, not able to confirm causal relations; a longitudinal analysis in this population will be possible after the completion of SHS Phase V.
In conclusion, this initial cross-sectional analysis of a large cohort of American Indians over a wide age range suggests a high rate of self-reported depressive symptoms in those with diabetes and an association with glycemic control. The current results suggest that screening for depression could be an important factor in diabetes care at initial onset and diagnosis. Furthermore, continued monitoring of diabetes patients’ level of depression is likely to be an essential aspect of comprehensive care.
Acknowledgments
The Strong Heart Study was supported by cooperative agreement grants (Nos. U01HL-41642, U01HL-41652, and U01HL-41654) from the National Heart, Lung and Blood Institute. The authors acknowledge the assistance and cooperation of the tribal leadership and community members, without whose support this study would not have been possible. We thank the Indian Health Service hospitals and clinics at each center, the directors, and their staffs. We gratefully acknowledge Rachel Schaperow, MedStar Research Institute, for editing the manuscript. The opinions expressed in this paper are those of the authors and do not necessarily reflect the views of the Indian Health Service.
References
- Anderson RJ, Clouse RE, Freeland KE, Lustman PJ. The prevalence of comorbid depression in adults with diabetes. Diabetes Care. 2001;24:1069–1078. doi: 10.2337/diacare.24.6.1069. [DOI] [PubMed] [Google Scholar]
- Barón AE, Manson SM, Ackerson LM, Brenneman DL. Depressive symptomatology in older American Indians with chronic disease: Some psychometric considerations. In: Attkinsson C, Zich J, editors. Screening for depression in primary care: Screening and detection. New York: Routledge, Chapman and Hall, Inc; 1990. [Google Scholar]
- Beals J, Manson SM, Keane EM, Dick RW. Factorial structure of the Center for Epidemiologic Studies Depression scale among American Indian college students. Psychological Assessment: A Journal of Consulting and Clinical Psychology. 1991;3:623–627. [Google Scholar]
- Beals J, Manson SM, Whitesell NR, Mitchell CM, Novins DK, Simpson S, et al. Prevalence of major depressive episode in two American Indian reservation populations: Unexpected findings with a structured interview. American Journal of Psychiatry. 2005;162:1713–1722. doi: 10.1176/appi.ajp.162.9.1713. [DOI] [PubMed] [Google Scholar]
- Dick RW, Beals J, Keane EM, Manson SM. Factorial structure of the CES-D among American Indian adolescents. Journal of Adolescence. 1994;17:73–79. [Google Scholar]
- Finkbonner B. Depression in American Indians and Alaska Natives: A review of Indian Health Service Policy and Services. 2002;27:9. [Google Scholar]
- Gohdes D, Bennet MB. Diabetes in American Indians and Alaskan Natives: An overview. Diabetes Care. 1993;16:239–243. doi: 10.2337/diacare.16.1.214. [DOI] [PubMed] [Google Scholar]
- Golden SH, Lazo M, Carnethon M, Bertoni AG, Schreiner PJ, Diez Roux AV, Lee HB, Lyketsos C. Examining a bidirectional association between depressive symptoms and diabetes. Journal of the American Medical Association. 2008;299:2751–2759. doi: 10.1001/jama.299.23.2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greden JF, Schwenk TL. Major Mood Disorders. In: Knesper DJ, Riba MB, Schwenk TL, editors. Primary Care Psychiatry. Philadelphia, PA: WB Saunders Co; 1997. p. 129. [Google Scholar]
- Jiang L, Beals J, Whitesell NR, Roubideaux Y, Manson SM. Association between diabetes and mental disorders in two American Indian reservation communities. Diabetes Care. 2007;30:9. doi: 10.2337/dc07-0097. [DOI] [PubMed] [Google Scholar]
- Lee ET, Welty TK, Fabsitz R, Cowan LD, Ngoc-ahh LE, Oopik AJ, Cucchiara AJ, Savage P, Howard BV. The Strong Heart Study. A study of cardiovascular disease in American Indians: design and methods. J of Epidemiology. 1990;132:1141–1155. doi: 10.1093/oxfordjournals.aje.a115757. [DOI] [PubMed] [Google Scholar]
- Li C, Ford ES, Strine TW, Mokdad AH. Prevalence of depression among U.S. adults with diabetes. Diabetes Care. 2008;31:105–107. doi: 10.2337/dc07-1154. [DOI] [PubMed] [Google Scholar]
- Lustman PJ, Anderson RJ, Freedland KE, De Groot M, Carney RM, Clouse RE. Depression and glycemic control: A meta-analytic review of the literature. Diabetes Care. 2000;23:934–942. doi: 10.2337/diacare.23.7.934. [DOI] [PubMed] [Google Scholar]
- Lustman PJ, Clouse RE. Depression in diabetic pts: The relationship between mood & glycemic control. J of DM & its Complications. 2005;19:113–122. doi: 10.1016/j.jdiacomp.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Lustman PJ, Clouse RE. Treatment of depression in diabetes: Impact on mood and medical outcome. J of Psychosomatic Research. 2002;53:917–924. doi: 10.1016/s0022-3999(02)00416-6. [DOI] [PubMed] [Google Scholar]
- Lustman PJ, Freedland Griffith LS, Clouse RE. Fluoxetine for depression in diabetes: a randomized double-blind placebo-controlled trial. Diabetes Care. 2000;23:618–623. doi: 10.2337/diacare.23.5.618. [DOI] [PubMed] [Google Scholar]
- Lustman PJ, Griffith LS, Clouse RE, Freedland KE, Eisen SA, Rubin EH, Carney RM, McGill JB. Effects of nortriptylene on depression and glucose regulation in diabetes: results of a double-blind, placebo-controlled trial. Psychosomatic Medicine. 1997;59:241–250. doi: 10.1097/00006842-199705000-00007. [DOI] [PubMed] [Google Scholar]
- Lustman PJ, Griffith LS, Freedland KE, Kissel SS, Clouse RE. Cognitive behavior therapy for depression in type-2 diabetes: a randomized controlled trial. Annals of Internal Medicine. 1998;129:613–621. doi: 10.7326/0003-4819-129-8-199810150-00005. [DOI] [PubMed] [Google Scholar]
- Manson SM, Ackerson LM, Dick RW, Baron AE, Fleming CM. Depressive symptoms among American Indian adolescents: Psychometric characteristics of the Center for Epidemiologic Studies Depression Scale (CES-D) Psychological Assessment. 1990;2:231–237. [Google Scholar]
- Manson SM, Kleinman DSM-IV, culture, and mood disorders: A critical reflection on recent progress. Transcultural Psychiatry. 1998;35:337–386. [Google Scholar]
- Morisaki N, Wantanabe S, Kobayashi J, Kanzaki T, Takahashi K, Yokote K, Tezuka M, Tashario J, Inadera H, Saito Y, Yoshida S, Shigemura K. Diabetic control and progression of retinopathy in elderly patients: five year follow up study. Journal of American Geriatric Society. 1994;42:142–145. doi: 10.1111/j.1532-5415.1994.tb04941.x. [DOI] [PubMed] [Google Scholar]
- Plaud JJ, Schweigman K, Welty TK. Health and depression among American Indians: Psychosocial data from the Strong Heart Study Phase II. International Journal of Rehabilitation and Health. 1997;3:51–59. [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Rubin RR, Ma Y, Marrero DG, Peyrot M, Barrett-Conner EL, Kahn SE, Haffner SM, Price DW, Knowler WC. Elevated depression symptoms, antidepressant,medicine use and risk of developing diabetes during the Diabetes Prevention Program. Diabetes Care. 2008;31:420–6. doi: 10.2337/dc07-1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahota PK, Knowler WC, Looker HC. Depression, diabetes, and glycemic control in an American Indian community. Journal of Clinical Psychiatry. doi: 10.4088/jcp.v69n0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh PK, Looker HC, Hanson RL, Krakoff J, Bennett PH, Knowler WC. Depression, diabetes, and glycemic control in Pima Indians. Diabetes Care. 2004;27:618–619. doi: 10.2337/diacare.27.2.618-a. [DOI] [PubMed] [Google Scholar]
- Somervell PD, Beals J, Kinzie JD, Boehnlein J, Leung P, Manson SM. Criterion validity of the Center for Epidemiologic Studies Depression Scale in a population sample from an American Indian village. Psychiatry Research. 1993;47:255–266. doi: 10.1016/0165-1781(93)90083-s. [DOI] [PubMed] [Google Scholar]
- Strong Heart Study Family Study Phase IV Operations Manual. 8:8. http://strongheart.ouhsc.edu/manual/PhaseIV/Volume8.pdf. [Google Scholar]
- Talbot F, Nouwen A. A review of the relationship between depression and diabetes in adults: Is there a link? Diabetes Care. 2000;23:1556–1562. doi: 10.2337/diacare.23.10.1556. [DOI] [PubMed] [Google Scholar]
- Weissman MM, Sholomskas D, Pottenger M, Prussoff BA, Locke BZ. Assessing depressive symptoms in five psychiatric populations: A validation study. American Journal of Epidemiology. 1977;106:203–214. doi: 10.1093/oxfordjournals.aje.a112455. [DOI] [PubMed] [Google Scholar]


