Abstract
Background
Suitable procedures for transport of blood samples from general practitioners to hospital laboratories are requested. Here we explore routine testing on samples stored and transported as whole blood in lithium-heparin or serum tubes.
Methods
Blood samples were collected from 106 hospitalized patients, and analyzed on Architect c8000 or Advia Centaur XP for 35 analytes at base line, and after storage and transport of whole blood in lithium-heparin or serum tubes at 21 ± 1°C for 10 h. Bias and imprecision (representing variation from analysis and storage) were calculated from values at baseline and after storage, and differences tested by paired t-tests. Results were compared to goals set by the laboratory.
Results
We observed no statistically significant bias and results within the goal for imprecision between baseline samples and 10-h samples for albumin, alkaline phosphatase, antitrypsin, bilirubin, creatinine, free triiodothyronine, γ-glutamyl transferase, haptoglobin, immunoglobulin G, lactate dehydrogenase, prostate specific antigen, total carbon dioxide, and urea. Alanine aminotransferase, amylase, C-reactive protein, calcium, cholesterol, creatine kinase, ferritin, free thyroxine, immunoglobulin A, immunoglobulin M, orosomucoid, sodium, transferrin, and triglycerides met goals for imprecision, though they showed a minor, but statistically significant bias in results after storage. Cobalamin, folate, HDL-cholesterol, iron, phosphate, potassium, thyroid stimulating hormone and urate warranted concern, but only folate and phosphate showed deviations of clinical importance.
Conclusions
We conclude that whole blood in lithium-heparin or serum tubes stored for 10 h at 21 ± 1°C, may be used for routine analysis without restrictions for all investigated analytes but folate and phosphate.
Keywords: Folic acid, laboratory test, phosphate, potassium, pre-analytical, sample transportation, storage temperature, storage time
Introduction
Centralized routine hospital laboratories perform an increasing number of tests on blood samples provided by general practitioners (GPs). It is important that the results obtained on these samples, are comparable to those obtained when venipuncture is performed at the hospital. In this context pre-analytical variations related to storage time and temperature as well as transportation conditions of the blood samples are of major importance [1,2].
Transportation of blood samples from the GPs office to the laboratories often relies on different types of logistics, generally at ambient temperature and with variation in the time-frame of the entire process. In the hospital setting pre-analytical conditions are less variable, whole blood is centrifuged soon after venipuncture and subsequent analysis performed within a short time frame post-centrifugation [3]. Furthermore primary tubes are used, in order to ensure correct sample identity.
Focusing on cost-effectiveness it has in our region been suggested that centrifugation of blood samples from GPs should be performed at the hospital laboratories, provided that this does not compromise the quality of test results.
A number of studies have explored to which extent it is possible to use whole blood samples, subject to prolonged storage, and thus allowing flexibility in the requirements regarding transportation of blood samples to the hospital laboratories [4–15]. Results from most studies indicate the necessity of more than one daily sample transportation from GPs office [4,6,8,9,11,13–15], but there are also studies supporting one daily transport [7,12]. However, the main part of the studies are relatively small covering up to 30 analytes examined in five to 15 persons [4,6–8,12]. Furthermore, most studies include blood from presumably healthy individuals [4,6–9,12,14,15].
Here we explore the feasibility of analyzing blood samples in lithium-heparin or serum tubes stored for 10 h at 21°C prior to centrifugation and cover 35 analytes with concentration both within and outside the reference interval.
Methods
Sample collection
A total of 106 adult non-fasting patients admitted to regional hospitals in Herning (n = 50) and Holstebro (n = 56), respectively, were included in the study. Venous blood samples were collected by experienced phlebotomists into two 7 mL lithium-heparin vacutainer tubes without gel separator for analysis on Architect c8000 (Holstebro) or two 5 mL serum vacutainer tubes without gel separator for analysis on Advia Centaur XP (Herning) (Table I). Tubes were from Becton Dickinson, BD Diagnostics, Brøndby, Denmark (ref 368884 [lithium-heparin] and 368815 [serum]).
Table I.
Analytes, number of samples and range of values.
| Short name | Analyte | Unit | IUPAC (NPU)† |
n | Baseline values Median (range) |
Local reference intervals (adults) |
|---|---|---|---|---|---|---|
| ALAT | Alanine aminotransferase | U/L | 19651 | 56 | 18 (6–173) | 10–70 |
| ALB | Albumin | g/L | 19673 | 56 | 37 (17–46) | 34–48 |
| AMYL | Amylase | U/L | 19653 | 56 | 23 (5–218) | 10–65 |
| ATRYP | Antitrypsin | g/L | 19692 | 34 | 1.83 (0.57–3.43) | 0.97–1.68 |
| B12* | Cobalamin | pmol/L | 01700 | 49 | 406 (137–1476) | 200–600 |
| BASP | Alkaline phosphatase | U/L | 19655 | 55 | 62 (33–503) | 35–105 |
| BILI | Bilirubin | μmol/L | 01370 | 56 | 9 (4–87) | 5–25 |
| CA | Calcium | mmol/L | 01443 | 56 | 2.33 (1.87–2.64) | 2.20–2.55 |
| CHOL | Cholesterol | mmol/L | 01566 | 56 | 4.4 (2.0–7.2) | < 5.0‡ |
| CK | Creatine kinase | U/L | 19656 | 55 | 74 (7–551) | 50–270 |
| CO2 | Total carbon dioxide | mmol/L | 01472 | 56 | 29 (16–41) | 23–32 |
| CREA | Creatinine | μmol/L | 18016 | 56 | 69 (31–215) | 45–105 |
| CRP | c-Reactive protein | mg/L | 19748 | 56 | 6.7 (0.2–217.5) | < 8.0 |
| F-T3* | Free triiodothyronine | pmol/L | 03625 | 50 | 3.7 (1.7–6.4) | 3.9–6.8 |
| F-T4* | Free thyroxine | pmol/L | 03579 | 50 | 18.3 (11.7–33.1) | 12.0–21.0 |
| Fe | Iron | μmol/L | 02508 | 55 | 12 (3–29) | 9–34 |
| FER* | Ferritin | μg/L | 19763 | 50 | 309 (35–3716) | 15–355 |
| FOL* | Folate | nmol/L | 02070 | 48 | 12 (2–39) | > 9 |
| GGT | γ-glutamyl transferase | U/L | 19657 | 55 | 32 (11–442) | 10–115 |
| HAPT | Haptoglobin | g/L | 19788 | 54 | 1.35 (0.20–4.33) | 0.35–2.05 |
| HDL-C | HDL-cholesterol | mmol/L | 01567 | 56 | 1.2 (0.2–2.1) | > 1.0‡ |
| IgA | Immunoglobulin A | g/L | 19795 | 56 | 1.94 (0.86–5.21) | 0.80–4.90 |
| IgG | Immunoglobulin G | g/L | 19814 | 56 | 9.9 (3.8–21.3) | 6.1–15.7 |
| IgM | Immunoglobulin M | g/L | 19825 | 55 | 0.82 (0.13–2.27) | 0.39–2.30 |
| K | Potassium | mmol/L | 03230 | 56 | 3.8 (3.1–4.8) | 3.5–4.6 |
| LD | Lactate dehydrogenase | U/L | 19658 | 56 | 173 (95–609) | 105–255 |
| Na | Sodium | mmol/L | 03429 | 56 | 139 (127–145) | 137–145 |
| OROS | Orosomucoid | g/L | 19873 | 54 | 0.84 (0.28–2.45) | 0.45–1.17 |
| P | Phosphate | mmol/L | 03096 | 56 | 1.02 (0.51–1.60) | 0.71–1.53 |
| PSA* | Prostate specific antigen | μg/L | 08669 | 31 | 0.8 (0.1–11.6) | < 0.3 |
| TGLY | Triglycerides | mmol/L | 04094 | 56 | 1.1 (0.4–6.2) | < 2.0‡ |
| TRANS | Transferrin | μmol/L | 03607 | 55 | 27 (9–47) | 24–41 |
| TSH* | Thyroid stimulating hormone | mIU/L | 03577 | 50 | 1.29 (0.020–8.72) | 0.300–4.50 |
| URATE | Uric acid | mmol/L | 03688 | 56 | 0.25 (0.12–0.73) | 0.15–0.48 |
| UREA | Urea | mmol/L | 01459 | 56 | 5.4 (2.6–25.7) | 2.6–8.1 |
Analysis performed on Advia Centaur XP using whole blood in serum tubes. For the other analytes analysis was performed on Architect c8000 using whole blood in lithium-heparin tubes.
IUPAC, International Union of Pure and Applied Chemistry; NPU, Nomenclature for Properties and Units. The NPU terminology is in national use in Denmark, for communication and recording of clinical laboratory information. http://www.ssi.dk/EnglishNPU.
Decision limit.
All samples for a specific analyte were collected on the same day in September 2011. Sample collection from each patient, otherwise undergoing venipuncture, was performed during morning routine, and samples were taken through the same venipuncture site. Samples were subjected to different settings of pre-analytical conditions as described in the section entitled Storage conditions.
Ethics
Procedures followed were in accordance with the ethical standards specified by the Helsinki Declaration of 1975, revised in 1983. The study was presented to and accepted by the Central Denmark Regional Science Ethics Committee as a technical and quality investigation (Case nr. 1-10-72-2-12). Participants gave their informed consent.
Storage conditions
Baseline samples were kept as whole blood (in lithium-heparin or serum tubes) at room temperature and centrifuged for 10 min at 2200 g and 20°C within 30–90 min after sample collection. The results for these samples were considered to represent the ‘true value’. 10 h samples, kept as whole blood (in lithium-heparin or serum tubes) were placed in racks in vertical upright position in transportation boxes and stored in the dark at a controlled temperature of 21.0 ± 1.0°C for 10 h. During the last 90 min of storage, 10-h samples were transported to the laboratory by car, air-conditioned to meet a temperature of 21.0 ± 1.0°C.
At all times storage temperature was monitored using temperature loggers (ICU Scandinavia AB, Aalborg, Denmark), which displayed a temperature span of 20.4–22.0°C during the described storage of 10 h samples.
Ten-hour samples were centrifuged as described for the baseline samples after the relevant storage period. Following centrifugation both baseline samples and 10 h samples were kept in the primary blood collection tubes at room temperature without light protection until analysis.
Biochemical analysis
Samples (n = 34–56, depending on analyte) were analyzed for 35 analytes (Table I) by single determination on automated analyzer systems Architect c8000 (Abbott Diagnostics, Copenhagen, Denmark) at the regional hospital in Holstebro, or on Advia Centaur XP (Siemens Healthcare Diagnostics, Ballerup, Denmark) at the regional hospital in Herning according to the manufacturers’ recommendations, and following the routine procedure for the analysis. Both baseline samples and samples processed after 10 h of storage were analyzed between 15 min and 3 h after centrifugation dependent on the analyte. 10 h samples were analyzed in random order as compared to the order of baseline samples. The 35 analytes are listed in Table I, which also lists the short names used.
Analysis methods were: Chemiluminescense immunoassay (B12, FER, FOL, F-T3, F-T4, PSA and TSH), photometry (ALAT, ALB, AMYL, BASP, BILI, CA, CHOL, CK, CO2, CREA, Fe, GGT, LD, P, TGLY, URATE, UREA), potentiometry (K, Na) and turbidimetry (ATRYP, CRP, HAPT, IgA, IgG, IgM, OROS, TRANS). For HDL-C a direct Chol-HDL method using accelerator selective detergent was used.
Reliability of the analytical tests was ensured through routine quality control determinations, including one daily measurement of quality control materials in relevant levels and evaluating according to specified limits of acceptance. All analytical tests were concluded reliable on the day of analysis as well as on the following day. For PSA, only values > 0.04 μg/L were included in the data evaluation (n = 31).
Quality goals and related statistics
The effect of sample storage including transportation at 21°C was evaluated concerning bias and imprecision (coefficient of variation [CV]). The mean deviation was calculated from the formula:
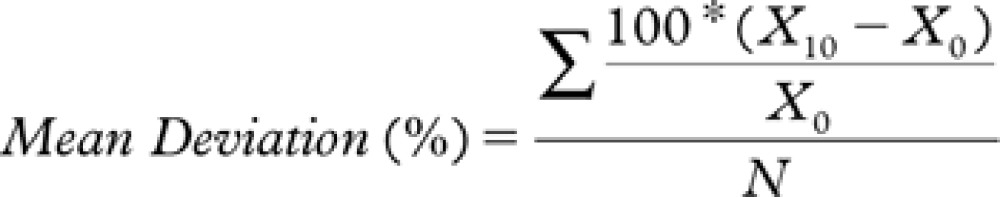 |
where: N = Total number of pairs; X10 = 10-h value; and X0 = Baseline value.
The mean deviation was then compared to the acceptable systematic difference (bias goal). We used values at baseline as compared to values obtained after 10 h of storage in order to calculate the CV of replicate measurements as previously described by Rodbard [16], and compared the results to the analytical- and the goal-CV. CV was calculated from the following formula.
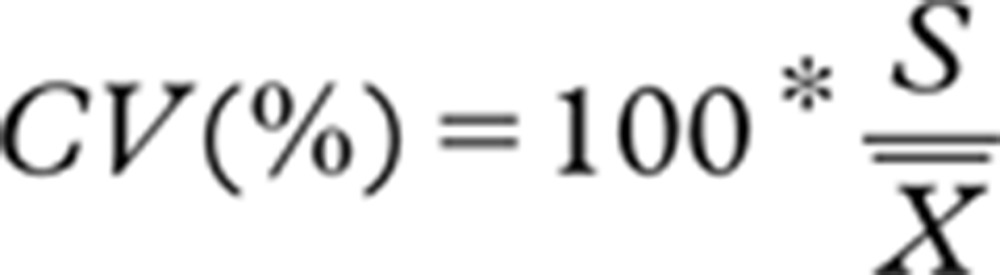 |
where:  = mean of all measurements (both baseline and 10-h values)
= mean of all measurements (both baseline and 10-h values)
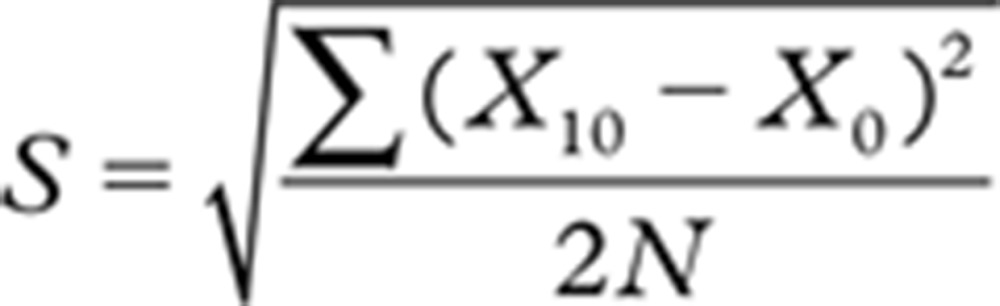 |
The precision we calculated thus represents variation from both analysis and storage. Analytical CV denotes the CV determined as the average analytical variation in laboratory routine quality control data in the year 2011. Bias- and CV goals refer to limits used in evaluating differences in laboratory routine quality control and are generally determined from within- and between-subject biological variation [17,18] or empirically. Data for goal bias as well as analytical- and goal-CV used in this study are indicated for each analyte in Table II.
Table II.
Imprecision (CV) and bias for 35 analytes. Results obtained after storage of whole blood at 21°C was compared to baseline results.
| CV (%) |
Bias (%) |
Paired t-test p value†† | |||
|---|---|---|---|---|---|
| Analyte | CV† | Analytical‡/goal§ | Mean deviation (95% CI)|| | Goal¶ | |
| ALAT | 2.4 | 2.7/9.0 | − 1.7 (− 2.9;− 0.4) | 12.0** | 0.0034 |
| ALB | 1.5 | 0.9/3.2** | 0.2 (0;0.5) | 2.2** | 0.067 |
| AMYL | 1.1 | 2.4/5.9 | − 0.8 (− 1.2;− 0.3) | 8.0 | < 0.0001 |
| ATRYP | 2.9 | 0.9/4.5 | − 0.7 (− 1.5;0.1) | 6.5 | 0.12 |
| B12* | 6.4 | 6.4/7.5 | − 3.2 (− 5.6;− 0.8) | 17.7 | 0.015 |
| BASP | 1.2 | 2.2/4.8 | 0.2 (− 0.3;0.7) | 6.4 | 0.31 |
| BILI | 2.1 | 3.2/6.4** | 1.0 (0.2;1.9) | 10.0** | 0.11 |
| CA | 1.1 | 1.0/2.5** | 1.0 (0.7;1.3) | 2.0** | < 0.0001 |
| CHOL | 1.3 | 0.5/3.0** | 0.6 (0.2;1.1) | 4.0 | 0.0085 |
| CK | 1.4 | 0.8/5.7 | − 0.7 (− 1.3;− 0.1) | 11.5 | 0.0023 |
| CO2 | 3.4 | 5.0/6.0** | 1.2 (0.0;2.4) | 5.0** | 0.16 |
| CREA | 1.1 | 1.3/2.7** | − 0.1 (− 0.6;0.3) | 3.8** | 0.50 |
| CRP | 1.8 | 1.8/5.0** | 2.6 (− 0.5;5.7) | 5.0** | 0.046 |
| F-T3* | 2.4 | 2.2/7.5** | 0.8 (− 0.3;1.8) | 7.2 | 0.28 |
| F-T4* | 4.6 | 4.6/7.5** | 2.1 (0.3;3.9) | 3.6** | 0.039 |
| Fe | 3.7 | 1.6/6.7** | 6.8 (5.8;8.2) | 8.8 | < 0.0001 |
| FER* | 7.0 | 4.3/7.1 | − 1.1 (− 2.7;0.5) | 5.2 | 0.039 |
| FOL* | 20.5 | 6.6/12.0 | − 13.7 (− 19.1;− 8.3) | 19.2 | 0.0007 |
| GGT | 2.6 | 1.6/3.5 | − 0.8 (− 2.1;0.6) | 5.4 | 0.62 |
| HAPT | 1.7 | 1.4/5.1 | 0.1 (− 0.4;0.6) | 10.4 | 0.72 |
| HDL-C | 2.9 | 1.8/3.6 | 4.6 (3.7;5.4) | 5.2 | < 0.0001 |
| IgA | 2.1 | 1.3/2.7 | 1.9 (1.4;2.3) | 9.1 | < 0.0001 |
| IgG | 1.0 | 0.9/2.3 | 0.2 (− 0.2;0.5) | 4.3 | 0.75 |
| IgM | 1.5 | 2.5/4.5** | 1.7 (0.9;2.5) | 11.9 | 0.0001 |
| K | 2.9 | 0.8/2.4 | 1.2 (0.1;2.2) | 1.8 | 0.040 |
| LD | 4.5 | 3.1/5.0** | 1.0 (− 1.3;3.1) | 4.3 | 0.55 |
| Na | 0.4 | 0.7/1.0** | 0.3 (0.1;0.4) | 0.6** | 0.0003 |
| OROS | 1.5 | 0.9/5.7 | 0.8 (0.1;1.5) | 6.8 | 0.017 |
| P | 9.0 | 1.8/4.3 | − 11.2 (− 12.4;− 10.0) | 3.2 | < 0.0001 |
| PSA* | 3.4 | 3.4/9.1 | − 0.4 (− 2.0;1.2) | 18.7 | 0.083 |
| TGLY | 2.0 | 1.0/5.3** | − 0.9 (− 1.7;− 0.1) | 5.4** | 0.018 |
| TRANS | 1.5 | 0.9/2.3 | 1.8 (1.4;2.2) | 2.6** | < 0.0001 |
| TSH* | 9.2 | 4.9/9.7 | 6.0 (3.4;8.7) | 7.8 | 0.0040 |
| URATE | 1.6 | 1.3/4.3** | 1.5 (0.9;2.1) | 4.8** | < 0.0001 |
| UREA | 1.0 | 1.9/6.2 | − 0.2 (− 0.6;0.2) | 5.5 | 0.072 |
Analysis performed on Advia Centaur XP using whole blood in serum tubes. For the other analytes analysis was performed on Architect c8000 using whole blood in lithium-heparin tubes.
CV calculation based on measurements at baseline and after 10 h of storage.
Annual (2011) average CV of laboratory routine quality control samples.
CV goals used in evaluating analytical variation in laboratory routine quality control. Goals were generally based on biological variation from http://westgard.com/biodatabase1.htm and calculated as described by Fraser et al. [18].
denotes goals where empirically based modifications to the three stated levels of performance were used.
Mean deviation % between baseline and 10-h values and 95% confidence interval (shown as confidence limits).
Bias goals used in evaluating systematic differences in laboratory routine quality control.
See §CV goals for further details.
p-values for testing baseline values against values obtained after 10 h of storage of whole blood.
For all analytes differences between baseline values and values obtained after 10 h of storage of whole blood were assessed for normality and concluded to meet the requirements for parametric statistical analysis. Student’s t-tests (Paired t-tests [two-tailed]) were used, the level of significance set at p < 0.05. All statistical analyses were performed using STATA (StataCorp LP, TX, USA) version 12.1.
Fractional Bland-Altman plots were depicted in order to evaluate study results, e.g. concentration dependent alterations. Baseline results were depicted as X-values, and fractions [(10-h storage values)/(baseline values)] as the Y-values.
Calculations were executed using Microsoft Office Excel 2003 software, and GraphPad version 4.0 was used for depicting the results.
Results
We examined the stability of 35 biochemical and immunological analytes as a function of storage of whole blood (in lithium-heparin or serum tubes) at 21°C. Our choice was to use samples from hospitalized patients in order to cover a wide range of values, including both normal as well as pathological levels (Table I).
We used the upper or lower measurement range values for the few results for ALAT, B12, CK, CRP and HAPT reported as above or below this range. For CK, CRP and HAPT values were below the lower measurement range for both baseline and 10-h samples (one sample set affected for each analyte). For ALAT three sets of samples showed results below the lower measurement range for 10-h samples, two of these also at baseline. For B12 two sets of samples showed results above the upper measurement range for baseline samples, one of these also for the 10-h sample.
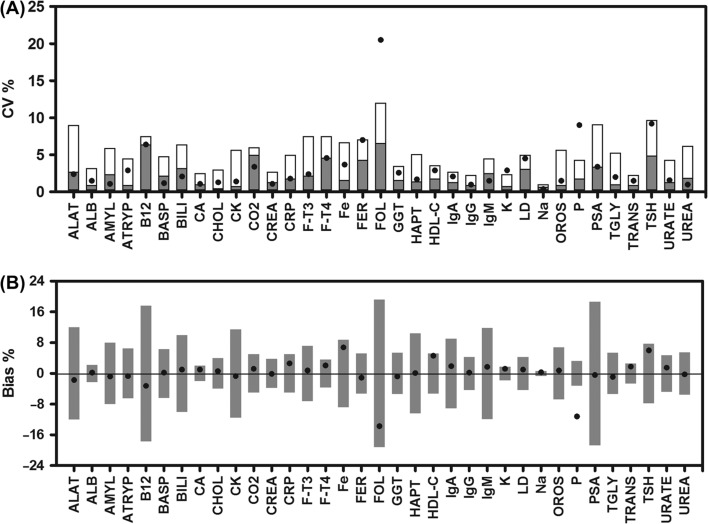
Thirteen of the analytes showed no statistically significant bias and mean CVs below or close to the analytical CV upon storage of whole blood for 10 h (ALB, ATRYP, BASP, BILI, CO2, CREA, F-T3, GGT, HAPT, IgG, LD, PSA, UREA), and thus will not be subject to any further discussion (Figure 1 and Table II). For most of the remaining analytes bias was statistically significant though quantitatively small (ALAT, AMYL, CA, CHOL, CK, CRP, F-T4, FER, IgA, IgM, K, Na, OROS, TGLY, TRANS and URATE). A more pronounced bias was observed for B12, Fe, FOL, HDL-C and TSH. Bias exceeding the bias goal was observed only for P (Figure 1 and Table II) and the 95% confidence interval (95% CI) of bias was below the bias goal for all but CRP, FT-4, HDL-C, K and TSH. Despite the changes in bias all but three analytes (FOL, K and P) met the criteria set forward for imprecision – the goal-CV (Figure 1 and Table II).
Figure 1.
Imprecision and bias calculated for results obtained at baseline and after 10 h of storage of whole blood (in lithium-heparin or serum tubes). Data on imprecision (A) shows CV calculated from results obtained at baseline and after storage of whole blood in lithium-heparin or serum tubes at 21°C for 10 h. The analytical imprecision is indicated as grey columns and the goal imprecision as the total height of the columns. Data on bias (B) shows the mean bias between baseline and results obtained after storage of whole blood for 10 h. The columns indicate bias goal. Data for analytical- and goal-CV as well as bias goal are indicated for each analyte in Table II.
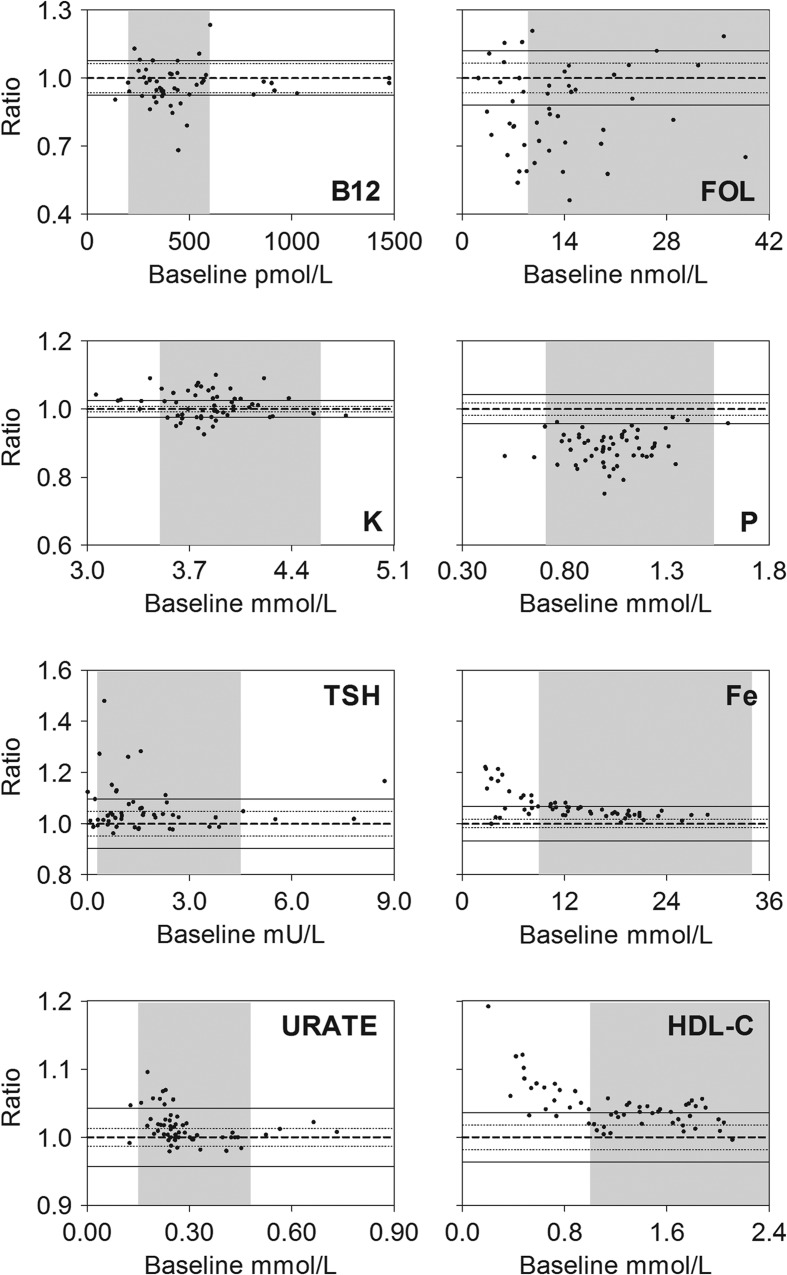
We also analyzed the results using fractional Bland-Altman plots, and present plots for B12, Fe, FOL, HDL-C, K, P, TSH, and URATE (Figure 2).
Figure 2.
Bland-Altman plots of results obtained after storage of whole blood (in lithium-heparin or serum tubes) for 10 h prior to analysis as compared to results at baseline. Fraction: Ratio between baseline results and results at 10 h. Indicated on each figure is the line of identity, i.e. baseline/10 h = 1.0 (bold dashed line), the analytical CV (thin dashed line), goal-CV (full drawn thin line) and the reference interval (shaded area). Data for analytical- and goal-CVs are indicated for each analyte in Table II.
B12 showed an average of around 3% decrease in values. For Fe, a concentration-dependent relative increase was observed. Thus, for values below 10 μmol/L, the average increase was approximately 11% after 10 h of storage. FOL decreased around 14%, whereas for HDL-cholesterol, a concentration-dependent relative increase was seen, the average increase being 7% for values below 1.0 mmol/L. For K, we observed a tendency to increased values after 10 h of storage. P showed an average of around 11% decrease in values throughout the measurement range. For TSH, we found a relative increase in values, especially in the lower measurement range, where values < 1.25 mU/L showed an average increase of 8% after 10 h of storage. This was however caused by few samples with a very high bias. For URATE, we observed a concentration dependent relative increase after 10 h of storage. For values in the lower part of the reference interval and below (values < 0.24 mmol/L) the average increase was 3%.
Discussion
We investigated the effect of storage of venous whole blood in lithium-heparin or serum tubes at a temperature of 21°C for 10 h, including 90 min of transportation by car. Our study included the 35 most frequently requested biochemical and immunological analytes from GPs. The results obtained covered a broad range of analyte concentrations, since samples were obtained from hospitalized patients rather than from volunteers (Table I). To the best of our knowledge this is the first study to include data on ATRYP, IgA, IgG, IgM, and OROS evaluating stability of whole blood at 21°C.
In our study some of the analytes showed a statistically significant but quantitatively small bias not depending on the concentration of the analyte. Only few analytes showed more pronounced changes (B12, Fe, FOL, HDL-C, P, and TSH); however, the observed bias were smaller than the bias goal for all but P.
In addition we reported concentration-dependent statistically significant relative changes for Fe, HDL-C, TSH, and URATE. For these analytes we found spuriously high results in the lower level of the reference interval and below (Figure 2). The increased level for Fe was likely to be caused by leakage from the red blood cells, while the deviations for HDL-C, TSH, and URATE remained unexplained. Though these differences may be of importance in the research setting, we judged them to be of no importance in the clinical setting. For B12 we judged the observed decrease in concentration upon storage to be of limited importance.
We estimated the mean CV for all analytes studied based on comparison of baseline values with values obtained after 10 h of storage. Obviously CVs calculated in this manner will mirror not only the analytical imprecision but also any observed bias. The fact that the mean CV exceeded the goal-CV only for FOL, K and P underscored that the observed bias was unlikely to be of clinical importance.
Previous relatively small studies covering up to 30 analytes, have shown that prolonged (up to 24 h) storage of blood samples from presumed healthy individuals introduces considerable changes for several analytes, both when samples of whole blood are stored at room temperature (20–25°C) and especially when stored at temperatures well above room temperature [4–8]. Particular critical analytes are ALAT, K, LD, and P, which in most studies are reported as stable only for a few hours at room temperature and above (up to 12 h depending on analyte and study) [4,8–10,12,15,19].
The studies were undertaken on serum [5–9,15,19] or plasma [7–10,12], in tubes with [5,7,10,15] or without gel separator [6,8,9,19]. The most extensive study performed so far was based on whole blood (in lithium heparin tubes with gel) drawn from a large cohort of patients (n = 406) at GPs offices [11]. Analyzing samples from 100 patients, the authors concluded that all but two (K and P) out of 21 analytes tested were stable for 8 h at 21°C pre- centrifugation, while K and P met quality goals only after storage for up to 4 h. The study included a transportation process of 10–15 min duration, which was shorter than the relevant transportation time for most GPs. A recent study on patient samples (n = 100) stored as whole blood (in serum-separation tubes with gel) at 15–25°C for 6 h, including a 2-h transportation procedure, concluded that K, LD and P did not meet quality goals set by the authors, whereas FOL did [13].
Combined with the results obtained in our study, all of these results suggest that whole blood can be transported for analysis at the hospital laboratory. However, as evident from the above, some concern is still warranted for some analytes.
An increased level of K caused by leakage from blood cells has over the years been a major concern [20,21]. Also, the changes in K levels caused by the activity of the temperature-dependent Na+K+-ATPase has to be considered. Transporting samples at low temperatures leads to increased K levels, and at high temperatures K levels are typically decreased [12,14,22].
Tanner et al. [14] studying serum obtained from gel tubes reported temperature-dependent changes in K concentrations, and concluded that storage at 25°C was superior to 15°C and 35°C. However, accepting a 7% deviation, K only remained stable for 4 h. Temperature-dependent changes in K concentrations in lithium-heparin whole blood stored for 2–12 h at temperatures ranging from 17–25°C have also been presented by Stahl et al. [12]. They observed the mean difference from initial values to be lowest at 20°C, and accepting a 10% deviation, concluded K being stable in whole blood for 12 h at 20°C.
Our study showed less deviations in K concentrations upon storage of lithium-heparin whole blood, than observed in other studies on patient whole blood samples [11,21,23]. In our study, only one out of 56 samples showed K values with more than 10% deviation (10.1%) to baseline samples – a criteria used by both Jensen et al. [11] and Nybo et al. [23]. Nybo et al., however, observed approximately 40% of samples deviating more than ∼10% after 6 h of storage at room temperature, and applying the same criteria to the data presented by Seamark et al. [21] only 70–80% of the samples showed acceptable values for K after storage at temperatures between 15 and 30°C for up to 12 h. Jensen et al., who stored samples at 21 ± 1°C, reported 12–13% of samples showing more than a ∼10% deviation to baseline after 8 h of storage. One possible explanation for the discrepancies was a difference in the design of our study as compared to the others. The three studies [11,21,23] all using lithium-heparin whole blood samples separated plasma after centrifugation of baseline samples, whereas we followed daily routine and performed the analysis on primary tubes soon after centrifugation without separation of plasma. Based on our data, we concluded that K can be analyzed on blood stored for up to 10 h at 21 ± 1°C.
In agreement with others, studying the stability of P in lithium-heparin whole blood stored at 21 ± 1°C for periods between 4 and 12 h [8,10–12], we observed a considerable mean decrease to baseline samples (11.2%) in P after 10 h of storage.
Storage at room temperature has shown a biphasic change in the concentration of P. Decreased levels are reported after storage up to 8–16 h, and increased concentrations after 24–48 h of storage [4,7,9,14]. The decrease in the concentration of P is likely to be caused by a consumption of inorganic P during intracellular phosphorylation of glucose, whereas an increase could be ascribed to hydrolysis of organic phosphates and leakage from the red blood cells, red blood cells having a 7-fold higher concentration of P as compared to plasma [4,14,19].
We observed a large average decrease (13.7%) in the FOL level after storage of whole blood in serum tubes. It is well known that FOL is unstable, undergoing oxidative cleavage in stored blood samples [24]. In addition, FOL has been described to be light-sensitive [25]. Our results on FOL are in accordance with previous studies on the stability of human whole blood stored at 21°C and above for periods between 2 and 96 h [9,26,27].
The current study was planned to mirror the routine setting of the clinical biochemistry laboratory, and thus did not allow refined conclusions concerning the influence of 10 h storage of whole blood. To do so, several issues should be taken into account. Plasma/serum should be separated as soon as possible after blood collection and analysis of the baseline sample should be done not only at baseline but also at the same time as the 10-h sample. In addition, in a refined study, it may be of relevance to use goals for bias and CV defined solely by biological variation rather than based on a combined knowledge of biological variation and analytical performance in daily practice.
Based on the results, it is our conclusion that storage of whole blood in lithium-heparin or serum tubes at 21 ± 1°C for up to 10 h induces changes that are of no importance in the clinical setting, and that caution is warranted only for FOL and P. In the research setting time- and concentration-dependent changes may be of importance, notably if samples are to be retrieved both in the GPs office and at the hospital. For daily clinical practice, we believe that the use of whole blood samples is superior to the alternative, involving centrifugation and further handling of the blood samples in the GPs office.
Acknowledgements
We wish to thank our phlebotomy staff for their assistance with the specimen collection and analysis. We also thank Karin Biering, MHSc, Department of Occupational Medicine, Regional Hospital West Jutland, Herning, Denmark, for assistance regarding statistical analyses. Financial support from the Central Denmark Region is acknowledged.
Footnotes
Declaration of interest The authors report no conflict of interest. The authors alone are responsible for the content and writing of the paper.
References
- 1.Lippi G, Chance JJ, Church S, Dazzi P, Fontana R, Giavarina D, Grankvist K, Huisman W, Kouri T, Palicka V, Plebani M, Puro V, Salvagno GL, Sandberg S, Sikaris K, Watson I, Stankovic AK, Simundic A-M. Preanalytical quality improvement: from dream to reality. Clin Chem Lab Med. 2011;49:1113–26. doi: 10.1515/CCLM.2011.600. [DOI] [PubMed] [Google Scholar]
- 2.Plebani M. Errors in clinical laboratories or errors in laboratory medicine? Clin Chem Lab Med. 2006;44:750–9. doi: 10.1515/CCLM.2006.123. [DOI] [PubMed] [Google Scholar]
- 3.CLSI. Approved Guideline. 4th ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2010. Procedures for the Handling and Processing of Blood Specimens for Common Laboratory Tests. CLSI document H18-A4. [Google Scholar]
- 4.Ono T, Kitaguchi K, Takehara M, Shiiba M, Hayami K. Serum-constituents analyses: effect of duration and temperature of storage of clotted blood. Clin Chem. 1981; 27:35–8. [PubMed] [Google Scholar]
- 5.Rehak NN, Chiang BT. Storage of whole blood: effect of temperature on the measured concentration of analytes in serum. Clin Chem. 1988;34:2111–4. [PubMed] [Google Scholar]
- 6.Laessig RH, Indriksons AA, Hassemer DJ, Paskey TA, Schwartz TH. Changes in serum chemical values as a result of prolonged contact with the clot. Am J Clin Pathol. 1976; 66:598–604. doi: 10.1093/ajcp/66.3.598. [DOI] [PubMed] [Google Scholar]
- 7.Boyanton BL, Jr, Blick KE. Stability studies of twenty-four analytes in human plasma and serum. Clin Chem. 2002;48:2242–7. [PubMed] [Google Scholar]
- 8.Foucher B, Pina G, Desjeux G, Prevosto JM, Chaulet JF, Cheminel V. [Influence of temperature and delayed centrifugation: stability studies of 28 analytes currently analysed] Ann Biol Clin (Paris) 2005;63:93–100. [PubMed] [Google Scholar]
- 9.Oddoze C, Lombard E, Portugal H. Stability study of 81 analytes in human whole blood, in serum and in plasma. Clin Biochem. 2012;45:464–9. doi: 10.1016/j.clinbiochem.2012.01.012. [DOI] [PubMed] [Google Scholar]
- 10.Leino A, Koivula MK. Stability of chemical and immunochemical analytes in uncentrifuged plasma samples. Ann Clin Biochem. 2009;46:159–61. doi: 10.1258/acb.2008.008212. [DOI] [PubMed] [Google Scholar]
- 11.Jensen EA, Stahl M, Brandslund I, Grinsted P. Stability of heparin blood samples during transport based on defined pre-analytical quality goals. Clin Chem Lab Med. 2008;46:225–34. doi: 10.1515/CCLM.2008.053. [DOI] [PubMed] [Google Scholar]
- 12.Stahl M, Brandslund I. Controlled storage conditions prolong stability of biochemical components in whole blood. Clin Chem Lab Med. 2005;43:210–5. doi: 10.1515/CCLM.2005.036. [DOI] [PubMed] [Google Scholar]
- 13.Dialma P, Piaulenne S, Baty S, Zeitoun T. [Preanalytical phase and accreditation: acceptance criteria for samples of multisite laboratory] Ann Biol Clin (Paris) 2013;71:121–8. doi: 10.1684/abc.2012.0780. [DOI] [PubMed] [Google Scholar]
- 14.Tanner M, Kent N, Smith B, Fletcher S, Lewer M. Stability of common biochemical analytes in serum gel tubes subjected to various storage temperatures and times pre-centrifugation. Ann Clin Biochem. 2008;45:375–9. doi: 10.1258/acb.2007.007183. [DOI] [PubMed] [Google Scholar]
- 15.Kang HJ, Jeon SY, Park JS, Yun JY, Kil HN, Hong WK, Lee MH, Kim JW, Jeon JP, Han BG. Identification of clinical biomarkers for pre-analytical quality control of blood samples. Biopreserv Biobank. 2013;11:94–100. doi: 10.1089/bio.2012.0051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rodbard D. Statistical quality control and routine data processing for radioimmunoassays and immunoradiometric assays. Clin Chem. 1974;20:1255–70. [PubMed] [Google Scholar]
- 17.Fraser CG, Petersen PH, Ricos C, Haeckel R. Proposed quality specifications for the imprecision and inaccuracy of analytical systems for clinical chemistry. Eur J Clin Chem Clin Biochem. 1992;30:311–7. [PubMed] [Google Scholar]
- 18.Fraser CG, Hyltoft PP, Libeer JC, Ricos C. Proposals for setting generally applicable quality goals solely based on biology. Ann Clin Biochem. 1997;34((Pt 1)):8–12. doi: 10.1177/000456329703400103. [DOI] [PubMed] [Google Scholar]
- 19.Zhang DJ, Elswick RK, Miller WG, Bailey JL. Effect of serum-clot contact time on clinical chemistry laboratory results. Clin Chem. 1998;44:1325–33. [PubMed] [Google Scholar]
- 20.Webster JH, Neff J, Schiaffino SS, Richmond AM. Evaluation of serum potassium levels. Am J Clin Pathol. 1952;22:833–42. doi: 10.1093/ajcp/22.9.833. [DOI] [PubMed] [Google Scholar]
- 21.Seamark D, Backhouse S, Barber P, Hichens J, Salzmann M, Powell R. Transport and temperature effects on measurement of serum and plasma potassium. J R Soc Med. 1999;92:339–41. doi: 10.1177/014107689909200703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sinclair D, Briston P, Young R, Pepin N. Seasonal pseudohyperkalaemia. J Clin Pathol. 2003;56:385–8. doi: 10.1136/jcp.56.5.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nybo M, Hansen AB, Pedersen B, Jørgensen PJ, Kristensen SR. Præanalytisk variation af P-Kalium-ion, stofkonc.: relevans for primærsektoren? [Preanalytical variation of plasma potassium: Importance for the general practitioners?] Klinisk Kemi i Norden. 2004;16:16–22. [Google Scholar]
- 24.Hannisdal R, Svardal A, Ueland PM. Measurement of folate in fresh and archival serum samples as p-aminobenzoylglutamate equivalents. Clin Chem. 2008;54:665–72. doi: 10.1373/clinchem.2007.100511. [DOI] [PubMed] [Google Scholar]
- 25.Mastropaolo W, Wilson MA. Effect of light on serum B12 and folate stability. Clin Chem. 1993;39:913. [PubMed] [Google Scholar]
- 26.Chu SY, MacLeod J. Effect of three-day clot contact on results of common biochemical tests with serum. Clin Chem. 1986;32:2100. [PubMed] [Google Scholar]
- 27.van Eijsden M, van der Wal MF, Hornstra G, Bonsel GJ. Can whole-blood samples be stored over 24 hours without compromising stability of C-reactive protein, retinol, ferritin, folic acid, and fatty acids in epidemiologic research? Clin Chem. 2005;51:230–2. doi: 10.1373/clinchem.2004.042234. [DOI] [PubMed] [Google Scholar]