Abstract
Calcineurin signaling plays diverse roles in fungi in regulating stress responses, morphogenesis and pathogenesis. Although calcineurin signaling is conserved among fungi, recent studies indicate important divergences in calcineurin-dependent cellular functions among different human fungal pathogens. Fungal pathogens utilize the calcineurin pathway to effectively survive the host environment and cause life-threatening infections. The immunosuppressive calcineurin inhibitors (FK506 and cyclosporine A) are active against fungi, making targeting calcineurin a promising antifungal drug development strategy. Here we summarize current knowledge on calcineurin in yeasts and filamentous fungi, and review the importance of understanding fungal-specific attributes of calcineurin to decipher fungal pathogenesis and develop novel antifungal therapeutic approaches.
Keywords: calcineurin, fungi, cell wall, virulence, septum, Aspergillus, Candida
1. Introduction
The importance of protein phosphorylation and dephosphorylation is well-recognized in cellular functions. The significance of fungal protein phosphorylation by protein kinases in signal transduction has paved the way for understanding the equally important protein phosphatases and reversible phosphorylation as critical for integration of cellular events. Among the two major families of serine/threonine phosphatases, the regulatory role of calcineurin, a Ca2+/calmodulin (CaM)-regulated serine/threonine protein phosphatase (also known as protein phosphatase 2B) is emerging. Calcineurin is a metalloenzyme with a binuclear metal center made of Fe3+and Zn2+ (Aramburu et al., 2001; Hemenway and Heitman, 1999). It is a heterodimer consisting of two subunits, the α-catalytic subunit, termed calcineurin A (~60 kDa) that binds to CaM, and a regulatory β-subunit, termed calcineurin B (~19 kDa) (Rusnak and Mertz, 2000).
Calcineurin homologs are widely distributed in different organisms, ranging from parasites, fungi, plants and invertebrates to the higher vertebrates (Naderer et al., 2011; Rusnak and Mertz, 2000; Singh et al., 2014). In mammalian cells, three genes encoding the calcineurin catalytic subunit (CnAα, CnAβ, and CnAγ) and two genes encoding the calcineurin regulatory subunit (CnB1 and CnB2) have been identified (Rusnak and Mertz, 2000) that have been implicated in diverse processes such as neuronal metabolism, T-lymphocyte proliferation, immune suppression via its downstream transcription factor NFAT (nuclear factor of activated T cells), and also in the regulation of intracellular Ca2+ release channels (Heineke and Ritter, 2012; Musson et al., 2012; Williams and Gooch, 2012). Calcineurin is the only phosphatase recognized in fungi that is regulated by Ca2+ and CaM, and its principal target, Crz1 (homolog of NFAT), has also been well studied in Saccharomyces cerevisiae (Stathopoulos-Gerontides et al., 1999).
Drugs such as cyclosporin A (CsA) and tacrolimus (FK506) inhibit calcineurin function by first binding to their respective intracellular receptors, the immunophilins cyclophilin A (CyA) and FK506-binding protein (FKBP12). Their immunosuppressive activity has revolutionized modern transplantation to prevent graft rejection (van Rossum et al., 2010). While defects in calcineurin signaling have been linked to various diseases in humans, including Alzheimer’s, Down syndrome, cardiac hypertrophy, diabetes mellitus, skin disorders, and others (Harris et al., 2005; Heit, 2007; Reese and Taglialatela, 2011; Reynolds and Al-Daraji, 2002), the fact that calcineurin is essential for fungal growth, stress responses, mating and pathogenesis has generated great interest among medical mycologists (Bastidas et al., 2008; Chen et al., 2010). Cellular functions of calcineurin in various fungal species are summarized in Table 1. There is a growing population of drug resistant isolates of Cryptococcus, Candida, and Aspergillus species (Cowen and Steinbach, 2008), and recently emerging Zygomycete species, Mucor circinelloides and Rhizopus oryzae (Shirazi and Kontoyiannis, 2013), which are all known causes of high mortality in immunocompromised patients. The increasing incidence of these difficult to treat invasive fungal infections, coupled with the current limited armamentarium of antifungals, makes the as yet incompletely explored calcineurin pathway an attractive novel alternative for antifungal targeting (Steinbach et al., 2007b). Along with salient well-known findings, here we review the latest discoveries on calcineurin signaling in fungi, with particular emphasis on how this pathway can be exploited for future antifungal-specific drug design for the treatment of invasive fungal infections.
Table 1.
Roles of calcineurin in yeasts and fungi
2. Calcineurin signaling in fungi
Calcineurin has been well characterized in the model yeasts S. cerevisiae and Schizosaccharomyces pombe, where it is required for adaptation to a variety of environmental stresses, cation homeostasis, morphogenesis, cell wall integrity, and mating (Cyert et al., 1991; Matsumoto et al., 2002; Mendoza et al., 1996; Mendoza et al., 1994; Sugiura et al., 2002). In S. cerevisiae, the calcineurin catalytic subunit is encoded by two genes, CNA1 and CNA2, and a single regulatory subunit (CNB1) (Cyert, 2003). S. pombe and most other filamentous fungal species contain a single gene each encoding the catalytic subunit and the regulatory subunit of calcineurin (Yoshida et al., 1994). Calcineurin participates in the morphogenesis of S. pombe by altering septal positioning and aberrant spindle body organization (Lu et al., 2002; Yoshida et al., 1994). Calcineurin also regulates growth at alkaline pH, elevated temperature, membrane stress and virulence in the pathogenic yeasts Candida albicans and Cryptococcus neoformans (Chen et al., 2010; Kozubowski and Heitman, 2012). In C. albicans, calcineurin is also important for maintenance of colony morphology and dimorphic switching, in addition to regulating several downstream target genes, including PMC1, the vacuolar calcium P-type ATPase, and FKS1, the β-1,3 glucan synthase subunit (Sanglard et al., 2003). In the model filamentous fungi Neurospora crassa, calcineurin was shown to influence the tip high calcium gradient necessary for growing hyphal tip and normal vegetative growth (Kothe and Free, 1998; Prokisch et al., 1997). The calcineurin catalytic subunit encoding cDNA from the filamentous fungus Aspergillus oryzae also complemented the S. cerevisiae calcineurin mutant (Δcna1Δcna2), which was not viable in the presence of high concentrations of NaCl (1.2 M) and at alkaline pH (Juvvadi et al., 2001), indicating that the heterologously expressed calcineurin is capable of protecting the yeast against alkaline pH and high salt concentrations. Subsequently calcineurin was also implicated in the regulation growth under alkaline pH, high salt concentrations and heat stress conditions in filamentous fungi (Juvvadi et al., 2003). The only well-known mechanism regarding calcineurin controlling the expression of several genes is through the dephosphorylation of the Zn-finger transcription factor Crz1p/Tcn1p, triggering its translocation into the nucleus to activate gene expression (Stathopoulos-Gerontides et al., 1999), yet knowledge on its posttranscriptional roles and interacting proteins leading to regulation is limited. A recent study also provided a novel insight into the relationship between calcineurin signaling and cellular iron, membrane lipid homeostasis and drug susceptibility of C. albicans (Hameed et al., 2011).
While calcineurin has been linked to several stress response pathways in S. cerevisiae, its importance for light signaling has only been recently explored. The calcineurin-dependent transcription factor, Crz1p, was shown to translocate into the nucleus and activate gene expression during illumination (Bodvard et al., 2013). In comparison to the oscillatory nuclear-cytoplasmic shuttling of the Msn2p and Msn4p transcription factors that are the major stress response regulators in the budding yeast (Estruch, 2000), Crz1p exhibited permanent nuclear localization, which is different than the previously reported response following exposure to high extracellular Ca2+ levels (Cai et al., 2008). More detailed description on calcineurin roles in fungal stress responses, pathogenesis and drug resistance are provided below.
3. Calcineurin in pH stress, temperature stress response and cation homeostasis
Based on the sensitivity of the yeast cnb1 mutants to alkalization, which was recapitulated by treatment of the wild-type cells with FK506, calcineurin is required for high pH tolerance (Nakamura et al., 1993). Subsequently, calcineurin-mediated dephosphorylation of the Crz1 transcription factor and its entry into the nucleus induces the expression of several alkaline pH responsive genes (Serrano et al., 2002; Stathopoulos-Gerontides et al., 1999). Calcium entry through the Cch1–Mid1 channels in response to increased pH may activate calcineurin (Viladevall et al., 2004). However, the CRZ1 mutant did not exhibit sensitivity to alkaline pH (Stathopoulos and Cyert, 1997), revealing that calcineurin may also regulate pH stress response in a Crz1-independent manner by interacting with other alkaline responsive genes, such as HPH1 and HPH2, which were reported as substrates of calcineurin (Heath et al., 2004). As mentioned earlier heterologous expression of filamentous fungal calcineurin rescued the yeast calcineurin mutant against alkaline pH (Juvvadi et al., 2001). Subsequent study also showed the importance of calcineurin in the regulation growth under alkaline pH in filamentous fungi (Juvvadi et al., 2003). In C. albicans, previous studies also revealed a novel requirement for calcineurin for growth at both alkaline and acidic pH (Bader et al., 2006; Kullas et al., 2007).
While the S. cerevisiae calcineurin catalytic subunit null mutant was able to grow at higher temperature (Liu et al., 1991), the C. neoformans calcineurin catalytic subunit disruption strain was not viable in conditions that mimicked the host environment (37° C, 5% CO2 or alkaline pH) and was avirulent (Odom et al., 1997). In contrast to C. neoformans, calcineurin was dispensable for survival of C. albicans at 37 or 42° C, and the C. albicans cnb1 mutant strains had no defects in germination and filamentous growth (Blankenship et al., 2003). Although the C. albicans cnb1 mutant strain showed filamentous growth in vivo, it failed to grow in a mouse model of disseminated candidiasis and was unable to survive in serum, revealing evolutionarily distinct control aspects of calcineurin over virulence of two divergent fungal pathogens via distinct mechanisms (Blankenship and Heitman, 2005; Blankenship et al., 2003; Kraus et al., 2003). To some extent, the host niche is also an important factor for demonstrating the requirement of calcineurin for virulence, as demonstrated in vaginal or pulmonary candidiasis models (Bader et al., 2006). This highlights that although the calcineurin signal transduction pathway seems conserved, one cannot extrapolate from what is known in the model yeasts, or even from other human fungal pathogens, to completely establish calcineurin-dependent cellular functions in all fungal pathogens.
The requirement of calcineurin for NaCl and LiCl tolerance in S. cerevisiae was demonstrated wherein the cnb1 mutant accumulated high levels of lithium as a consequence of decrease in the expression of the ENA1 gene and failure in K+ transport system (Mendoza et al., 1994). This again was attributed to the alterations in cytoplasmic Ca2+ as a trigger for the salt adaptation process and suggested that calcineurin coordinated the NaCl stress adaptation process by efficient control of the expression of the Na+ efflux and the K+ uptake system (Matsumoto et al., 2002). Supporting these observations, constitutive expression of active calcineurin resulted in higher tolerance to even toxic levels of Na+ and K+ by promoting the expression of ENA1 and resulted in elongated and unipolar budding, providing evidence for calcineurin control of polarity establishment (Mendoza et al., 1996). It was recently found that Ypi1, a regulatory subunit of type 1 protein phosphatase Glc7, is also dependent on calcineurin for modulating the cation tolerance in S. cerevisiae (Marquina et al., 2012). Consistent with S. cerevisiae cnb1 mutants, the C. albicans CNA mutant was more susceptible to calcium, lithium or sodium cations (Sanglard et al., 2003).
The deletion of S. pombe PMR1, encoding the P-type Ca2+/Mn2+ ATPase, caused rounded cell morphology and the expression of PMR1 was calcineurin-dependent, revealing the role for calcineurin in Mn2+ homeostasis (Maeda et al., 2004). In addition, the deletion of A. fumigatus pmrA, encoding a golgi Ca2+/Mn2+ P-type ATPase, showed a basal growth defect and cationic tolerance associated with increased expression of calcineurin pathway genes (Pinchai et al., 2010). The functions of three calcium transporters, pmcA, pmcB, and pmcC (PMC1 homologs) were also investigated and cyclosporine A was able to modulate the sensitivity of these mutant strains to calcium and manganese salts, revealing their dependence on calcineurin (Dinamarco et al., 2012).
The deletion of A. fumigatus crzA also conferred a severe growth defect in the presence of increased concentrations of calcium and manganese, in addition to decreased conidiation, abnormal conidial surface morphology, altered mRNA expression of calcium transporters and a virulence defect (Cramer et al., 2008; Soriani et al., 2008). Similarly, two independent investigations have revealed that the A. nidulans crzA (CRZ1 homolog) mutant exhibited sensitivity to alkaline pH, high calcium and manganese concentrations, and mediated the expression of P-type calcium-ATPase homologous genes (Hagiwara et al., 2008; Spielvogel et al., 2008). In continuation of these results, the nucleo-cytoplasmic shuttling of CrzA and its regulation by phosphorylation/dephosphorylation in response to Ca2+ and alkaline pH and the involvement of casein kinase 1 and glycogen synthase kinase-3β in its regulation was recently elucidated (Hernández-Ortiz and Espeso, 2013). Characterization of extragenic suppressors of the A. nidulans crzA null mutant identified mutations in cnaB, the folA gene encoding dihydroneopterin aldolase, an enzyme involved in folic acid biosynthesis, and scrC, a gene with as yet unknown function (Almeida et al., 2013).
4. Calcineurin control of hyphal growth and cell wall integrity
Calcineurin appears to control many aspects of fungal growth. The expression of a constitutively active calcineurin A subunit resulted in cell elongation with a unipolar budding pattern in S. cerevisiae (Mendoza et al., 1996). In the fission yeast, S. pombe, the calcineurin A null mutant showed a cell polarity defect along with delayed cytokinesis, resulting in multi-septate cells, and overexpression of calcineurin caused abnormality in cell shape (Yoshida et al., 1994). In C. neoformans, calcineurin is required for hyphal elongation during mating, cell fusion and asexual haploid fruiting of MATα cells in response to nitrogen limitation (Cruz et al., 2001). Furthermore, a phospholipid binding protein, Cts1 (calcineurin temperature suppressor), was shown to control septal positioning and cell separation in coordination with calcineurin (Fox et al., 2003). Increased expression of FKS1, encoding a component of β-1,3-glucan synthase complex that is responsible for cell wall biosynthesis, was evident in the C. neoformans cnb1 mutant (Kraus et al., 2003). Distinct roles for calcineurin control over stress, mating and filamentation became evident by studying the function of the calcipressin family member, Cbp1/Rcn1, shown to play a role in mating in C. neoformans but not required for survival at high temperature (Fox and Heitman, 2005; Gorlach et al., 2000).
Previous studies in filamentous fungi revealed the importance of calcineurin for cell cycle progression in A. nidulans (Nanthakumar NN, 1996), hyphal branching in N. crassa (Kothe and Free, 1998; Prokisch et al., 1997), stress adaptation in A. oryzae (Juvvadi et al., 2003), sclerotial development in Sclerotinia scleotiorum (Harel et al., 2006) and formation of the infectious structure called “appressorium” in the plant pathogen Magnaporthe oryzae (Choi, 2009). In the necrotrophic fungus Botrytis cinerea, deletion of crz1 caused defects in cell wall and membrane integrity, resulting in slowed growth rate, conidiation and hyphal penetration of the leaves in addition to an impaired response to cationic stress (Schumacher et al., 2008). While in the plant pathogenic basidiomycete Ustilago maydis mutation of the calcineurin catalytic subunit caused multiple budding and reduced mating (Egan et al., 2009), in Ustilago hordei the calcineurin pathway is required for a variety of environmental stresses, including sensitivity to pH, temperature, cations, acid and oxidative stresses (Cervantes-Chávez et al., 2010). Furthermore, alterations in cell morphology as a consequence of defects in cell-wall integrity were notable in addition to delayed mating and decreased virulence (Cervantes-Chávez et al., 2010). The M. oryzae crz1 mutant exhibited defective growth in presence of cell wall inhibitors and reduced pathogenicity along with down regulation of the FKS1 and the chitin synthase genes, CHS2 and CHS4 (Choi et al., 2009). The Rho1 GTPase regulates cell wall glucan synthesis, and the absence of calcineurin was lethal in a temperature sensitive mutant strain of S. pombe carrying the rho1-596 allele which displayed reduced Rho1 GTPase activity concomitant with severe cell wall defects (Viana et al., 2013).
Calcineurin and some of its downstream pathway genes have been well studied in the model fungus, A. nidulans, and the human fungal pathogen, A. fumigatus. Though calcineurin is not an essential gene in A. fumigatus, it is a key phosphatase involved in regulating the differentiation and fitness of this opportunistic pathogen (Ferreira et al., 2007; Steinbach et al., 2006). A. fumigatus lacking either the calcineurin catalytic subunit or the regulatory subunit exhibited defective hyphal morphology related to apical extension and branching (Juvvadi et al., 2011; Steinbach et al., 2006). The deletion of A. fumigatus crzA also conferred a severe growth defect, decreased conidiation, abnormal conidial surface morphology and a virulence defect (Cramer et al., 2008; Soriani et al., 2008).
Transcriptional profiling of cell wall biosynthesis genes revealed decreased fksA expression, which correlated with reduced β-1,3-glucan content in the A. fumigatus ΔcnaA strain but not in the ΔcrzA strain, indicating that CrzA may not have a significant role in the regulation of β-1,3-glucan biosynthesis in A. fumigatus (Cramer et al., 2008). Although the ΔpmrA strain had higher β-glucan and chitin content, it was hypersensitive to cell wall inhibitors and remained virulent, suggesting calcineurin control of pmrA in cation homeostasis and cell wall integrity. Deletion of A. fumigatus cbpA, belonging to the calcipressin family, resulted in reduced hyphal growth and limited attenuated virulence (Pinchai et al., 2009). Based on the phenotypes obtained by deleting the calcineurin genes in filamentous fungi, its importance for regulating hyphal growth and development was demonstrated, but the exact mechanisms involved remain less well characterized. In the A. nidulans ΔcnaA mutant, calcineurin dependency of flbA, brlA and wetA genes in directing the induction of asexual development was reported (Soriani et al., 2008). The concerted action of calcineurin with the high affinity Ca2+ channel proteins, CchA and MidA, has also been shown to be required for conidiation in A. nidulans (Wang et al., 2012).
While calcineurin is required for virulence of A. fumigatus, the calcineurin complex (CnaA and CnaB) also selectively localizes at the hyphal tips and septa to direct proper hyphal growth and regular septum formation. Additionally, the regulatory subunit (CnaB) is absolutely essential for activation of the catalytic subunit (CnaA) in vivo (Juvvadi et al., 2008; Juvvadi et al., 2011). Because septa are cross walls that are laid down at regular intervals during hyphal extension and are central to filamentous fungal life style, septal localization of the calcineurin complex may be required for biochemical processes linking cell wall biosynthesis at the septum. Furthermore, the continued presence of the calcineurin complex around the septal pore, confirmed by live cell imaging, implicates its role in cell wall repair mechanisms. Co-localization of actin with the calcineurin complex was also observed during early stage of septation process in A. fumigatus (Juvvadi et al., 2011). In S. cerevisiae, the link between actin polarization and calcineurin through antagonizing the phosphorylation of Slm proteins (slm1p and slm2p) in response to heat shock was also shown (Daquinag et al., 2007). A recent study analyzing the function of a plasma membrane associated kin1 kinase (a member of the eukaryotic PAR-1/MARK/MELK kinase family) in S. pombe showed that Kin1 deletion is synthetic lethal with the calcineurin A mutant and resulted in misfolding of contractile actomyosin rings and promoted multi-septation (Cadou et al., 2013).
One important area of potential clinical relevance is the concept of the “paradoxical effect” following treatment with caspofungin, a β-1,3-glucan synthesis inhibitor (Wiederhold et al., 2004), whereby a compensatory increase in chitin content occurs, linking the two cell wall components under a stress response (Steinbach et al., 2007a; Stevens et al., 2006). It appears that the glucan-chitin interaction is controlled or affected by calcineurin signaling through the transcriptional regulation of chitin synthases (Fortwendel et al., 2010). Analyses of the cell wall composition in the A. fumigatus ΔcnaA ΔcnaB strain (Juvvadi et al., 2011) showed that the cell wall was thicker in all the calcineurin deletion mutants and displayed abnormal septa. Septum formation in the double mutant was not coordinated from either side of the hyphal wall in a regulated manner, which resulted in an incomplete septum. It was also shown that if calcineurin is eliminated, there is a compensatory increase in chitin content in response to reduced β-1,3-glucan biosynthesis. This apparent cell wall stress response is ideal for potential future combination therapy in which calcineurin, β-1,3-glucan, and chitin targets can all be blocked by drugs, and supports a multi-faceted attack on cell wall targets and hyphal growth in A. fumigatus.
Recent investigations into functional domains in A. fumigatus calcineurin have revealed important domains for localization and function of calcineurin at the septum (Juvvadi et al., 2013). While CaM, the key protein known to activate calcineurin, is not required for septal localization of CnaA, it is required for calcineurin function at the hyphal septum and the PxIxIT substrate binding motif in CnaA is required for its localization at the hyphal septum.
While in the higher eukaryotes calcineurin has been shown to regulate vesicular transport and to dephosphorylate several proteins required for clathrin-mediated vesicle recycling (Marks and McMahon, 1998) we do not have any such findings in filamentous fungi except for the identified clathrin AP-1 and AP-2 complex homologs in S. cerevisiae and S. pombe and the link between calcineurin and intracellular trafficking (Cheng et al., 2002; Kita et al., 2004; Sugiura et al., 2002; Valdivia et al., 2002). Studies on FK506-sensitive mutants of S. pombe resulted in the isolation of several mutants showing defects in cell wall integrity and a majority of these genes were related to membrane trafficking (Ma et al., 2011). Based on the dynamic localization of calcineurin as punctate structures at the hyphal tips and their movement to septation sites (Juvvadi et al., 2011), in the future it will be interesting to investigate the role for calcineurin in membrane trafficking in filamentous fungi.
5. Regulation of fungal calcineurin by phosphorylation
Although various functional aspects of calcineurin have been studied in several organisms and the functional domains described, few studies have focused on phosphorylation of calcineurin and the in vivo consequence of mutations in its key domains. Post-translational modifications involving protein phosphorylation/dephosphorylation are important events regulating protein function in vivo, either by activation or inhibition of activity of the protein. While one report on phosphorylation of mammalian calcineurin by casein kinase I in vitro showed no apparent change in its activity (Singh and Wang, 1987), another report on mammalian calcineurin phosphorylation by CaM-dependent protein kinase II and protein kinase C in the CaMBD (Ser411) revealed that the phosphorylation partially inactivated calcineurin and the binding by CaM inhibited the phosphorylation in vitro (Hashimoto et al., 1988; Hashimoto and Soderling, 1989; Martensen et al., 1989). On the contrary, calcineurin phosphorylation by CDS1 (chk2) in S. pombe at the same residue in the CaMBD (Ser459) and at Ser521 in the auto-inhibitory domain (AID) resulted in calcineurin activation (Kume et al., 2011). None of these residues are conserved in calcineurin A in A. fumigatus and other filamentous fungi.
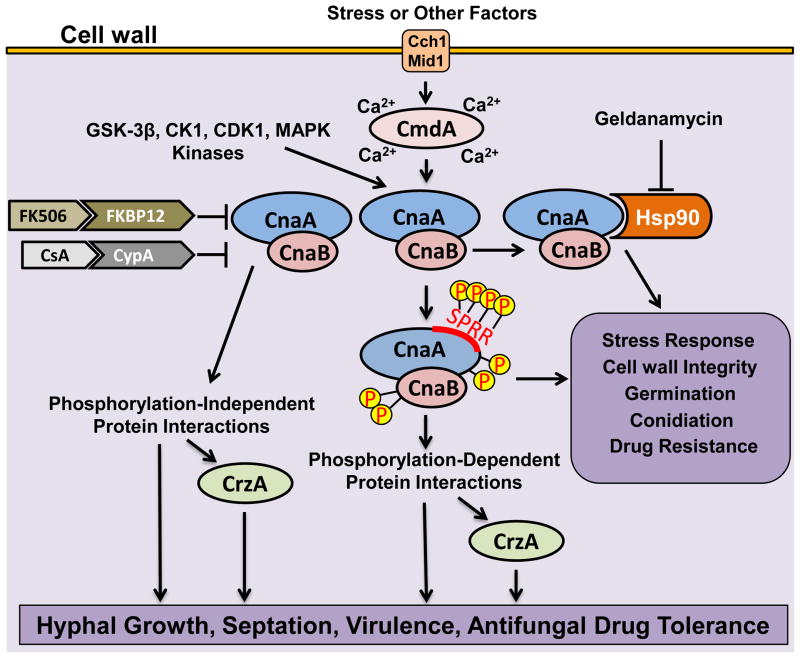
During the course of analyzing the various conserved regions in CnaA, we recently identified a filamentous fungal-specific novel linker domain between the CnBBH and the CaMBD which we designated as the “Serine Proline Rich Region (SPRR)” (Juvvadi et al., 2013). A phylogenic tree constructed based on the variable linker domain sequences and the flanking highly conserved CnBBH and CaMBD sequences from yeasts to human clearly distinguished the filamentous fungal calcineurins from other organisms based on this novel linker region, revealing the possibility of filamentous fungi acquiring this domain during evolution. Based on the CnaA-CnaB structural model we created, the SPRR seems to be present outside the core binding region between CnaA and CnaB and the preponderance of proline and serine residues in this linker creates a hydrophobic environment which could lead to binding to other as yet unknown substrates. This SPRR region was phosphorylated at all 4 clustered serine residues. Though mutations in this region did not affect septal localization of CnaA, they caused hyphal growth and virulence defects, implicating the importance of calcineurin phosphorylation for its function. Phosphorylation at the serine residues in proximity to the proline residues may induce secondary structure conformation in the molecule facilitating binding to other proteins. In vitro phosphorylation assays revealed GSK3β, CK1, CDK1 and MAPK as potential kinases that might phosphorylate CnaA in vivo. In addition to the SPRR, CnaA was also phosphorylated at 2 serine residues at its C-terminus as well as at 2 serine residues in the CnaB N-terminus. FK506 treatment caused reduction in phosphorylation of specific serine residues in CnaA SPRR and CnaB, revealing a new mode of calcineurin inhibition by FK506. It remains to be determined if phosphorylation/dephosphorylation of calcineurin is a dynamic process and is developmental stage-dependent or altered in response to stress conditions.
Heterologous expression of CnaA homologs from other closely related filamentous fungi, N. crassa and M. grisea, also revealed the phosphorylation of serine residues within the SPRR, further evidence that filamentous fungal calcineurins have diverged from the yeasts and other systems. We postulate that conservation of this unique SPRR domain in CnaA among filamentous fungi is evolutionarily significant; filamentous fungi may have acquired this unique domain and that phosphorylation in this domain is another novel mode of calcineurin regulation. Based on these novel findings in the regulation of A. fumigatus calcineurin, we expect that calcineurin interacts with its substrates in a phosphorylated/dephosphorylated state to regulate different cellular functions (Figure 1). It has to be determined if CrzA is activated by phosphorylated calcineurin or dephosphorylated calcineurin, or if both pathways can exist in the control of hyphal growth, septation, virulence and drug resistance. Analyzing phosphorylation-dependent interactants of calcineurin will help identify target proteins that can be exploited as additional fungal-specific therapeutic targets.
Figure 1.
Novel aspects of calcineurin regulation in A. fumigatus. Calcineurin, a heterodimer comprising of the catalytic subunit (CnaA) and the regulatory subunit (CnaB), is activated by Ca2+-calmodulin (CmdA) in response to increase in intracellular Ca2+ by the activity of calcium channels (Cch1/Mid1) and other intracellular stores (not shown). Calcineurin function is inhibited by the binding of the immunophilin-immunosuppresant complexes (FK506-FKBP12 and CsA-CypA). CnaA is phosphorylated at four serine residues in the Serine Proline Rich Region (SPRR) and also at two serine residues in the C-terminus, and two serine residues in the N-terminus of CnaB by the activity of kinases (GSK-3β, CK1, CDK1, MAPK). The phosphorylated calcineurin complex may bind to other substrates and regulate a subset of cellular functions through the transcription factor CrzA or independent of CrzA. Similarly, the unphosphorylated calcineurin complex may also exist and interact with substrates in a phosphorylation-independent manner to regulate other subsets of cellular functions in a CrzA-dependent or independent manner. Calcineurin also orchestrates its function through interaction with the heat shock protein Hsp90, which is a target of geldanamycin. The activated calcineurin-Hsp90 complex is involved in the regulation of stress response, cell wall integrity, growth and drug resistance.
6. Calcineurin in membrane stress and as a drug target
The pathogenic basidiomycete C. neoformans can survive at host temperature via calcineurin-mediated regulatory mechanisms and requires calcineurin for virulence (Brown et al., 2007; Odom et al., 1997). Only recently, in an effort to identify the mechanism governing the role for calcineurin in regulating the growth and virulence of C. neoformans at high temperatures, the interaction of the calcineurin catalytic subunit with the Sec28 (COPI) and Sec13 (COPII) components of membrane trafficking was elucidated (Kozubowski et al., 2011b). When the cryptococcal cells were incubated at 37 °C, the cytosolic Cna1 relocated to endoplasmic reticulum-associated puncta and the mother bud neck. Subsequently, a transcription-independent role of calcineurin was demonstrated by showing the co-localization of calcineurin with P-bodies and stress granules, revealing the association of calcineurin with sites of mRNA processing (Kozubowski et al., 2011a). Recently, the C. neoformans Crz1 ortholog (Crz1/Sp1) was characterized and the CRZ1/SP1 mutant (Δcrz1) was susceptible to cell wall inhibitors concomitant with reduced expression of the gene encoding Chs6 (Lev et al., 2012). Supporting this observation, defects in plasma membrane architecture was also reported in calcineurin mutants of C. gattii and C. neoformans (Chen et al., 2013).
Though calcineurin is not an essential gene in C. albicans, mutation of CNB1 caused hypersensitivity to fluconazole, revealing its role in mediating survival during membrane stress (Cruz et al., 2002). Examination of several azole drugs, including voriconazole and posaconazole, in combination with FK506 and cyclosporine A showed clear synergism. In contrast, there was no difference in sensitivity of the cnb1 mutant compared to the wild type strain following treatment with nikkomycin Z, a chitin synthase inhibitor, or the β-1,3 glucan synthase inhibitor caspofungin, indicating that calcineurin in C. albicans is required specifically for cell survival during membrane stress but not cell wall stress (Cruz et al., 2002). By constructing a C. albicans homozygous calcineurin A mutant, calcineurin was found to be required for survival of C. albicans in presence of various antifungals and in particular to fluconazole as evidenced by constitutive expression of activated calcineurin (Sanglard et al., 2003). This was further linked to the RTA2 gene encoding a flippase as the target for calcineurin-mediated azole resistance via its transcriptional factor Crz1p (Jia et al., 2009; Jia et al., 2012). Furthermore, although C. albicans RTA2 by itself was not involved in virulence (Jia et al., 2012), the deletion of CRZ1 caused a moderate virulence defect and resulted in hypersensitivity to alkaline cations and membrane stress conditions induced by SDS and azoles, indicating that calcineurin controls additional components in this signaling pathway (Karababa et al., 2006; Santos and Larrinoa, 2005). Similarly, the importance of calcineurin for cell wall integrity and tolerance to azoles and echinocandins was reported in the emerging pathogenic Candida species, C. glabrata, C. dubliniensis and C. lusitaniae (Chen et al., 2011; Chen et al., 2012; Miyazaki et al., 2013; Miyazaki et al., 2010; Zhang et al., 2012). In S. cerevisiae the CRZ1 deleted strain was hypersensitive to chitosan and FK506 treatment yielded a similar response, indicating that calcineurin pathway along with Rlm1p control cell wall integrity upon cell wall stress (Zakrzewska et al., 2005).
Beginning with S. cerevisiae, wherein the role for calcineurin in endoplasmic stress response was demonstrated and linked to Ca2+ influx leading to calcineurin activation and prevention of apoptosis and increased drug resistance (Bonilla et al., 2002), recent work investigating the link between Hsp90 (Heat shock protein 90) and calcineurin in C. albicans revealed that activation of calcineurin could up-regulate apoptosis and that calcineurin was downstream player of Hsp90 in regulating echinocandin resistance (Dai et al., 2012). More recently, it was reported that calcineurin inhibition induced caspase-like activity, elevated intracellular Ca2+ leading to increased apoptosis, and enhanced in vitro activity of azoles against R. oryzae, Cunninghamella bertholletiae and M. circinelloides through the apoptotic pathway (Shirazi and Kontoyiannis, 2013).
Current antifungal classes target the cell wall (echinocandin) and cell membrane (azole, polyene), but due to limited efficacy and emerging resistance may need adjunctive agents focused on separate cellular pathways that can be used in combination therapy. This highlights the need to develop new therapeutic strategies for invasive fungal disease, including agents with novel mechanisms of action. Studies on heat-shock protein 90 (Hsp90) and the connection to calcineurin have been emerging in C. albicans and A. fumigatus. Hsp90 stabilizes calcineurin, allowing for calcineurin-dependent stress responses required to survive exposure to antifungal drugs. Hsp90 inhibition abrogates calcineurin dependent stress responses, changing fungistatic drugs to fungicidal agents.
7. Chaperoning of calcineurin by the heat-shock protein 90 (Hsp90) in cell wall integrity and antifungal drug resistance
Hsp90 is a molecular chaperone that is essential and highly conserved among eukaryotes. It has a regulatory role in the folding, maturation, and activation of multiple client proteins (Pearl and Prodromou, 2006; Zhao et al., 2005). The interaction between Hsp90 and calcineurin was first described in a S. cerevisiae strain overexpressing hsc82 (the constitutively expressed homolog of Hsp90) that exhibited similar phenotypic characteristics as calcineurin defective mutants with respect to salt stress sensitivity and calcium tolerance (Imai and Yahara, 2000). Concomitant overexpression of cna2 (catalytic subunit of calcineurin) abolished this phenotype. Furthermore, the level of calcineurin-dependent gene expression was reduced in the hsc82 overexpressing strain. Physical interaction was demonstrated by the co-immunoprecipitation of cna2 with hsp82 and hsc82 (Imai and Yahara, 2000). This complex dissociated upon exposure to salt stress, which suggests that cna2p is maintained in an inactive state when bound to Hsp90 and that a shift in this equilibrium, triggered by the calcium-calmodulin complex, is required for stress responses. The physical interaction of Hsp90 and calcineurin was also demonstrated by reciprocal co-immunoprecipitation analyses in C. albicans (Singh et al., 2009). Hsp90 inhibitors (geldanamycin, radicicol) achieved a similar drastic reduction of calcineurin activation as cyclosporine A and genetic repression of Hsp90 was associated with decreased calcineurin levels (Singh et al., 2009).
Hsp90 was shown to have a key role in the acquisition and evolution of resistance to azoles and echinocandins, and growing evidence suggests that this inherent resistance mechanism is mediated via calcineurin (Cowen et al., 2006; Cowen and Lindquist, 2005; Cowen et al., 2009; Singh et al., 2009). In the yeasts, calcineurin inhibition phenocopies Hsp90 inhibition, resulting in the abolition of fluconazole and echinocandin resistance (Cowen et al., 2006; Cowen and Lindquist, 2005; Singh et al., 2009). Cowen and colleagues further genetically dissected the role of the Hsp90-calcineurin axis in antifungal resistance. Deletions of the calcineurin catalytic (cna1, cna2) or regulatory (cnb1) subunits in an erg3 defective S. cerevisiae mutant in which azole resistance is Hsp90-dependent (i.e. can be completely abolished by Hsp90 inhibition) could also abrogate resistance (Cowen and Lindquist, 2005). Genetic work in this Δerg3 background also supports the role of downstream effectors of calcineurin (crz1p, hph1p) in this response (Cowen et al., 2006). Partial or complete loss of azole resistance was observed in the Δerg3Δcrz1 and Δerg3Δhph1 mutants, respectively. Overall, CRZ1 deletion resulted in an only partial loss of tolerance to both fluconazole and micafungin compared to CNA1 deletion, which further supports that crz1p contributes to this response but is not the only downstream effector (Cowen et al., 2006; Singh et al., 2009). Inhibition of Hsp90 blocked fluconazole- and micafungin-induced calcineurin activation, as did calcineurin inhibition (Singh et al., 2009). The fact that activation was measured via an UTR2p-lacZ reporter containing CDRE elements that are activated by crz1p is an additional argument supporting the key role of crz1p in this response.
In A. fumigatus, the ΔcnaA and ΔcrzA strains were more sensitive to high temperature (44°C) when grown in the presence of CsA and also upon treatment with the anti-Hsp90 drug radicicol (Soriani et al., 2008). The interaction between Hsp90 and calcineurin has been recently investigated by our group after the creation of various genetically modified strains for Hsp90 repression or localization (Lamoth et al., 2012; Lamoth et al., 2013a) (Figure 1). Genetic or pharmacologic repression of Hsp90 resulted in various similar phenotypic consequences compared to calcineurin deletion, such as impaired hyphal elongation, decreased β-glucan content of the cell wall, defective sporulation, and increased susceptibility to caspofungin with abolition of the paradoxical effect of this drug (i.e. resistance at high caspofungin concentrations) (Fortwendel et al., 2010; Fortwendel et al., 2009; Juvvadi et al., 2011; Lamoth et al., 2012; Lamoth et al., 2013a; Steinbach et al., 2006; Steinbach et al., 2007a). Supporting these observations, there was down-regulation of the conidiation-specific transcription factors brlA, wetA under Hsp90 repression (Lamoth et al., 2012). Thus, the Hsp90-calcineurin pathway could possibly be one pathway of control of conidiation in Aspergillus species, as the transcription of the flbA, brlA and wetA was also decreased in the A. nidulans ΔcnaA mutant (Soriani et al., 2008).
Akin to the Hsp90 repression strain, the A. fumigatus ΔcrzA strain exhibited the same sporulation and cell wall defects, and was also hypersensitive to caspofungin and incapable of generating a paradoxical response at high caspofungin concentrations (Cramer et al., 2008; Fortwendel et al., 2010; Fortwendel et al., 2009). Both Hsp90 and calcineurin expression increased in a similar proportion upon exposure to increasing concentrations of caspofungin (Lamoth et al., 2013a). A 100-bp proximal sequence of the hsp90 promoter containing two putative CDRE elements was found to be essential to trigger a 1.5-fold increase of hsp90 expression that was required for the paradoxical effect (Lamoth et al., 2013a).
Subcellular localization analysis of A. fumigatus Hsp90 using an EGFP-tagged strain showed that Hsp90 was abundantly distributed within the cytosol under standard growth conditions and moved to the nucleus under heat stress or to the cell wall, septa and hyphal tips in response to the cell wall stress induced by caspofungin (Lamoth et al., 2012). These results suggest the possibility of Hsp90-calcineurin interactions under cell wall stress conditions, as both the calcineurin A and B subunits were shown to localize at the septum and to play a key role in septum formation and cell wall integrity (Juvvadi et al., 2011; Steinbach et al., 2006).
Taken together, these data support the existence of the Hsp90-calcineurin-crzA axis in cell wall repair mechanisms and echinocandin resistance in A. fumigatus, and highlight a novel role of this pathway in the sporulation process which is specific to molds. However, important differences regarding the role of Hsp90 and calcineurin in azole resistance were observed between A. fumigatus and C. albicans/S. cerevisiae. Genetic or pharmacologic inhibition of Hsp90 or calcineurin did not (or only modestly) impact azole susceptibility (Lamoth et al., 2012; Lamoth et al., 2013a; Steinbach et al., 2004a). The ergosterol biosynthetic pathways and mechanisms of azole resistance are multiple and complex, and may substantially differ between molds and yeasts (Ferreira et al., 2005). Further investigation of the molecular network of the Hsp90-calcineurin pathway might provide greater insights on antifungal resistance mechanisms among fungi.
8. Calcineurin inhibitors: antifungal activity and perspectives for future clinical application
Invasive fungal infections are one of the most frequent causes of death in immunocompromised patients (Kontoyiannis et al., 2010). Genetic studies in fungi have clearly established the molecular mechanism of calcineurin inhibition by the cyclophilin A– cyclosporine A (CsA) and FKBP12–FK506 complexes. However, the currently available anti-calcineurin agents, CsA and FK506, are not ideal for treating invasive fungal infections due to their immunosuppressive effects because of host calcineurin cross-reactivity (Ho et al., 1996). In C. albicans, calcineurin inhibitors displayed no inherent antifungal effect, but were synergistic in combination with fluconazole in vitro and in vivo at concentrations achievable in humans (Maesaki et al., 1998; Marchetti et al., 2000a; Marchetti et al., 2000b). However, these calcineurin inhibitors are active in vitro against A. fumigatus as monotherapy and potentiate the effect of caspofungin (High, 1994; Kontoyiannis et al., 2003; Steinbach et al., 2004a; Steinbach et al., 2004b). While CsA has less potent antifungal activity, FK506 is active against fungi within the human therapeutic range of concentrations. Calcineurin inhibitor antifungal activity was also demonstrated against various azole- and echinocandin-resistant A. fumigatus clinical isolates (Lamoth et al., 2013b). Similar drug interactions were observed with the Hsp90 inhibitors geldanamycin and its derivatives 17-DMAG and 17-AAG against A. fumigatus (Lamoth et al., 2012; Lamoth et al., 2013b), further supporting the intimate link between Hsp90 and calcineurin in antifungal drug resistance. A positive interaction between FK506 and geldanamycin achieving a fungicidal effect was also described (Lamoth et al., 2013b).
A single study assessed the effect of CsA, FK506 and sirolimus in a murine model of invasive aspergillosis and was not conclusive (High and Washburn, 1997). While CsA was associated with decreased median survival, FK506 and sirolimus resulted in similar survival curves compared to the untreated group. Some retrospective clinical analyses also suggested a lower incidence of invasive aspergillosis with lower mortality rates among patients treated with FK506 compared to those receiving CsA (Singh et al., 2003; Torre-Cisneros et al., 1991).
Conclusion
To date, it is unlikely that the current calcineurin inhibitors will gain approval for a clinical application as antifungal agents. New and more potent fungal-specific calcineurin inhibitors are required that do not cross-react with human calcineurin and do not induce immunosuppression. Calcineurin plays diverse and distinct roles among the different pathogenic yeasts and filamentous fungi. The recent identification of key regions for calcineurin function that are unique to fungi and absent in humans is a promising step towards the development of such novel antifungal therapies (Juvvadi et al., 2013). Certainly the development of novel non-immunosuppressive analogs of the calcineurin inhibitors CsA and FK506 that would retain antifungal activity hold promise as better antifungal drugs. Identification of fungal-specific domains in calcineurin and an in-depth understanding of the calcineurin pathway and its specific interactants in each of these pathogenic fungi will help in effective designing of better drugs and also identifying new targets for combating fungal diseases. Elucidation of the complete crystal structure of the calcineurin complexes in these different fungal pathogens would be very useful in future development of novel drugs that can specifically target the pathogen.
Highlights.
Calcineurin pathway is an important signaling cascade in regulating stress responses and growth in the yeasts and filamentous fungi
Calcineurin is important for virulence of several plant and human fungal pathogens
Currently available calcineurin inhibitors are active in vitro against major invasive fungal pathogens but their immunosuppressive properties limit usage as antifungal agents in humans
Understanding calcineurinfunctionality in fungi might reveal novel fungal-specific antimicrobials
Acknowledgments
Research in our laboratory is supported by grants from the NIH/NIAID (R56 AI077648-01A2; R21 AI097541-01A1). FL is funded by the Swiss Foundation for Medical-Biological Grants and the Swiss National Science Foundation (PASMP3-142746). We apologize to colleagues for not citing other relevant articles in this review due to space limitation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Almeida RS, Loss O, Colabardini AC, Brown NA, Bignell E, Savoldi M, Pantano S, Goldman MHS, Arst HN, Goldman GH. Genetic Bypass of Aspergillus nidulans crzA Function in Calcium Homeostasis. G3: Genes|Genomes|Genetics. 2013;3:1129–1141. doi: 10.1534/g3.113.005983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aramburu J, Rao A, Klee CB. Calcineurin: From structure to function. In: Earl RS, Chock PB, editors. Current Topics in Cellular Regulation. Academic Press; 2001. pp. 237–295. [DOI] [PubMed] [Google Scholar]
- Bader T, Schröppel K, Bentink S, Agabian N, Köhler G, Morschhäuser J. Role of Calcineurin in Stress Resistance, Morphogenesis, and Virulence of a Candida albicans Wild-Type Strain. Infection and Immunity. 2006;74:4366–4369. doi: 10.1128/IAI.00142-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastidas RJ, Reedy JL, Morales-Johansson H, Heitman J, Cardenas ME. Signaling cascades as drug targets in model and pathogenic fungi. Curr Opin Investig Drugs. 2008;9:856–864. [PMC free article] [PubMed] [Google Scholar]
- Blankenship JR, Heitman J. Calcineurin Is Required for Candida albicans To Survive Calcium Stress in Serum. Infection and Immunity. 2005;73:5767–5774. doi: 10.1128/IAI.73.9.5767-5774.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blankenship JR, Wormley FL, Boyce MK, Schell WA, Filler SG, Perfect JR, Heitman J. Calcineurin Is Essential for Candida albicans Survival in Serum and Virulence. Eukaryotic Cell. 2003;2:422–430. doi: 10.1128/EC.2.3.422-430.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodvard K, Jörhov A, Blomberg A, Molin M, Käll M. The Yeast Transcription Factor Crz1 Is Activated by Light in a Ca2+/Calcineurin-Dependent and PKA-Independent Manner. PLoS ONE. 2013;8:e53404. doi: 10.1371/journal.pone.0053404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilla M, Nastase KK, Cunningham KW. Essential role of calcineurin in response to endoplasmic reticulum stress. EMBO J. 2002;21:2343–2353. doi: 10.1093/emboj/21.10.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SM, Campbell LT, Lodge JK. Cryptococcus neoformans, a fungus under stress. Current Opinion in Microbiology. 2007;10:320–325. doi: 10.1016/j.mib.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadou A, Couturier A, Le Goff C, Xie L, Paulson JR, Le Goff X. The Kin1 kinase and the calcineurin phosphatase cooperate to link actin ring assembly and septum synthesis in fission yeast. Biology of the Cell. 2013;105:129–148. doi: 10.1111/boc.201200042. [DOI] [PubMed] [Google Scholar]
- Cai L, Dalal CK, Elowitz MB. Frequency-modulated nuclear localization bursts coordinate gene regulation. Nature. 2008;455:485–490. doi: 10.1038/nature07292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervantes-Chávez JA, Ali S, Bakkeren G. Response to Environmental Stresses, Cell-wall Integrity, and Virulence Are Orchestrated Through the Calcineurin Pathway in Ustilago hordei. Molecular Plant-Microbe Interactions. 2010;24:219–232. doi: 10.1094/MPMI-09-10-0202. [DOI] [PubMed] [Google Scholar]
- Chen YL, Brand A, Morrison EL, Silao FGS, Bigol UG, Malbas FF, Nett JE, Andes DR, Solis NV, Filler SG, Averette A, Heitman J. Calcineurin Controls Drug Tolerance, Hyphal Growth, and Virulence in Candida dubliniensis. Eukaryotic Cell. 2011;10:803–819. doi: 10.1128/EC.00310-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YL, Konieczka JH, Springer DJ, Bowen SE, Zhang J, Silao FGS, Bungay AAC, Bigol UG, Nicolas MG, Abraham SN, Thompson DA, Regev A, Heitman J. Convergent Evolution of Calcineurin Pathway Roles in Thermotolerance and Virulence in Candida glabrata. G3: Genes|Genomes|Genetics. 2012;2:675–691. doi: 10.1534/g3.112.002279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YL, Kozubowski L, Cardenas ME, Heitman J. On the Roles of Calcineurin in Fungal Growth and Pathogenesis. Current Fungal Infection Reports. 2010;4:244–255. [Google Scholar]
- Chen YL, Lehman VN, Lewit Y, Averette AF, Heitman J. Calcineurin Governs Thermotolerance and Virulence of Cryptococcus gattii. G3: Genes|Genomes|Genetics. 2013;3:527–539. doi: 10.1534/g3.112.004242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H, Sugiura R, Wu W, Fujita M, Lu Y, Sio SO, Kawai R, Takegawa K, Shuntoh H, Kuno T. Role of the Rab GTP-Binding Protein Ypt3 in the Fission Yeast Exocytic Pathway and Its Connection to Calcineurin Function. Molecular Biology of the Cell. 2002;13:2963–2976. doi: 10.1091/mbc.01-09-0463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Kim Y, Kim S, Park J, Lee YH. MoCRZ1, a gene encoding a calcineurin-responsive transcription factor, regulates fungal growth and pathogenicity of Magnaporthe oryzae. Fungal Genetics and Biology. 2009;46:243–254. doi: 10.1016/j.fgb.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Choi JH, Kim Yang Seon, Lee Yong-Hwan. Functional Analysis of MCNA, a Gene Encoding a Catalytic Subunit of Calcineurin, in the Rice Blast Fungus Magnaporthe oryzae. J Microbiol Biotechnol. 2009;19:11–16. [PubMed] [Google Scholar]
- Cowen LE, Carpenter AE, Matangkasombut O, Fink GR, Lindquist S. Genetic architecture of Hsp90-dependent drug resistance. Eukaryot Cell. 2006;5:2184–2188. doi: 10.1128/EC.00274-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowen LE, Lindquist S. Hsp90 potentiates the rapid evolution of new traits: drug resistance in diverse fungi. Science. 2005;309:2185–2189. doi: 10.1126/science.1118370. [DOI] [PubMed] [Google Scholar]
- Cowen LE, Singh SD, Kohler JR, Collins C, Zaas AK, Schell WA, Aziz H, Mylonakis E, Perfect JR, Whitesell L, Lindquist S. Harnessing Hsp90 function as a powerful, broadly effective therapeutic strategy for fungal infectious disease. Proc Natl Acad Sci U S A. 2009;106:2818–2823. doi: 10.1073/pnas.0813394106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowen LE, Steinbach WJ. Stress, Drugs, and Evolution: the Role of Cellular Signaling in Fungal Drug Resistance. Eukaryotic Cell. 2008;7:747–764. doi: 10.1128/EC.00041-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer RA, Jr, Perfect BZ, Pinchai N, Park S, Perlin DS, Asfaw YG, Heitman J, Perfect JR, Steinbach WJ. Calcineurin target CrzA regulates conidial germination, hyphal growth, and pathogenesis of Aspergillus fumigatus. Eukaryot Cell. 2008;7:1085–1097. doi: 10.1128/EC.00086-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz MC, Fox DS, Heitman J. Calcineurin is required for hyphal elongation during mating and haploid fruiting in Cryptococcus neoformans. EMBO J. 2001;20:1020–1032. doi: 10.1093/emboj/20.5.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz MC, Goldstein AL, Blankenship JR, Del Poeta M, Davis D, Cardenas ME, Perfect JR, McCusker JH, Heitman J. Calcineurin is essential for survival during membrane stress in Candida albicans. EMBO J. 2002;21:546–559. doi: 10.1093/emboj/21.4.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cyert MS. Calcineurin signaling in Saccharomyces cerevisiae: how yeast go crazy in response to stress. Biochemical and Biophysical Research Communications. 2003;311:1143–1150. doi: 10.1016/s0006-291x(03)01552-3. [DOI] [PubMed] [Google Scholar]
- Cyert MS, Kunisawa R, Kaim D, Thorner J. Yeast has homologs (CNA1 and CNA2 gene products) of mammalian calcineurin, a calmodulin-regulated phosphoprotein phosphatase. Proceedings of the National Academy of Sciences. 1991;88:7376–7380. doi: 10.1073/pnas.88.16.7376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai B, Wang Y, Li D, Xu Y, Liang R, Zhao L, Cao Y, Jia J, Jiang Y. Hsp90 Is Involved in Apoptosis of Candida albicans by Regulating the Calcineurin-Caspase Apoptotic Pathway. PLoS ONE. 2012;7:e45109. doi: 10.1371/journal.pone.0045109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daquinag A, Fadri M, Jung SY, Qin J, Kunz J. The Yeast PH Domain Proteins Slm1 and Slm2 Are Targets of Sphingolipid Signaling during the Response to Heat Stress. Molecular and Cellular Biology. 2007;27:633–650. doi: 10.1128/MCB.00461-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinamarco TM, Freitas FZ, Almeida RS, Brown NA, dos Reis TF, Ramalho LNZ, Savoldi M, Goldman MHS, Bertolini MC, Goldman GH. Functional Characterization of an Aspergillus fumigatus Calcium Transporter (PmcA) that Is Essential for Fungal Infection. PLoS ONE. 2012;7:e37591. doi: 10.1371/journal.pone.0037591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan JD, García-Pedrajas MD, Andrews DL, Gold SE. Calcineurin Is an Antagonist to PKA Protein Phosphorylation Required for Postmating Filamentation and Virulence, While PP2A Is Required for Viability in Ustilago maydis. Molecular Plant-Microbe Interactions. 2009;22:1293–1301. doi: 10.1094/MPMI-22-10-1293. [DOI] [PubMed] [Google Scholar]
- Estruch F. Stress-controlled transcription factors, stress-induced genes and stress tolerance in budding yeast. FEMS Microbiology Reviews. 2000;24:469–486. doi: 10.1111/j.1574-6976.2000.tb00551.x. [DOI] [PubMed] [Google Scholar]
- Ferreira ME, Colombo AL, Paulsen I, Ren Q, Wortman J, Huang J, Goldman MH, Goldman GH. The ergosterol biosynthesis pathway, transporter genes, and azole resistance in Aspergillus fumigatus. Med Mycol. 2005;43(Suppl 1):S313–319. doi: 10.1080/13693780400029114. [DOI] [PubMed] [Google Scholar]
- Ferreira MEdS, Heinekamp T, Härtl A, Brakhage AA, Semighini CP, Harris SD, Savoldi M, de Gouvêa PF, Goldman MHdS, Goldman GH. Functional characterization of the Aspergillus fumigatus calcineurin. Fungal Genetics and Biology. 2007;44:219–230. doi: 10.1016/j.fgb.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Fortwendel JR, Juvvadi PR, Perfect BZ, Rogg LE, Perfect JR, Steinbach WJ. Transcriptional regulation of chitin synthases by calcineurin controls paradoxical growth of Aspergillus fumigatus in response to caspofungin. Antimicrob Agents Chemother. 2010;54:1555–1563. doi: 10.1128/AAC.00854-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortwendel JR, Juvvadi PR, Pinchai N, Perfect BZ, Alspaugh JA, Perfect JR, Steinbach WJ. Differential effects of inhibiting chitin and 1,3-{beta}-D-glucan synthesis in ras and calcineurin mutants of Aspergillus fumigatus. Antimicrob Agents Chemother. 2009;53:476–482. doi: 10.1128/AAC.01154-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox DS, Cox GM, Heitman J. Phospholipid-Binding Protein Cts1 Controls Septation and Functions Coordinately with Calcineurin in Cryptococcus neoformans. Eukaryotic Cell. 2003;2:1025–1035. doi: 10.1128/EC.2.5.1025-1035.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox DS, Heitman J. Calcineurin-Binding Protein Cbp1 Directs the Specificity of Calcineurin-Dependent Hyphal Elongation during Mating in Cryptococcus neoformans. Eukaryotic Cell. 2005;4:1526–1538. doi: 10.1128/EC.4.9.1526-1538.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorlach J, Fox DS, Cutler NS, Cox GM, Perfect JR, Heitman J. Identification and characterization of a highly conserved calcineurin binding protein, CBP1/calcipressin, in Cryptococcus neoformans. EMBO J. 2000;19:3618–3629. doi: 10.1093/emboj/19.14.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara D, Kondo A, Fujioka T, Abe K. Functional analysis of C2H2 zinc finger transcription factor CrzA involved in calcium signaling in Aspergillus nidulans. Curr Genet. 2008;54:325–338. doi: 10.1007/s00294-008-0220-z. [DOI] [PubMed] [Google Scholar]
- Hameed S, Dhamgaye S, Singh A, Goswami SK, Prasad R. Calcineurin Signaling and Membrane Lipid Homeostasis Regulates Iron Mediated MultiDrug Resistance Mechanisms in Candida albicans. PLoS ONE. 2011;6:e18684. doi: 10.1371/journal.pone.0018684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harel A, Bercovich S, Yarden O. Calcineurin Is Required for Sclerotial Development and Pathogenicity of Sclerotinia sclerotiorum in an Oxalic Acid-Independent Manner. Molecular Plant-Microbe Interactions. 2006;19:682–693. doi: 10.1094/MPMI-19-0682. [DOI] [PubMed] [Google Scholar]
- Harris CD, Ermak G, Davies KJA. Multiple roles of the DSCR1 (Adapt78 or RCAN1) gene and its protein product Calcipressin 1 (or RCAN1) in disease. Cellular and Molecular Life Sciences CMLS. 2005;62:2477–2486. doi: 10.1007/s00018-005-5085-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto Y, King MM, Soderling TR. Regulatory interactions of calmodulin-binding proteins: phosphorylation of calcineurin by autophosphorylated Ca2+/calmodulin-dependent protein kinase II. Proceedings of the National Academy of Sciences. 1988;85:7001–7005. doi: 10.1073/pnas.85.18.7001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto Y, Soderling TR. Regulation of calcineurin by phosphorylation. Identification of the regulatory site phosphorylated by Ca2+/calmodulin-dependent protein kinase II and protein kinase C. Journal of Biological Chemistry. 1989;264:16524–16529. [PubMed] [Google Scholar]
- Heath VL, Shaw SL, Roy S, Cyert MS. Hph1p and Hph2p, Novel Components of Calcineurin-Mediated Stress Responses in Saccharomyces cerevisiae. Eukaryotic Cell. 2004;3:695–704. doi: 10.1128/EC.3.3.695-704.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heineke J, Ritter O. Cardiomyocyte calcineurin signaling in subcellular domains: From the sarcolemma to the nucleus and beyond. Journal of Molecular and Cellular Cardiology. 2012;52:62–73. doi: 10.1016/j.yjmcc.2011.10.018. [DOI] [PubMed] [Google Scholar]
- Heit JJ. Calcineurin/NFAT signaling in the β-cell: From diabetes to new therapeutics. BioEssays. 2007;29:1011–1021. doi: 10.1002/bies.20644. [DOI] [PubMed] [Google Scholar]
- Hemenway C, Heitman J. Calcineurin. Cell Biochem Biophys. 1999;30:115–151. doi: 10.1007/BF02737887. [DOI] [PubMed] [Google Scholar]
- Hernández-Ortiz P, Espeso EA. Phospho-regulation and nucleocytoplasmic trafficking of CrzA in response to calcium and alkaline-pH stress in Aspergillus nidulans. Molecular Microbiology. 2013;89:532–551. doi: 10.1111/mmi.12294. [DOI] [PubMed] [Google Scholar]
- High KP. The antimicrobial activities of cyclosporine, FK506, and rapamycin. Transplantation. 1994;57:1689–1700. [PubMed] [Google Scholar]
- High KP, Washburn RG. Invasive aspergillosis in mice immunosuppressed with cyclosporin A, tacrolimus (FK506), or sirolimus (rapamycin) J Infect Dis. 1997;175:222–225. doi: 10.1093/infdis/175.1.222. [DOI] [PubMed] [Google Scholar]
- Ho S, Clipstone N, Timmermann L, Northrop J, Graef I, Fiorentino D, Nourse J, Crabtree GR. The Mechanism of Action of Cyclosporin A and FK506. Clinical Immunology and Immunopathology. 1996;80:S40–S45. doi: 10.1006/clin.1996.0140. [DOI] [PubMed] [Google Scholar]
- Imai J, Yahara I. Role of HSP90 in salt stress tolerance via stabilization and regulation of calcineurin. Mol Cell Biol. 2000;20:9262–9270. doi: 10.1128/mcb.20.24.9262-9270.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia XM, Wang Y, Jia Y, Gao PH, Xu YG, Wang L, Cao YY, Cao YB, Zhang LX, Jiang YY. RTA2 is involved in calcineurin-mediated azole resistance and sphingoid long-chain base release in Candida albicans. Cell Mol Life Sci. 2009;66:122–134. doi: 10.1007/s00018-008-8409-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Tang RJ, Wang L, Zhang X, Wang Y, Jia XM, Jiang YY. Calcium-Activated-Calcineurin Reduces the In Vitro and In Vivo Sensitivity of Fluconazole to Candida albicans via Rta2p. PLoS ONE. 2012;7:e48369. doi: 10.1371/journal.pone.0048369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juvvadi PR, Arioka M, Nakajima H, Kitamoto K. Cloning and sequence analysis of cnaA gene encoding the catalytic subunit of calcineurin from Aspergillus oryzae. FEMS Microbiology Letters. 2001;204:169–174. doi: 10.1111/j.1574-6968.2001.tb10881.x. [DOI] [PubMed] [Google Scholar]
- Juvvadi PR, Fortwendel JR, Pinchai N, Perfect BZ, Heitman J, Steinbach WJ. Calcineurin Localizes to the Hyphal Septum in Aspergillus fumigatus: Implications for Septum Formation and Conidiophore Development. Eukaryotic Cell. 2008;7:1606–1610. doi: 10.1128/EC.00200-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juvvadi PR, Fortwendel JR, Rogg LE, Burns KA, Randell SH, Steinbach WJ. Localization and activity of the calcineurin catalytic and regulatory subunit complex at the septum is essential for hyphal elongation and proper septation in Aspergillus fumigatus. Molecular Microbiology. 2011;82:1235–1259. doi: 10.1111/j.1365-2958.2011.07886.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juvvadi PR, Gehrke C, Fortwendel JR, Lamoth F, Soderblom EJ, Cook EC, Hast MA, Asfaw YG, Moseley MA, Creamer TP, Steinbach WJ. Phosphorylation of Calcineurin at a Novel Serine-Proline Rich Region Orchestrates Hyphal Growth and Virulence in Aspergillus fumigatus. PLoS Pathog. 2013;9:e1003564. doi: 10.1371/journal.ppat.1003564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juvvadi PR, Kuroki Y, Arioka M, Nakajima H, Kitamoto K. Functional analysis of the calcineurin-encoding gene cnaA from Aspergillus oryzae: evidence for its putative role in stress adaptation. Archives of Microbiology. 2003;179:416–422. doi: 10.1007/s00203-003-0546-3. [DOI] [PubMed] [Google Scholar]
- Karababa M, Valentino E, Pardini G, Coste AT, Bille J, Sanglard D. CRZ1, a target of the calcineurin pathway in Candida albicans. Molecular Microbiology. 2006;59:1429–1451. doi: 10.1111/j.1365-2958.2005.05037.x. [DOI] [PubMed] [Google Scholar]
- Kita A, Sugiura R, Shoji H, He Y, Deng L, Lu Y, Sio SO, Takegawa K, Sakaue M, Shuntoh H, Kuno T. Loss of Apm1, the μ1 Subunit of the Clathrin-Associated Adaptor-Protein-1 Complex, Causes Distinct Phenotypes and Synthetic Lethality with Calcineurin Deletion in Fission Yeast. Molecular Biology of the Cell. 2004;15:2920–2931. doi: 10.1091/mbc.E03-09-0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kontoyiannis DP, Lewis RE, Osherov N, Albert ND, May GS. Combination of caspofungin with inhibitors of the calcineurin pathway attenuates growth in vitro in Aspergillus species. J Antimicrob Chemother. 2003;51:313–316. doi: 10.1093/jac/dkg090. [DOI] [PubMed] [Google Scholar]
- Kontoyiannis DP, Marr KA, Park BJ, Alexander BD, Anaissie EJ, Walsh TJ, Ito J, Andes DR, Baddley JW, Brown JM, Brumble LM, Freifeld AG, Hadley S, Herwaldt LA, Kauffman CA, Knapp K, Lyon GM, Morrison VA, Papanicolaou G, Patterson TF, Perl TM, Schuster MG, Walker R, Wannemuehler KA, Wingard JR, Chiller TM, Pappas PG. Prospective Surveillance for Invasive Fungal Infections in Hematopoietic Stem Cell Transplant Recipients, 2001–2006: Overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) Database. Clinical Infectious Diseases. 2010;50:1091–1100. doi: 10.1086/651263. [DOI] [PubMed] [Google Scholar]
- Kothe GO, Free SJ. Calcineurin Subunit B Is Required for Normal Vegetative Growth inNeurospora crassa. Fungal Genetics and Biology. 1998;23:248–258. doi: 10.1006/fgbi.1998.1037. [DOI] [PubMed] [Google Scholar]
- Kozubowski L, Aboobakar EF, Cardenas ME, Heitman J. Calcineurin Colocalizes with P-Bodies and Stress Granules during Thermal Stress in Cryptococcus neoformans. Eukaryotic Cell. 2011a;10:1396–1402. doi: 10.1128/EC.05087-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozubowski L, Heitman J. Profiling a killer, the development of Cryptococcus neoformans. FEMS Microbiology Reviews. 2012;36:78–94. doi: 10.1111/j.1574-6976.2011.00286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozubowski L, Thompson JW, Cardenas ME, Moseley MA, Heitman J. Association of Calcineurin with the COPI Protein Sec28 and the COPII Protein Sec13 Revealed by Quantitative Proteomics. PLoS ONE. 2011b;6:e25280. doi: 10.1371/journal.pone.0025280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus PR, Fox DS, Cox GM, Heitman J. The Cryptococcus neoformans MAP kinase Mpk1 regulates cell integrity in response to antifungal drugs and loss of calcineurin function. Molecular Microbiology. 2003;48:1377–1387. doi: 10.1046/j.1365-2958.2003.03508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullas AL, Martin SJ, Davis D. Adaptation to environmental pH: integrating the Rim101 and calcineurin signal transduction pathways. Molecular Microbiology. 2007;66:858–871. doi: 10.1111/j.1365-2958.2007.05929.x. [DOI] [PubMed] [Google Scholar]
- Kume K, Koyano T, Kanai M, Toda T, Hirata D. Calcineurin ensures a link between the DNA replication checkpoint and microtubule-dependent polarized growth. Nat Cell Biol. 2011;13:234–242. doi: 10.1038/ncb2166. [DOI] [PubMed] [Google Scholar]
- Lamoth F, Juvvadi PR, Fortwendel JR, Steinbach WJ. Heat Shock Protein 90 Is Required for Conidiation and Cell Wall Integrity in Aspergillus fumigatus. Eukaryot Cell. 2012;11:1324–1332. doi: 10.1128/EC.00032-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamoth F, Juvvadi PR, Gehrke C, Asfaw YG, Steinbach WJ. Transcriptional Activation of Heat-Shock Protein 90 (Hsp90) Mediated Via a Proximal Promoter Region Confers Caspofungin Resistance in Aspergillus fumigatus. J Infect Dis. 2013a doi: 10.1093/infdis/jit530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamoth F, Juvvadi PR, Gehrke C, Steinbach WJ. In Vitro Activity of Calcineurin and Heat Shock Protein 90 Inhibitors against Aspergillus fumigatus Azole- and Echinocandin-Resistant Strains. Antimicrobial Agents and Chemotherapy. 2013b;57:1035–1039. doi: 10.1128/AAC.01857-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev S, Desmarini D, Chayakulkeeree M, Sorrell TC, Djordjevic JT. The Crz1/Sp1 Transcription Factor of Cryptococcus neoformans Is Activated by Calcineurin and Regulates Cell Wall Integrity. PLoS ONE. 2012;7:e51403. doi: 10.1371/journal.pone.0051403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Ishii S, Tokai M, Tsutsumi H, Ohki O, Akada R, Tanaka K, Tsuchiya E, Fukui S, Miyakawa T. The Saccharomyces cerevisiae genes (CMP1 andCMP2) encoding calmodulin-binding proteins homologous to the catalytic subunit of mammalian protein phosphatase 2B. Molec Gen Genet. 1991;227:52–59. doi: 10.1007/BF00260706. [DOI] [PubMed] [Google Scholar]
- Lu Y, Sugiura R, Yada T, Cheng H, Sio SO, Shuntoh H, Kuno T. Calcineurin is implicated in the regulation of the septation initiation network in fission yeast. Genes to Cells. 2002;7:1009–1019. doi: 10.1046/j.1365-2443.2002.00582.x. [DOI] [PubMed] [Google Scholar]
- Ma Y, Jiang W, Liu Q, Ryuko S, Kuno T. Genome-Wide Screening for Genes Associated with FK506 Sensitivity in Fission Yeast. PLoS ONE. 2011;6:e23422. doi: 10.1371/journal.pone.0023422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda T, Sugiura R, Kita A, Saito M, Deng L, He Y, Yabin L, Fujita Y, Takegawa K, Shuntoh H, Kuno T. Pmr1, a P-type ATPase, and Pdt1, an Nramp homologue, cooperatively regulate cell morphogenesis in fission yeast: The importance of Mn2+ homeostasis. Genes to Cells. 2004;9:71–82. doi: 10.1111/j.1356-9597.2004.00699.x. [DOI] [PubMed] [Google Scholar]
- Maesaki S, Marichal P, Hossain MA, Sanglard D, Vanden Bossche H, Kohno S. Synergic effects of tactolimus and azole antifungal agents against azole-resistant Candida albican strains. J Antimicrob Chemother. 1998;42:747–753. doi: 10.1093/jac/42.6.747. [DOI] [PubMed] [Google Scholar]
- Marchetti O, Entenza JM, Sanglard D, Bille J, Glauser MP, Moreillon P. Fluconazole plus cyclosporine: a fungicidal combination effective against experimental endocarditis due to Candida albicans. Antimicrob Agents Chemother. 2000a;44:2932–2938. doi: 10.1128/aac.44.11.2932-2938.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti O, Moreillon P, Glauser MP, Bille J, Sanglard D. Potent synergism of the combination of fluconazole and cyclosporine in Candida albicans. Antimicrob Agents Chemother. 2000b;44:2373–2381. doi: 10.1128/aac.44.9.2373-2381.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks B, McMahon HT. Calcium triggers calcineurin-dependent synaptic vesicle recycling in mammalian nerve terminals. Current Biology. 1998;8:740–749. doi: 10.1016/s0960-9822(98)70297-0. [DOI] [PubMed] [Google Scholar]
- Marquina M, González A, Barreto L, Gelis S, Muñoz I, Ruiz A, Álvarez MC, Ramos J, Ariño J. Modulation of Yeast Alkaline Cation Tolerance by Ypi1 Requires Calcineurin. Genetics. 2012;190:1355–1364. doi: 10.1534/genetics.112.138370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martensen TM, Martin BM, Kincaid RL. Identification of the site on calcineurin phosphorylated by calcium/CaM-dependent kinase II: modification of the CaM-binding domain. Biochemistry. 1989;28:9243–9247. doi: 10.1021/bi00450a002. [DOI] [PubMed] [Google Scholar]
- Matsumoto TK, Ellsmore AJ, Cessna SG, Low PS, Pardo JM, Bressan RA, Hasegawa PM. An Osmotically Induced Cytosolic Ca2+ Transient Activates Calcineurin Signaling to Mediate Ion Homeostasis and Salt Tolerance of Saccharomyces cerevisiae. Journal of Biological Chemistry. 2002;277:33075–33080. doi: 10.1074/jbc.M205037200. [DOI] [PubMed] [Google Scholar]
- Mendoza I, Quintero FJ, Bressan RA, Hasegawa PM, Pardo JM. Activated Calcineurin Confers High Tolerance to Ion Stress and Alters the Budding Pattern and Cell Morphology of Yeast Cells. Journal of Biological Chemistry. 1996;271:23061–23067. doi: 10.1074/jbc.271.38.23061. [DOI] [PubMed] [Google Scholar]
- Mendoza I, Rubio F, Rodriguez-Navarro A, Pardo JM. The protein phosphatase calcineurin is essential for NaCl tolerance of Saccharomyces cerevisiae. Journal of Biological Chemistry. 1994;269:8792–8796. [PubMed] [Google Scholar]
- Miyazaki T, Nakayama H, Nagayoshi Y, Kakeya H, Kohno S. Dissection of Ire1 Functions Reveals Stress Response Mechanisms Uniquely Evolved in Candida glabrata. PLoS Pathog. 2013;9:e1003160. doi: 10.1371/journal.ppat.1003160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki T, Yamauchi S, Inamine T, Nagayoshi Y, Saijo T, Izumikawa K, Seki M, Kakeya H, Yamamoto Y, Yanagihara K, Miyazaki Y, Kohno S. Roles of Calcineurin and Crz1 in Antifungal Susceptibility and Virulence of Candida glabrata. Antimicrobial Agents and Chemotherapy. 2010;54:1639–1643. doi: 10.1128/AAC.01364-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musson REA, Cobbaert CM, Smit NPM. Molecular Diagnostics of Calcineurin-Related Pathologies. Clinical Chemistry. 2012;58:511–522. doi: 10.1373/clinchem.2011.167296. [DOI] [PubMed] [Google Scholar]
- Naderer T, Dandash O, McConville MJ. Calcineurin is required for Leishmania major stress response pathways and for virulence in the mammalian host. Molecular Microbiology. 2011;80:471–480. doi: 10.1111/j.1365-2958.2011.07584.x. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Liu Y, Hirata D, Namba H, Harada S, Hirokawa T, TM Protein phosphatase type 2B (calcineurin)-mediated, FK506-sensitive regulation of intracellular ions in yeast is an important determinant for adaptation to high salt stress conditions. EMBO J. 1993;12:4063–4071. doi: 10.1002/j.1460-2075.1993.tb06090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanthakumar NNDJ, Means AR. Role of Ca++/calmodulin binding proteins in Aspergillus nidulans cell cycle regulation. Prog Cell Cycle Res. 1996;2:217–228. doi: 10.1007/978-1-4615-5873-6_21. [DOI] [PubMed] [Google Scholar]
- Odom A, Muir S, Lim E, Toffaletti DL, Perfect J, Heitman J. Calcineurin is required for virulence of Cryptococcus neoformans. EMBO J. 1997;16:2576–2589. doi: 10.1093/emboj/16.10.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl LH, Prodromou C. Structure and mechanism of the Hsp90 molecular chaperone machinery. Annu Rev Biochem. 2006;75:271–294. doi: 10.1146/annurev.biochem.75.103004.142738. [DOI] [PubMed] [Google Scholar]
- Pinchai N, Juvvadi PR, Fortwendel JR, Perfect BZ, Rogg LE, Asfaw YG, Steinbach WJ. The Aspergillus fumigatus P-Type Golgi Apparatus Ca2+/Mn2+ ATPase PmrA Is Involved in Cation Homeostasis and Cell Wall Integrity but Is Not Essential for Pathogenesis. Eukaryotic Cell. 2010;9:472–476. doi: 10.1128/EC.00378-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinchai N, Perfect BZ, Juvvadi PR, Fortwendel JR, Cramer RA, Asfaw YG, Heitman J, Perfect JR, Steinbach WJ. Aspergillus fumigatus Calcipressin CbpA Is Involved in Hyphal Growth and Calcium Homeostasis. Eukaryotic Cell. 2009;8:511–519. doi: 10.1128/EC.00336-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prokisch H, Yarden O, Dieminger M, Tropschug M, Barthelmess IB. Impairment of calcineurin function in Neurospora crassa reveals its essential role in hyphal growth, morphology and maintenance of the apical Ca2+ gradient. Molec Gen Genet. 1997;256:104–114. doi: 10.1007/s004380050551. [DOI] [PubMed] [Google Scholar]
- Reese LC, Taglialatela G. A Role for Calcineurin in Alzheimer’s Disease. Current Neuropharmacology. 2011;9:685–692. doi: 10.2174/157015911798376316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds NJ, Al-Daraji WI. Calcineurin inhibitors and sirolimus: mechanisms of action and applications in dermatology. Clinical and Experimental Dermatology. 2002;27:555–561. doi: 10.1046/j.1365-2230.2002.01148.x. [DOI] [PubMed] [Google Scholar]
- Rusnak F, Mertz P. Calcineurin: Form and Function. Physiological Reviews. 2000;80:1483–1521. doi: 10.1152/physrev.2000.80.4.1483. [DOI] [PubMed] [Google Scholar]
- Sanglard D, Ischer F, Marchetti O, Entenza J, Bille J. Calcineurin A of Candida albicans: involvement in antifungal tolerance, cell morphogenesis and virulence. Molecular Microbiology. 2003;48:959–976. doi: 10.1046/j.1365-2958.2003.03495.x. [DOI] [PubMed] [Google Scholar]
- Santos M, Larrinoa I. Functional characterization of the Candida albicans CRZ1 gene encoding a calcineurin-regulated transcription factor. Curr Genet. 2005;48:88–100. doi: 10.1007/s00294-005-0003-8. [DOI] [PubMed] [Google Scholar]
- Schumacher J, de Larrinoa IF, Tudzynski B. Calcineurin-Responsive Zinc Finger Transcription Factor CRZ1 of Botrytis cinerea Is Required for Growth, Development, and Full Virulence on Bean Plants. Eukaryotic Cell. 2008;7:584–601. doi: 10.1128/EC.00426-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano R, Ruiz A, Bernal D, Chambers JR, Ariño J. The transcriptional response to alkaline pH in Saccharomyces cerevisiae: evidence for calcium-mediated signalling. Molecular Microbiology. 2002;46:1319–1333. doi: 10.1046/j.1365-2958.2002.03246.x. [DOI] [PubMed] [Google Scholar]
- Shirazi F, Kontoyiannis DP. The Calcineurin Pathway Inhibitor Tacrolimus Enhances The In Vitro Activity Of Azoles Against Mucorales Via Apoptosis. Eukaryotic Cell. 2013 doi: 10.1128/EC.00138-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh N, Avery RK, Munoz P, Pruett TL, Alexander B, Jacobs R, Tollemar JG, Dominguez EA, Yu CM, Paterson DL, Husain S, Kusne S, Linden P. Trends in risk profiles for and mortality associated with invasive aspergillosis among liver transplant recipients. Clin Infect Dis. 2003;36:46–52. doi: 10.1086/345441. [DOI] [PubMed] [Google Scholar]
- Singh S, More KR, Chitnis CE. Role of calcineurin and actin dynamics in regulated secretion of microneme proteins in Plasmodium falciparum merozoites during erythrocyte invasion. Cellular Microbiology. 2014;16:50–63. doi: 10.1111/cmi.12177. [DOI] [PubMed] [Google Scholar]
- Singh SD, Robbins N, Zaas AK, Schell WA, Perfect JR, Cowen LE. Hsp90 governs echinocandin resistance in the pathogenic yeast Candida albicans via calcineurin. PLoS Pathog. 2009;5:e1000532. doi: 10.1371/journal.ppat.1000532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh TJ, Wang JH. Phosphorylation of calcineurin by glycogen synthase (casein) kinase-1. Biochemistry and Cell Biology. 1987;65:917–921. doi: 10.1139/o87-118. [DOI] [PubMed] [Google Scholar]
- Soriani FM, Malavazi I, Da Silva Ferreira ME, Savoldi M, Von Zeska Kress MR, De Souza Goldman MH, Loss O, Bignell E, Goldman GH. Functional characterization of the Aspergillus fumigatus CRZ1 homologue, CrzA. Molecular Microbiology. 2008;67:1274–1291. doi: 10.1111/j.1365-2958.2008.06122.x. [DOI] [PubMed] [Google Scholar]
- Spielvogel A, Findon H, Arst HN, Araújo-bazán L, Hernández-ortíz P, Stahl U, Meyer V, Espeso EA. Two zinc finger transcription factors, CrzA and SltA, are involved in cation homoeostasis and detoxification in Aspergillus nidulans. Biochem J. 2008;414:419–429. doi: 10.1042/BJ20080344. [DOI] [PubMed] [Google Scholar]
- Stathopoulos-Gerontides A, Guo JJ, Cyert MS. Yeast calcineurin regulates nuclear localization of the Crz1p transcription factor through dephosphorylation. Genes & Development. 1999;13:798–803. doi: 10.1101/gad.13.7.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stathopoulos AM, Cyert MS. Calcineurin acts through the CRZ1/TCN1-encoded transcription factor to regulate gene expression in yeast. Genes & Development. 1997;11:3432–3444. doi: 10.1101/gad.11.24.3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach WJ, Cramer RA, Jr, Perfect BZ, Asfaw YG, Sauer TC, Najvar LK, Kirkpatrick WR, Patterson TF, Benjamin DK, Jr, Heitman J, Perfect JR. Calcineurin controls growth, morphology, and pathogenicity in Aspergillus fumigatus. Eukaryot Cell. 2006;5:1091–1103. doi: 10.1128/EC.00139-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach WJ, Cramer RA, Jr, Perfect BZ, Henn C, Nielsen K, Heitman J, Perfect JR. Calcineurin inhibition or mutation enhances cell wall inhibitors against Aspergillus fumigatus. Antimicrob Agents Chemother. 2007a;51:2979–2981. doi: 10.1128/AAC.01394-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach WJ, Reedy JL, Cramer RA, Perfect JR, Heitman J. Harnessing calcineurin as a novel anti-infective agent against invasive fungal infections. Nat Rev Micro. 2007b;5:418–430. doi: 10.1038/nrmicro1680. [DOI] [PubMed] [Google Scholar]
- Steinbach WJ, Schell WA, Blankenship JR, Onyewu C, Heitman J, Perfect JR. In vitro interactions between antifungals and immunosuppressants against Aspergillus fumigatus. Antimicrob Agents Chemother. 2004a;48:1664–1669. doi: 10.1128/AAC.48.5.1664-1669.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinbach WJ, Singh N, Miller JL, Benjamin DK, Jr, Schell WA, Heitman J, Perfect JR. In vitro interactions between antifungals and immunosuppressants against Aspergillus fumigatus isolates from transplant and nontransplant patients. Antimicrob Agents Chemother. 2004b;48:4922–4925. doi: 10.1128/AAC.48.12.4922-4925.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens DA, Ichinomiya M, Koshi Y, Horiuchi H. Escape of Candida from Caspofungin Inhibition at Concentrations above the MIC (Paradoxical Effect) Accomplished by Increased Cell Wall Chitin; Evidence for β-1,6-Glucan Synthesis Inhibition by Caspofungin. Antimicrobial Agents and Chemotherapy. 2006;50:3160–3161. doi: 10.1128/AAC.00563-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiura R, Sio SO, Shuntoh H, Kuno T. Calcineurin phosphatase in signal transduction: lessons from fission yeast. Genes to Cells. 2002;7:619–627. doi: 10.1046/j.1365-2443.2002.00557.x. [DOI] [PubMed] [Google Scholar]
- Torre-Cisneros J, Manez R, Kusne S, Alessiani M, Martin M, Starzl TE. The spectrum of aspergillosis in liver transplant patients: comparison of FK 506 and cyclosporine immunosuppression. Transplant Proc. 1991;23:3040–3041. [PMC free article] [PubMed] [Google Scholar]
- Valdivia RH, Baggott D, Chuang JS, Schekman RW. The Yeast Clathrin Adaptor Protein Complex 1 Is Required for the Efficient Retention of a Subset of Late Golgi Membrane Proteins. Developmental Cell. 2002;2:283–294. doi: 10.1016/s1534-5807(02)00127-2. [DOI] [PubMed] [Google Scholar]
- van Rossum HH, de Fijter JW, van Pelt J. Pharmacodynamic Monitoring of Calcineurin Inhibition Therapy: Principles, Performance, and Perspectives. Therapeutic Drug Monitoring. 2010;32:3–10. doi: 10.1097/FTD.1090b1013e3181c1090eecb. [DOI] [PubMed] [Google Scholar]
- Viana RA, Pinar M, Soto T, Coll PM, Cansado J, Pérez P. Negative Functional Interaction Between Cell Integrity MAPK Pathway and Rho1 GTPase in Fission Yeast. Genetics. 2013 doi: 10.1534/genetics.113.154807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viladevall L, Serrano R, Ruiz A, Domenech G, Giraldo J, Barceló A, Ariño J. Characterization of the Calcium-mediated Response to Alkaline Stress in Saccharomyces cerevisiae. Journal of Biological Chemistry. 2004;279:43614–43624. doi: 10.1074/jbc.M403606200. [DOI] [PubMed] [Google Scholar]
- Wang S, Cao J, Liu X, Hu H, Shi J, Zhang S, Keller NP, Lu L. Putative Calcium Channels CchA and MidA Play the Important Roles in Conidiation, Hyphal Polarity and Cell Wall Components in Aspergillus nidulans. PLoS ONE. 2012;7:e46564. doi: 10.1371/journal.pone.0046564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiederhold NP, Kontoyiannis DP, Chi J, Prince RA, Tam VH, Lewis RE. Pharmacodynamics of Caspofungin in a Murine Model of Invasive Pulmonary Aspergillosis: Evidence of Concentration-Dependent Activity. Journal of Infectious Diseases. 2004;190:1464–1471. doi: 10.1086/424465. [DOI] [PubMed] [Google Scholar]
- Williams CR, Gooch JL. Calcineurin inhibitors and immunosuppression – a tale of two isoforms. Expert Reviews in Molecular Medicine. 2012;14:null–null. doi: 10.1017/erm.2012.8. [DOI] [PubMed] [Google Scholar]
- Yoshida T, Toda T, Yanagida M. A calcineurin-like gene ppb1+ in fission yeast: mutant defects in cytokinesis, cell polarity, mating and spindle pole body positioning. Journal of Cell Science. 1994;107:1725–1735. doi: 10.1242/jcs.107.7.1725. [DOI] [PubMed] [Google Scholar]
- Zakrzewska A, Boorsma A, Brul S, Hellingwerf KJ, Klis FM. Transcriptional Response of Saccharomyces cerevisiae to the Plasma Membrane-Perturbing Compound Chitosan. Eukaryotic Cell. 2005;4:703–715. doi: 10.1128/EC.4.4.703-715.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Silao FGS, Bigol UG, Bungay AAC, Nicolas MG, Heitman J, Chen YL. Calcineurin Is Required for Pseudohyphal Growth, Virulence, and Drug Resistance in Candida lusitaniae. PLoS ONE. 2012;7:e44192. doi: 10.1371/journal.pone.0044192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Davey M, Hsu YC, Kaplanek P, Tong A, Parsons AB, Krogan N, Cagney G, Mai D, Greenblatt J, Boone C, Emili A, Houry WA. Navigating the chaperone network: an integrative map of physical and genetic interactions mediated by the hsp90 chaperone. Cell. 2005;120:715–727. doi: 10.1016/j.cell.2004.12.024. [DOI] [PubMed] [Google Scholar]