Abstract
Chronic alcohol abuse is a major risk factor for hepatocellular carcinoma (HCC), the third leading cause of cancer deaths worldwide. Alcohol can also function synergistically with other risk factors to cause HCC. Hence, alcohol consumption is a major factor affecting hepatic carcinogenesis in millions and the cause of a substantial public health burden. Chronic alcohol consumption interferes with several host anti-tumor mechanisms, thereby facilitating hepatocyte proliferation and tumorigenesis. This review summarizes the major mechanisms of alcohol-induced HCC. These include pathways of ethanol metabolism, alcohol-induced oxidative stress and hypomethylation of DNA, and interplay of alcohol with iron elevation, retinoid metabolism, the immune system, inflammatory pathways, and neoangiogenesis. The relevance of each pathway in affecting HCC transformation is a topic of intense investigation. Ongoing research will enhance our insight into the alcohol-induced occurrence of HCC and offer hope in developing better therapeutics.
Keywords: Alcohol, HCC, Carcinogenesis
Introduction
Primary liver cancer is the fifth most common cancer worldwide and the third leading cause of cancer mortality [1]. More than 80 % of primary liver tumors are hepatocellular carcinomas (HCC) [2]. There are several established risk factors for HCC with distinct geographical patterns of incidence (Table 1) [3–5]. Interestingly, the highest attributable risk for HCC in the US and Europe is heavy alcohol intake [6, 7]. Alcohol consumption is common in the western world and rising in Asia, suggesting it will continue to be a prevalent cause of HCC [8]. In fact, epidemiological studies reveal that consumption of 80 > of alcohol/day for 10 years is an independent risk factor [9]. Data also suggest that alcohol can function synergistically with viral hepatitis and other risk factors to induce HCC [10, 11]. This review focuses on nine molecular mechanisms associated with alcohol-induced carcinogenesis (Table 2; Fig. 1).
Table 1. Risk factors associated with HCC.
| Demographics | Age >50, male gender, Asian ethnicity |
| Infections | Hepatitis B and C viruses |
| Underlying diseases | Cirrhosis, obesity, type II diabetes mellitus, hemochromatosis |
| Toxins | Aflatoxins |
| Habits | Tobacco use, alcohol use |
| Drugs | Oral contraceptive use |
Table 2. Mechanisms of alcohol-induced HCC.
| Mechanisms | Mediators |
|---|---|
| Acetaldehyde formation | ↑N2-propano-2′-deoxyguanosine, ↑N2-ethyl-2′-deoxyguanosine, ↑O6-methylguanosyl transferase adduct |
| Cytochrome P4502E1 induction | ↑Reactive oxygen species |
| Reduced antioxidants | ↓Glutathione |
| Hypomethylation | ↓S-adenosylmethionine |
| Iron overload | ↑Iron uptake, ↑reactive oxygen species |
| Reduced retinoic acid | ↑Activated protein 1 (c-jun, c-phos) |
| Immune surveillance | ↓Natural killer cells, toll-like receptor 4, Nanog |
| Neoangiogenesis | ↑Vasoconstriction, portal pressure |
| Inflammation | ↑Lipopolysaccharides, ↑transforming growth factor- β, ↑interleukin 6, ↑tumor necrosis factor-α |
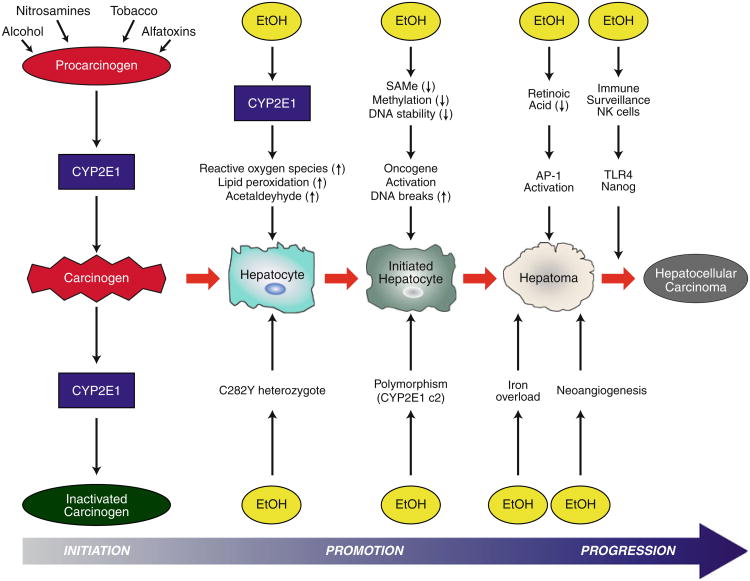
Fig. 1.
Interactions between alcohol-induced mechanisms leading to hepatic carcinogenesis. Alcohol mediates several mechanisms of hepatocyte proliferation and carcinogenesis. These include pathways of ethanol metabolism, which generate acetaldehyde and induction of CYP2E1, both of which promote generation of reactive oxygen species, lipid peroxidation, and DNA strand breaks. Alcohol leads to hypomethylation of DNA and also harms the retinoic acid pathway; both of these promote oncogene activation and induce carcinogenesis. Several host factors, for example gene polymorphisms (C282Y heterozygosity and CYP2E1 c2) lead to enhanced incidence of carcinogenesis. Iron overload and neoangiogenesis are both associated with alcoholic liver disease and HCC. Finally, the immune system is of major importance, with NK cells having a protective effect and TLR4/Nanog promoting the alcohol-induced occurrence of HCC
Acetaldehyde-mediated pathways
In hepatocytes, ethanol is metabolized to acetaldehyde by both alcohol dehydrogenase (ADH) and cytochrome P450 2E1 (CYP2E1). Acetaldehyde is a toxic compound with direct mutagenic and carcinogenic effects in vivo and in vitro [12–14]. During moderate alcohol intake, acetal-dehyde is metabolized to acetate by aldehyde dehydrogenase (ALDH). However, chronic alcohol abuse leads to induction of CYP2E1 and high levels of acetaldehyde production [15].
Acetaldehyde can form DNA adducts, for example N2-ethyl-2′-deoxyguanosine (N2-Et-dG) and N2-propano-2′-deoxyguanosine (PdG) which alter the integrity of hepatocyte DNA [16]. PdG, in particular, is capable of initiating replication errors [17]. Acetaldehyde can also form stable adducts with proteins, thereby altering protein structure and function. For example, adducts formed with O6-methylguanosyl transferase impair DNA repair mechanisms and could mediate carcinogenesis [18]. In addition, acetaldehyde damages mitochondria, resulting in inhibition of fatty acid oxidation [19]. Acetaldehyde stimulates collagen synthesis in hepatic stellate cells, an important step toward cirrhosis [20] and the eventual development of HCC.
Induction of CYP2E1 and reactive oxygen species generation
Chronic consumption of alcohol results in a 10–20 fold induction of CYP2E1. This cytochrome is involved in the activation of a variety of procarcinogens, for example aflatoxins, nitrosamines, and polycyclic hydrocarbons, to carcinogens. Induction of CYP2E1 by ethanol is also associated with the generation of hydroxyethyl radicals (HER), which bind to carcinogens, forming neoantigens [21] and leading to lipid peroxidation [22]. Studies report lower levels of oxidized DNA products in the blood of CYP2E1 knock-out mice compared with wild-type mice [23], and profound hepatic injury has been observed in CYP2E1 transgenic mice [24]. In humans, induction of CYP2E1 after ethanol ingestion results in increased levels of reactive oxygen species (ROS), lipid peroxidation, malondialdehyde (MDA), and 4-hydroxynonenol (4-HNE) [25]. Of these, 4-HNE has been shown to result in mutations at codon 249 of p53 tumor suppressor gene, blocking apoptosis of cells and affording them proliferative advantage [26]. Together, these products lead to the formation of DNA adducts with mutagenic potential that promote hepatic carcinogenesis.
Effects on antioxidant defense mechanisms
The augmented generation of ROS as a by-product of alcohol metabolism results in an increased requirement for antioxidants glutathione and α-tocopherol. In this regard, alcohol consumption has been shown to reduce hepatic glutathione concentrations [27], resulting in significant mitochondrial dysfunction and lipid peroxidation [27]. Alcohol also results in a reduction of α-tocopherol levels [28], and superoxide dismutase, catalase, and glutathione reductase activity [29], leading to enhanced lipid peroxidation and hepatic injury [30]. Together, these changes over time result in impaired DNA repair mechanisms and hepatic carcinogenesis [18].
Hypomethylation and DNA stability
DNA methylation is an important epigenetic factor controlling gene expression, in which hypomethylation is associated with increased gene expression. In alcohol-induced cirrhosis, reduced levels of the methyl group donor S-adenosylmethionine (SAMe) and increased levels of S-adenosylhomocysteine (SAH) result in a decrease in the SAMe/SAH ratio by a factor of 2.5 [31]. In rats, diet-induced SAMe deficiency leads to hypomethylation of oncogenes and DNA strand breaks, factors promoting carcinogenesis [32]. Further research is necessary to elucidate the effects of altered DNA methylation on HCC in humans.
Effects of iron overload
Lauret et al. [33] showed that HCC patients with alcohol-induced cirrhosis had a greater frequency (20.9 %) of heterozygosity for the hemochromatosis gene (C282Y allele) than did patients with alcohol-induced cirrhosis without HCC (4.4 %). Heterozygosity for C282Y leads to slightly higher iron levels, which may be important during heavy alcohol consumption. Notably, chronic alcohol abuse increases intestinal iron absorption and hepatic iron stores. The higher risk of HCC associated with increased iron stores can possibly be attributed to the generation of ROS from free iron, which can occur directly through the Fenton reaction or indirectly through induction of lipid peroxidation [34]. Increased iron stores can cause DNA strand breaks, p53 mutation, and DNA adducts, factors that promote HCC [35]. Thus, this overload of iron deposition due to alcohol consumption is a likely factor in the development of HCC.
Interference with retinoid metabolism
Retinoic acid (RA), which is synthesized from retinol, has many effects on cell growth and differentiation [36]. Chronic alcohol consumption is associated with reduced serum and hepatic vitamin A levels [37] and with increased mobilization of retinol from the liver to other tissues [38]. Ethanol is a competitive inhibitor of hepatic retinol oxidation, reducing RA production [39]. Furthermore, ethanol-induced enhancement of CYP2E1 results in an increase in RA catabolism and a subsequent decline in RA levels in the liver [40]. Finally, ethanol exposure results in functional down-regulation of RA receptors and a subsequent increase in activator protein-1 or AP-1 (c-fos and c-jun) transcriptional complex [39]. Together, these changes favor hepatic proliferation and carcinogenesis [40].
Immunological mechanism of alcohol-induced carcinogenesis
Several studies have revealed the pivotal importance of natural killer (NK) cells in immune surveillance of tumors [41] in animal models. NK cell number and lytic function are substantially reduced in cirrhotics [42], particularly those with a history of alcohol use [43]. Consequently, diminished NK cell function is likely to be important in the development of progressive liver fibrosis and HCC.
Increased incidence of HCC has been observed among HCV core transgenic mice fed with ethanol [44]. A recent study showed that NS5A transgenic mice fed with ethanol developed alcoholic steatohepatitis [45]. However, the same effect was not observed for NS5A Tg mice deficient in TLR4, suggesting the involvement of TLR4 in mediating the effects of alcohol and NS5A [45]. NS5A Tg mice fed an alcohol diet developed HCC, in contrast with wild-type or NS5A TLR4 −/− mice [45]. Interestingly, these NS5A Tg mice fed ethanol expressed increasing amounts of Nanog, a stem cell marker. No induction of Nanog was observed in mice of TLR4 −/− background. Collectively, these results suggest the crucial involvement of Nanog in induction of HCC mediated by alcohol, dependent on TLR4 signaling. Finally, transplantation of p53-deficient hepatic stem cells transfected with either Nanog or TLR4 resulted in HCC, which was abrogated by simultaneous expression of short hairpin RNA against Nanog. These results, also, suggest crucial involvement of Nanog in mediating tumorigenesis.
Neoangiogenesis
Hepatic foci are monoclonal lesions in the liver characterized by high rates of proliferation, changes in cell morphology, and reduced hepatocyte function. Neoangiogenesis seems to be necessary for progression of hepatic foci to HCC, a highly vascular tumor [46]. Although little is known about the direct affects of alcohol on neoangiogenesis, animal models show that ethanol leads to impaired endothelin and nitric oxide signaling, resulting in increased vasoconstriction and hepatic portal pressure [47, 48]. These alcohol-induced changes in microcirculation around transformed cells may be involved in initiation or maintenance of neoangiogenesis in hepatic foci. However, the involvement of ethanol in neoangiogenesis in HCC occurrence and/or progression warrants more detailed evaluation.
Inflammation
Ethanol consumption has systemic and intrahepatic effects that may affect HCC development. Heavy alcohol intake increases gut permeability to lipopolysaccharides (LPS) [49] increasing intrahepatic LPS levels. LPS binds to the CD14/TLR4 complex of Kupffer cells (KC), leading to release of pro-inflammatory cytokines, particularly tumor necrosis factor-α (TNF-α) and transforming growth factor-β (TGF-β) [50]. Intestinal sterilization of Gram-negative bacteria with antibiotics prevented ethanol-induced liver injury [51]. CD14/TLR4 knockout mice are also resistant to alcohol toxicity, indicating the importance of KCs to the pathogenesis of ethanol-related hepatic injury [52].
Interleukin 6 (IL-6) secreted by KCs is elevated in alcoholic patients with liver disease [53]. IL-6 promotes carcinogenesis by inhibiting hOGG1, a repair enzyme for 8-oxoguanosine adducts [54], and interfering with DNA repair. IL-6 upregulates mcl-1, an anti-apoptotic gene [55]. Further research is necessary to elucidate the extent of its importance to HCC development.
Conclusions
Chronic alcohol consumption is an important risk factor for HCC and hence a major public health burden. The mechanisms involved in alcohol-induced hepatic carcinogenesis are multifold and complex (Fig. 1). Alcohol use can directly induce hepatic carcinogenesis and enhance the possibility of occurrence of HCC in those with viral hepatitis. Further research on understanding the involvement of ethanol metabolism in carcinogenesis and identifying susceptible individuals is vital to reducing the morbidity and mortality associated with chronic liver disease and HCC.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH (National Institute of Allergy and Infectious Diseases, and the Clinical Research Center). The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
Footnotes
Conflict of interest: Sreetha Sidharthan and Shyam Kottilil have no conflicts of interest to report.
Contributor Information
Sreetha Sidharthan, Critical Care Medicine Department, Department of Health and Human Services, Clinical Center, National Institutes of Health, Bethesda, MD, USA.
Shyam Kottilil, Email: skottilil@niaid.nih.gov, Laboratory of Immunoregulation, Department of Health and Human Services, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bldg10, Rm.11N204, Bethesda, MD 20892, USA.
References
- 1.Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2:533–543. doi: 10.1016/S1470-2045(01)00486-7. [DOI] [PubMed] [Google Scholar]
- 2.Marrero JA. Hepatocellular carcinoma. Curr Opin Gastroenterol. 2005;21:308–312. doi: 10.1097/01.mog.0000159817.55661.ca. [DOI] [PubMed] [Google Scholar]
- 3.El-Serag HB, Mason AC. Risk factors for the rising rates of primary liver cancer in the United States. Arch Intern Med. 2000;160:3227–3230. doi: 10.1001/archinte.160.21.3227. [DOI] [PubMed] [Google Scholar]
- 4.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 5.Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127:S35–S50. doi: 10.1053/j.gastro.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 6.El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–50. doi: 10.1056/NEJM199903113401001. [DOI] [PubMed] [Google Scholar]
- 7.Taylor-Robinson SD, Foster GR, Arora S, Hargreaves S, Thomas HC. Increase in primary liver cancer in the UK, 1979–94. Lancet. 1997;350:1142–1143. doi: 10.1016/S0140-6736(05)63789-0. [DOI] [PubMed] [Google Scholar]
- 8.Morgan TR, Mandayam S, Jamal MM. Alcohol and hepatocellular carcinoma. Gastroenterology. 2004;127:S87–S96. doi: 10.1053/j.gastro.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 9.Donato F, Tagger A, Gelatti U, Parrinello G, Boffetta P, Albertini A, et al. Alcohol and hepatocellular carcinoma: the effect of lifetime intake and hepatitis virus infections in men and women. Am J Epidemiol. 2002;155:323–331. doi: 10.1093/aje/155.4.323. [DOI] [PubMed] [Google Scholar]
- 10.Bellentani S, Pozzato G, Saccoccio G, Crovatto M, Croce LS, Mazzoran L, et al. Clinical course and risk factors of hepatitis C virus related liver disease in the general population: report from the Dionysos study. Gut. 1999;44:874–880. doi: 10.1136/gut.44.6.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kew MC. Synergistic interaction between aflatoxin B1 and hepatitis B virus in hepatocarcinogenesis. Liver Int. 2003;23:405–409. doi: 10.1111/j.1478-3231.2003.00869.x. [DOI] [PubMed] [Google Scholar]
- 12.Dellarco VL. A mutagenicity assessment of acetaldehyde. Mutat Res. 1988;195:1–20. doi: 10.1016/0165-1110(88)90013-9. [DOI] [PubMed] [Google Scholar]
- 13.Helander A, Lindahl-Kiessling K. Increased frequency of acetaldehyde-induced sister-chromatid exchanges in human lymphocytes treated with an aldehyde dehydrogenase inhibitor. Mutat Res. 1991;264:103–107. doi: 10.1016/0165-7992(91)90124-m. [DOI] [PubMed] [Google Scholar]
- 14.Simanowski UA, Suter P, Russell RM, Heller M, Waldherr R, Ward R, et al. Enhancement of ethanol induced rectal mucosal hyper regeneration with age in F344 rats. Gut. 1994;35:1102–1106. doi: 10.1136/gut.35.8.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lieber CS, DeCarli LM. The role of the hepatic microsomal ethanol oxidizing system (MEOS) for ethanol metabolism in vivo. J Pharmacol Exp Ther. 1972;181:279–287. [PubMed] [Google Scholar]
- 16.Matsuda T, Terashima I, Matsumoto Y, Yabushita H, Matsui S, Shibutani S. Effective utilization of N2-ethyl-2′-deoxyguanosine triphosphate during DNA synthesis catalyzed by mammalian replicative DNA polymerases. Biochemistry. 1999;38:929–935. doi: 10.1021/bi982134j. [DOI] [PubMed] [Google Scholar]
- 17.Brooks PJ, Theruvathu JA. DNA adducts from acetaldehyde: implications for alcohol-related carcinogenesis. Alcohol. 2005;35:187–193. doi: 10.1016/j.alcohol.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 18.Espina N, Lima V, Lieber CS, Garro AJ. In vitro and in vivo inhibitory effect of ethanol and acetaldehyde on O6-methylguanine transferase. Carcinogenesis. 1988;9:761–766. doi: 10.1093/carcin/9.5.761. [DOI] [PubMed] [Google Scholar]
- 19.Lieber CS. Alcohol and the liver: 1994 update. Gastroenterology. 1994;106:1085–1105. doi: 10.1016/0016-5085(94)90772-2. [DOI] [PubMed] [Google Scholar]
- 20.Friedman SL. Seminars in medicine of the Beth Israel Hospital, Boston. The cellular basis of hepatic fibrosis. Mechanisms and treatment strategies. N Engl J Med. 1993;328:1828–1835. doi: 10.1056/NEJM199306243282508. [DOI] [PubMed] [Google Scholar]
- 21.Albano E, Goria-Gatti L, Clot P, Jannone A, Tomasi A. Possible role of free radical intermediates in hepatotoxicity of hydrazine derivatives. Toxicol Ind Health. 1993;9:529–538. doi: 10.1177/074823379300900312. [DOI] [PubMed] [Google Scholar]
- 22.Dupont I, Lucas D, Clot P, Menez C, Albano E. Cytochrome P4502E1 inducibility and hydroxyethyl radical formation among alcoholics. J Hepatol. 1998;28:564–671. doi: 10.1016/s0168-8278(98)80279-1. [DOI] [PubMed] [Google Scholar]
- 23.Bradford BU, Kono H, Isayama F, Kosyk O, Wheeler MD, Akiyama TE, et al. Cytochrome P450 CYP2E1, but not nicotinamide adenine dinucleotide phosphate oxidase, is required for ethanol-induced oxidative DNA damage in rodent liver. Hepatology. 2005;41:336–344. doi: 10.1002/hep.20532. [DOI] [PubMed] [Google Scholar]
- 24.Morgan K, French SW, Morgan TR. Production of a cytochrome P450 2E1 transgenic mouse and initial evaluation of alcoholic liver damage. Hepatology. 2002;36:122–134. doi: 10.1053/jhep.2002.33720. [DOI] [PubMed] [Google Scholar]
- 25.Aleynik SI, Leo MA, Aleynik MK, Lieber CS. Increased circulating products of lipid peroxidation in patients with alcoholic liver disease. Alcohol Clin Exp Res. 1998;22:192–196. [PubMed] [Google Scholar]
- 26.Hu W, Feng Z, Eveleigh J, Iyer G, Pan J, Amin S, et al. The major lipid peroxidation product, trans-4-hydroxy-2-nonenal, preferentially forms DNA adducts at codon 249 of human p53 gene, a unique mutational hotspot in hepatocellular carcinoma. Carcinogenesis. 2002;23:1781–1789. doi: 10.1093/carcin/23.11.1781. [DOI] [PubMed] [Google Scholar]
- 27.Fernandez-Checa JC, Kaplowitz N. Hepatic mitochondrial glutathione: transport and role in disease and toxicity. Toxicol Appl Pharmacol. 2005;204:263–273. doi: 10.1016/j.taap.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 28.Kawase T, Kato S, Lieber CS. Lipid peroxidation and antioxidant defense systems in rat liver after chronic ethanol feeding. Hepatology. 1989;10:815–821. doi: 10.1002/hep.1840100511. [DOI] [PubMed] [Google Scholar]
- 29.Rouach H, Fataccioli V, Gentil M, French SW, Morimoto M, Nordmann R. Effect of chronic ethanol feeding on lipid peroxidation and protein oxidation in relation to liver pathology. Hepatology. 1997;25:351–355. doi: 10.1002/hep.510250216. [DOI] [PubMed] [Google Scholar]
- 30.Polavarapu R, Spitz DR, Sim JE, Follansbee MH, Oberley LW, Rahemtulla A, Nanji AA. Increased lipid peroxidation and impaired antioxidant enzyme function is associated with pathological liver injury in experimental alcoholic liver disease in rats fed diets high in corn oil and fish oil. Hepatology. 1998;27:1317–1323. doi: 10.1002/hep.510270518. [DOI] [PubMed] [Google Scholar]
- 31.Seitz HK, Stickel F. Risk factors and mechanisms of hepatocarcinogenesis with special emphasis on alcohol and oxidative stress. Biol Chem. 2006;387:349–360. doi: 10.1515/BC.2006.047. [DOI] [PubMed] [Google Scholar]
- 32.Pogribny IP, Basnakian AG, Miller BJ, Lopatina NG, Poirier LA, James SJ. Breaks in genomic DNA and within the p53 gene are associated with hypomethylation in livers of folate/methyl-deficient rats. Cancer Res. 1995;55:1894–1901. [PubMed] [Google Scholar]
- 33.Lauret E, Rodriguez M, Gonzalez S, Linares A, Lopez-Vazquez A, Martinez-Borra J, et al. HFE gene mutations in alcoholic and virus-related cirrhotic patients with hepatocellular carcinoma. Am J Gastroenterol. 2002;97:1016–1021. doi: 10.1111/j.1572-0241.2002.05553.x. [DOI] [PubMed] [Google Scholar]
- 34.Petersen DR. Alcohol, iron-associated oxidative stress, and cancer. Alcohol. 2005;35:243–249. doi: 10.1016/j.alcohol.2005.03.013. [DOI] [PubMed] [Google Scholar]
- 35.Marrogi AJ, Khan MA, van Gijssel HE, Welsh JA, Rahim H, Demetris AJ, et al. Oxidative stress and p53 mutations in the carcinogenesis of iron overload-associated hepatocellular carcinoma. J Natl Cancer Inst. 2001;93:1652–1655. doi: 10.1093/jnci/93.21.1652. [DOI] [PubMed] [Google Scholar]
- 36.Chambon P. A decade of molecular biology of retinoic acid receptors. FASEB J. 1996;10:940–954. [PubMed] [Google Scholar]
- 37.Leo MA, Lieber CS. Hepatic vitamin A depletion in alcoholic liver injury. N Engl J Med. 1982;307:597–601. doi: 10.1056/NEJM198209023071006. [DOI] [PubMed] [Google Scholar]
- 38.Leo MA, Lieber CS. Alcohol, vitamin A, and beta-carotene: adverse interactions, including hepatotoxicity and carcinogenicity. Am J Clin Nutr. 1999;69:1071–1085. doi: 10.1093/ajcn/69.6.1071. [DOI] [PubMed] [Google Scholar]
- 39.Wang XD, Liu C, Chung J, Stickel F, Seitz HK, Russell RM. Chronic alcohol intake reduces retinoic acid concentration and enhances AP-1 (c-Jun and c-Fos) expression in rat liver. Hepatology. 1998;28:744–750. doi: 10.1002/hep.510280321. [DOI] [PubMed] [Google Scholar]
- 40.Chung J, Liu C, Smith DE, Seitz HK, Russell RM, Wang XD. Restoration of retinoic acid concentration suppresses ethanol-enhanced c-Jun expression and hepatocyte proliferation in rat liver. Carcinogenesis. 2001;22:1213–1219. doi: 10.1093/carcin/22.8.1213. [DOI] [PubMed] [Google Scholar]
- 41.Trinchieri G. Biology of natural killer cells. Adv Immunol. 1989;47:187–376. doi: 10.1016/S0065-2776(08)60664-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Laso FJ, Madruga JI, Giron JA, Lopez A, Ciudad J, San Miguel JF, et al. Decreased natural killer cytotoxic activity in chronic alcoholism is associated with alcohol liver disease but not active ethanol consumption. Hepatology. 1997;25:1096–1100. doi: 10.1002/hep.510250508. [DOI] [PubMed] [Google Scholar]
- 43.Cook RT, Garvey MJ, Booth BM, Goeken JA, Stewart B, Noel M. Activated CD-8 cells and HLA DR expression in alcoholics without overt liver disease. J Clin Immunol. 1991;11:246–253. doi: 10.1007/BF00918182. [DOI] [PubMed] [Google Scholar]
- 44.Koike K, Tsutsumi T, Miyoshi H, Shinzawa S, Shintani Y, Fujie H, et al. Molecular basis for the synergy between alcohol and hepatitis C virus in hepatocarcinogenesis. J Gastroenterol Hepatol. 2008;23(Suppl 1):S87–S91. doi: 10.1111/j.1440-1746.2007.05292.x. [DOI] [PubMed] [Google Scholar]
- 45.Machida K, Tsukamoto H, Mkrtchyan H, Duan L, Dynnyk A, Liu HM, et al. Toll-like receptor 4 mediates synergism between alcohol and HCV in hepatic oncogenesis involving stem cell marker Nanog. Proc Natl Acad Sci USA. 2009;106:1548–1553. doi: 10.1073/pnas.0807390106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ribatti D, Vacca A, Nico B, Sansonno D, Dammacco F. Angiogenesis and anti-angiogenesis in hepatocellular carcinoma. Cancer Treat Rev. 2006;32:437–444. doi: 10.1016/j.ctrv.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 47.Bauer I, Bauer M, Pannen BH, Leinwand MJ, Zhang JX, Clemens MG. Chronic ethanol consumption exacerbates liver injury following hemorrhagic shock: role of sinusoidal perfusion failure. Shock. 1995;4:324–331. doi: 10.1097/00024382-199511000-00003. [DOI] [PubMed] [Google Scholar]
- 48.Karaa A, Kamoun WS, Clemens MG. Chronic ethanol sensitizes the liver to endotoxin via effects on endothelial nitric oxide synthase regulation. Shock. 2005;24:447–454. doi: 10.1097/01.shk.0000180616.13941.7d. [DOI] [PubMed] [Google Scholar]
- 49.Bjarnason I, Peters TJ, Wise RJ. The leaky gut of alcoholism: possible route of entry for toxic compounds. Lancet. 1984;1:179–182. doi: 10.1016/s0140-6736(84)92109-3. [DOI] [PubMed] [Google Scholar]
- 50.Seki E, Brenner DA. Toll-like receptors and adaptor molecules in liver disease: update. Hepatology. 2008;48:322–335. doi: 10.1002/hep.22306. [DOI] [PubMed] [Google Scholar]
- 51.Adachi Y, Moore LE, Bradford BU, Gao W, Thurman RG. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology. 1995;108:218–224. doi: 10.1016/0016-5085(95)90027-6. [DOI] [PubMed] [Google Scholar]
- 52.Yin M, Bradford BU, Wheeler MD, Uesugi T, Froh M, Goyert SM, Thurman RG. Reduced early alcohol-induced liver injury in CD14-deficient mice. J Immunol. 2001;166:4737–4742. doi: 10.4049/jimmunol.166.7.4737. [DOI] [PubMed] [Google Scholar]
- 53.Urbaschek R, McCuskey RS, Rudi V, Becker KP, Stickel F, Urbaschek B, et al. Endotoxin, endotoxin-neutralizing-capacity, sCD14, sICAM-1, and cytokines in patients with various degrees of alcoholic liver disease. Alcohol Clin Exp Res. 2001;25:261–268. [PubMed] [Google Scholar]
- 54.Bartsch H, Nair J. Oxidative stress and lipid peroxidation-derived DNA-lesions in inflammation driven carcinogenesis. Cancer Detect Prev. 2004;28:385–3891. doi: 10.1016/j.cdp.2004.07.004. [DOI] [PubMed] [Google Scholar]
- 55.Lin MT, Juan CY, Chang KJ, Chen WJ, Kuo ML. IL-6 inhibits apoptosis and retains oxidative DNA lesions in human gastric cancer AGS cells through up-regulation of anti-apoptotic gene mcl-1. Carcinogenesis. 2001;22:1947–1953. doi: 10.1093/carcin/22.12.1947. [DOI] [PubMed] [Google Scholar]