Abstract
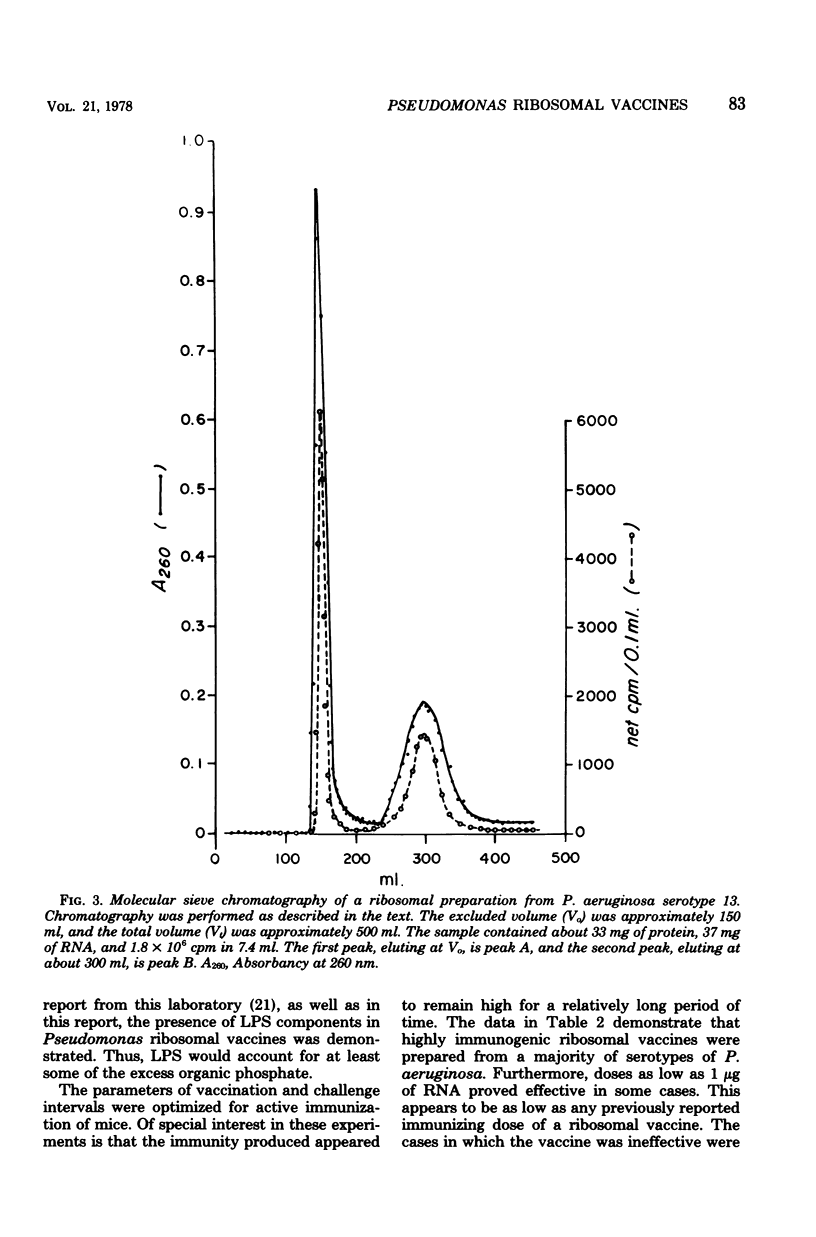
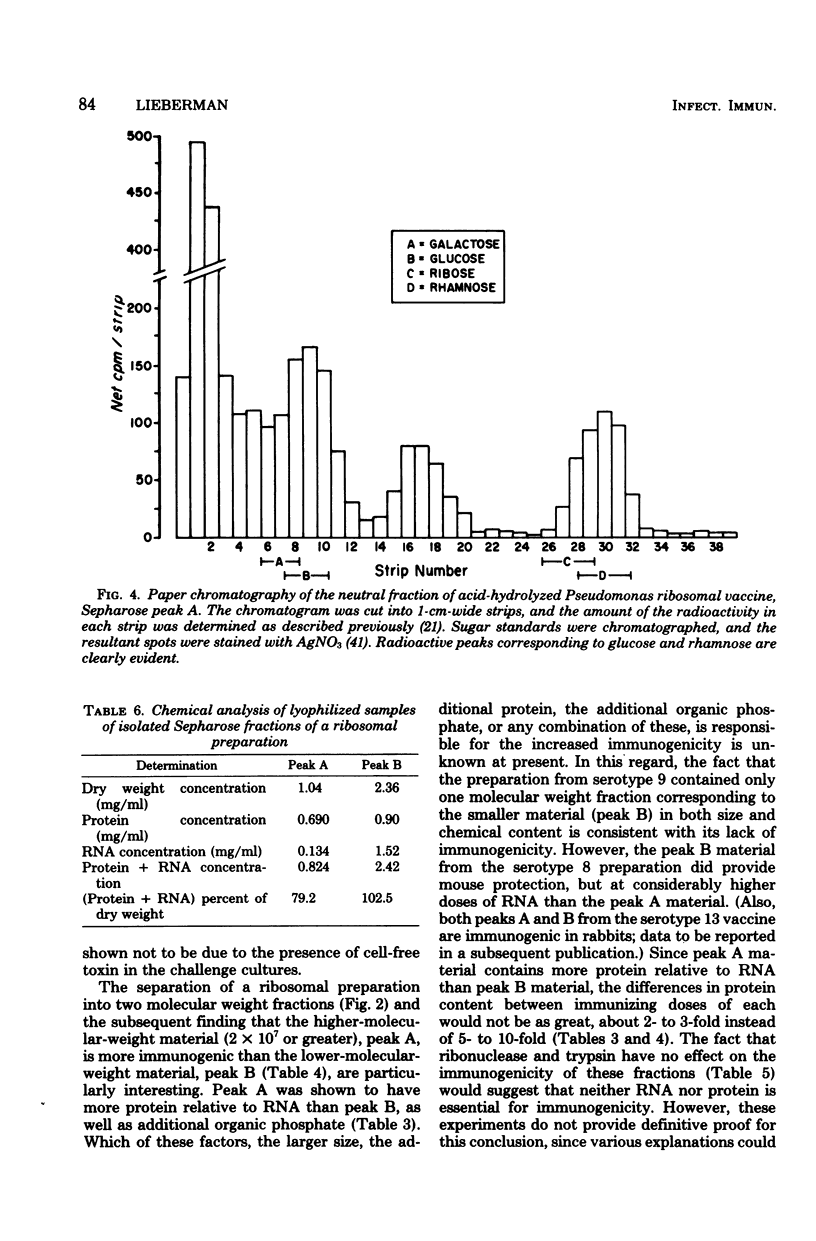
The preparation, properties, and immunogenicity of ribosomal vaccines from Pseudomonas aeruginosa are described. These preparations, containing protein and RNA, were tested for immunogenicity by active immunization of mice and subsequent challenge with homologous, live bacteria. The results demonstrated that vaccines prepared from a majority of serotypes used were immunogenic, i.e., afforded 60 to 100% mouse protection against a challenge inoculum containing 8 to 50 50% lethal doses. In some cases vaccine doses as low as 1 microgram of RNA provided 100% mouse protection. Molecular sieve chromatography of a highly immunogenic ribosomal preparation on Sepharose 4B demonstrated the presence of two molecular weight fractions: (i) peak A, an excluded peak (thus having a molecular weight of at least 2 times 10(7)), and (ii) peak B, considerably retarded, with an elution position corresponding to a molecular weight of about 2.2 X 10(6), approximating that of typical 70S ribosomes. Both peaks A and B were immunogenic; however, the immunogenicity of peak A was greater (i.e., a smaller immunizing dose was required) than that of peak B. Peak A was shown to contain components of lipopolysaccharide in addition to protein and RNA (which comprised 80% of the dry weight of peak A). On the other hand, peak B was shown to be free of lipopolysaccharide, and 100% of its dry weight consisted of protein and RNA.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham G. N., Scaletta C., Vaughan J. H. Modified diphenylamine reaction for increased sensitivity. Anal Biochem. 1972 Oct;49(2):547–549. doi: 10.1016/0003-2697(72)90460-5. [DOI] [PubMed] [Google Scholar]
- Andron LA I. I., Eigelsbach H. T. Biochemical and immunological properties of ribonucleic acid-rich extracts from Francisella tularensis. Infect Immun. 1975 Jul;12(1):137–142. doi: 10.1128/iai.12.1.137-142.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aprile M. A., Wardlaw A. C. Immunochemical studies on the lipopolysaccharides of Bordetella pertussis. Can J Microbiol. 1973 Feb;19(2):231–239. doi: 10.1139/m73-035. [DOI] [PubMed] [Google Scholar]
- Baba T. Immunogenic activity of a ribosomal fraction obtained from Pasteurella multocida. Infect Immun. 1977 Jan;15(1):1–6. doi: 10.1128/iai.15.1.1-6.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruneteau M., Volk W. A., Singh P. P., Lüderitz O. Structural investigations on the Salmonella T2 lipopolysaccharide. Eur J Biochem. 1974 Apr 16;43(3):501–508. doi: 10.1111/j.1432-1033.1974.tb03437.x. [DOI] [PubMed] [Google Scholar]
- Eisenstein T. K. Evidence for O antigens as the antigenic determinants in "ribosomal" vaccines prepared from Salmonella. Infect Immun. 1975 Aug;12(2):364–377. doi: 10.1128/iai.12.2.364-377.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel S., Sypherd P. S. Extraction and isolation of individual ribosomal proteins from Escherichia coli. J Bacteriol. 1968 Aug;96(2):358–364. doi: 10.1128/jb.96.2.358-364.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanos C., Rietschel E. T., Lüderitz O., Westphal O., Kim Y. B., Watson D. W. Biological activities of lipid A complexed with bovine-serum albumin. Eur J Biochem. 1972 Dec 4;31(2):230–233. doi: 10.1111/j.1432-1033.1972.tb02524.x. [DOI] [PubMed] [Google Scholar]
- HABS I. Untersuchungen über die O-Antigene von Pseudomonas aeruginosa. Z Hyg Infektionskr. 1957;144(3):218–228. [PubMed] [Google Scholar]
- Hanessian S., Regan W., Watson D., Haskell T. H. Isolation and characterization of antigenic components of a new heptavalent Pseudomonas vaccine. Nat New Biol. 1971 Feb 17;229(7):209–210. doi: 10.1038/newbio229209a0. [DOI] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Hoops P., Prather N. E., Berry J., Ravel J. M. Evidence for an extrinsic immunogen in effective ribosomal vaccines from Salmonella typhimurium. Infect Immun. 1976 Apr;13(4):1184–1192. doi: 10.1128/iai.13.4.1184-1192.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W. Ribosomal vaccines. I. Immunogenicity of ribosomal fractions isolated from Salmonella typhimurium and Yersinia pestis. Infect Immun. 1972 Jun;5(6):947–952. doi: 10.1128/iai.5.6.947-952.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson W. Ribosomal vaccines. II. Specificity of the immune response to ribosomal ribonucleic acid and protein isolated from Salmonella typhimurium. Infect Immun. 1973 Sep;8(3):395–400. doi: 10.1128/iai.8.3.395-400.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIM Y. B., WATSON D. W. INACTIVATION OF GRAM-NEGATIVE BACTERIAL ENDOTOXINS BY PAPAIN. Proc Soc Exp Biol Med. 1964 Jan;115:140–142. doi: 10.3181/00379727-115-28852. [DOI] [PubMed] [Google Scholar]
- Lieberman M. M. Direct evidence for the presence of lipopolysaccharide components in Pseudomonas ribosomal vaccine. Infect Immun. 1977 Aug;17(2):471–473. doi: 10.1128/iai.17.2.471-473.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn M., Tewari R. P., Solotorovsky M. Immunoprotective activity of ribosomes from Haemophilus influenzae. Infect Immun. 1977 Feb;15(2):453–460. doi: 10.1128/iai.15.2.453-460.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis J. M., Bigley N. J. Cytophilic macroglobulin reactive with bacterial protein in mice immunized with ribonucleic acid-protein fractions of virulent Salmonella typhimurium. Infect Immun. 1972 Sep;6(3):390–397. doi: 10.1128/iai.6.3.390-397.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W. J., Marcus S. Detoxified bacterial endotoxns. II. Preparation and biological properties of chemically modified crude endotoxins from Salmonella typhimurium. J Bacteriol. 1966 May;91(5):1750–1758. doi: 10.1128/jb.91.5.1750-1758.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myerowitz R. L., Medeiros A. A., O'Brien T. F. Recent experience with bacillemia due to gram-negative organisms. J Infect Dis. 1971 Sep;124(3):239–246. doi: 10.1093/infdis/124.3.239. [DOI] [PubMed] [Google Scholar]
- NOLL H., BRAUDE A. I. Preparation and biological properties of a chemically modified Escherichia coli endotoxin of high immunogenic potency and low toxicity. J Clin Invest. 1961 Nov;40:1935–1951. doi: 10.1172/JCI104419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistole T. G., Marcus S. Chemically-modified crude endotoxins as possible typhoid vaccines. Immunol Commun. 1973;2(1):43–56. doi: 10.3109/08820137309022880. [DOI] [PubMed] [Google Scholar]
- Prigal S. J., Herp A., Gerstein J. The detoxification of lipopolysaccharides derived from bacterial endotoxins by ferric chloride. J Reticuloendothel Soc. 1973 Sep;14(3):250–257. [PubMed] [Google Scholar]
- Richards G. M. Modifications of the diphenylamine reaction giving increased sensitivity and simplicity in the estimation of DNA. Anal Biochem. 1974 Feb;57(2):369–376. doi: 10.1016/0003-2697(74)90091-8. [DOI] [PubMed] [Google Scholar]
- Rietschel E. T., Gottert H., Lüderitz O., Westphal O. Nature and linkages of the fatty acids present in the lipid-A component of Salmonella lipopolysaccharides. Eur J Biochem. 1972 Jul 13;28(2):166–173. doi: 10.1111/j.1432-1033.1972.tb01899.x. [DOI] [PubMed] [Google Scholar]
- Schalla W. O., Johnson W. Immunogenicity of ribosomal vaccines isolated from group A, type 14 Streptococcus pyogenes. Infect Immun. 1975 Jun;11(6):1195–1202. doi: 10.1128/iai.11.6.1195-1202.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sensakovic J. W., Bartell P. F. The slime of Pseudomonas aeruginosa: biological characterization and possible role in experimental infection. J Infect Dis. 1974 Feb;129(2):101–109. doi: 10.1093/infdis/129.2.101. [DOI] [PubMed] [Google Scholar]
- Smith R. A., Bigley N. J. Detection of delayed hypersensitivity in mice injected with ribonucleic acid-protein fractions of Salmonella typhimurium. Infect Immun. 1972 Sep;6(3):384–389. doi: 10.1128/iai.6.3.384-389.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. L., Wysocki J. A., Bruun J. N., De Courcy S. J., Jr, Blakemore W. S., Mudd S. Efficacy of ribosomal preparations from Pseudomonas aeruginosa to protect against intravenous Pseudomonas challenge in mice. J Reticuloendothel Soc. 1974 Jan;15(1):22–30. [PubMed] [Google Scholar]
- Swendsen C. L., Johnson W. Humoral immunity to Streptococcus pneumoniae induced by a pneumococcal ribosomal protein fraction. Infect Immun. 1976 Aug;14(2):345–354. doi: 10.1128/iai.14.2.345-354.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Thomas D. W., Weiss E. Response of mice to injection of ribosomal fraction from group B Neisseria meningitidis. Infect Immun. 1972 Sep;6(3):355–363. doi: 10.1128/iai.6.3.355-363.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson H. C., Eisenstein T. K. Biological properties of an immunogenic pneumococcal subcellular preparation. Infect Immun. 1976 Mar;13(3):750–757. doi: 10.1128/iai.13.3.750-757.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang J. C., Wang C. S., Alaupovic P. Degradative effect of phenol on endotoxin and lipopolysaccharide preparations from Serratia marcescens. J Bacteriol. 1974 Feb;117(2):786–795. doi: 10.1128/jb.117.2.786-795.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneman M. R., Berry L. J. Serum-mediated resistance induced with immunogenic preparations of Salmonella typhimurium. Infect Immun. 1971 Oct;4(4):374–380. doi: 10.1128/iai.4.4.374-380.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venneman M. R. Purification of immunogenically active ribonucleic acid preparations of Salmonella typhimurium: molecular-sieve and anion-exchange chromatography. Infect Immun. 1972 Mar;5(3):269–282. doi: 10.1128/iai.5.3.269-282.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volk W. A., Salomonsky N. L., Hunt D. Xanthomonas sinensis cell wall lipopolysaccharide. I. Isolation of 4,7-anhydro- and 4,8-anhydro-3-deoxy-octulosonic acid following acid hydrolysis of Xanthomonas sinensis lipopolysaccharide. J Biol Chem. 1972 Jun 25;247(12):3881–3887. [PubMed] [Google Scholar]
- Wesley J., Fisher A., Fisher M. W. Immunization against Pseudomonas in infection after thermal injury. J Infect Dis. 1974 Nov;130 (Suppl)(0):S152–S158. doi: 10.1093/infdis/130.supplement.s152. [DOI] [PubMed] [Google Scholar]
- Winston S., Berry L. J. Immunity induced by ribosomal extracts from Staphylococcus aureus. J Reticuloendothel Soc. 1970 Jul;8(1):66–73. [PubMed] [Google Scholar]
- YOUMANS A. S., YOUMANS G. P. IMMUNOGENIC ACTIVITY OF A RIBOSOMAL FRACTION OBTAINED FROM MYCOBACTERIUM TUBERCULOSIS. J Bacteriol. 1965 May;89:1291–1298. doi: 10.1128/jb.89.5.1291-1298.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youmans A. S., Youmans G. P. Immunogenic mycobacterial ribosomal and ribonucleic Acid preparations: chemical and physical characteristics. Infect Immun. 1970 Nov;2(5):659–668. doi: 10.1128/iai.2.5.659-668.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youmans A. S., Youmans G. P. Preparation of highly immunogenic ribosomal fractions of Mycobacterium tuberculosis by use of sodium dodecyl sulfate. J Bacteriol. 1966 Jun;91(6):2139–2145. doi: 10.1128/jb.91.6.2139-2145.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]


