Abstract
Background: Chemotherapy with cisplatin (CP) is accompanied with nephrotoxicity.
Objectives: In the current study, pomegranate flower extract (PFE) has been evaluated as an antioxidant agent against CP-induced-renal toxicity.
Materials and Methods: Thirty two male Wistar rats were divided into five groups (6-8 in each group). The animals in groups 1 to 3 received PFE (25 mg/kg), PFE (50 mg/kg), and placebo (saline), respectively for 9 days, and onset of the day 3, they also received CP (2.5 mg/kg/day). Groups 4 and 5 were treated with PFE (25 and 50 mg/kg/day) for 9 days. Finally, the animals were sacrificed at day 9 after collecting blood samples. Kidneys were removed, weighted, and underwent histopathological investigation.
Results: The mean serum level of creatinine in group 3 (treated with CP and placebo) increased significantly (p<0.05), but the value decreased significantly (p<0.05) in group 1. Kidney weight in group 1 was lower than KW in groups 2 and 3, however it was significant when compared with group 2 (p<0.05). The serum nitrite level in group 2 was non-significantly lower than that in other groups, and no significant changes were observed in serum levels of malondialdehyde (MDA). Tissue level of nitrite was significantly decreased in the positive control and high dose of PFE plus CP-treated groups (p<0.05). Among CP-treated groups, low dose of PFE significantly improved kidney nitrite level (p<0.05). The results from histopathological staining indicated less tissue damage in group 1 when compared with group 3.
Conclusions: It seems that low dose of PFE plays a protective role against CP-induced renal toxicity in rats.
Keywords: Pomegranate flower extract, Cisplatin, Nephrotoxicity
Implication for health policy/practice/research/medical education:
The low dose of pomegranate flower extract (25 mg/kg) provided protective effects and ameliorated cisplatin-induced nephrotoxicity through its antioxidant effects. Therefore, co-administration of pomegranate flower extract with cisplatin might be beneficial for these patients.
1. Background
Cisplatin (Cis-diamminedichloroplatinum, CP) has been successfully used as a chemotherapeutic agent in the treatment of solid tumors (1). Despite its potent anti-neoplastic action, it has many side effects such as hepatotoxicity, ototoxicity, neurotoxicity, gastrotoxicity, myelosuppression, and allergic reactions (2). The major side effect of CP is nephrotoxicity. Nephrotoxicity is a result of proximal tubular cell injury which occurs with high prevalence in the initial days of CP chemotherapy. The mechanism of CP-induced nephrotoxicity might be related to oxidative stress, apoptosis or an inflammatory response (2). CP-induced oxidative stress and inflammatory response in the kidney may partially be prevented by several chemical and natural compounds such as L-arginine, losartan and erythropoietin (3-6). Oxidative stress is responsible for a variety of degenerative processes in some diseases and is initiated by free radicals which cause protein and DNA damage along with lipid peroxidation (7,8). Most herbs have antioxidant properties. Morus alba leaves have been reported to be effective against CP-induced nephrotoxicity (6). Also, the olive leaves have chemical content and antioxidant properties that remove free oxygen radicals (9). Pomegranate parts including leaf, bark, root, fruit, juice and seed contain ingredients that can be effective as antimicrobial and antioxidant agents (10). Pomegranate fruit and juice have anti-fungal (10) and anti-inflammatory properties (11) and reduce blood pressure (12). The major phenolic composition of pomegranate flower extract (PFE) is gallic acid, which has protective effect in kidney disturbances (13). The aqueous extract of pomegranate was investigated in gentamicin-induced nephrotoxicity model and its protective effects was reported (14).
2. Objectives
Considering the antioxidant properties of pomegranate, we examined the effects of PFE on CP-induced nephrotoxicity in the current study.
3. Materials and Methods
3. 1. Animals
This is an experimental study in which 32 adult male (170-200 g) Wistar rats (Animal Center, Isfahan University of Medical Sciences, Isfahan, Iran) were used. The rats were housed at the temperature of 23-25 °C and had free access to water and rat chow. The rats were acclimatized to this diet for at least one week prior to the experiment. The experimental procedures were in advance approved by the Isfahan University of Medical Sciences Ethics Committee.
3.2. Drugs
CP was purchased from EBEWE Pharma Ges.m.b.H (Unterach, Austria), and ketamine HCl was obtained from Rotexmedica (Trittau, Germany).
3.3. PFE preparation
Five hundred grams of pomegranate flower was provided and powdered. Hydroalcoholic extract was prepared by ethanol: water (70:30) mixture using percolation method. Hydroalcoholic extract was concentrated and dried to obtain 123 g pure powder.
3.4. Measurement of extract total phenolic content
Total phenolic content of the extract was assessed by the Folin-Ciocalteu method. Briefly, 20 µl extract 85% plus 1.58 ml deionized water and 100 µl Folin-Ciocalteu reagents were mixed. After 30 s, 30 µl Na2CO3 was added to the mixture. Then, the mixture was incubated at 20 °C for 2 h. Finally, the absorbance was read in 765 nm.
3.5. PFE analysis
PFE analysis indicated that the major phenolic content of this extract is gallic acid (9.98%).
3.6. Experimental protocol
The animals were randomly assigned to five groups and were treated as follows: Group 1 (n= 6) received PFE (25 mg/kg/day) for 9 consecutive days and from day 3 CP was added to the treatment (2.5 mg/kg/day). Group 2 (n= 6) had treatment the same as group 1with PFE at the dose of 50 mg/kg/day. Group 3, as the positive control group, (n= 8) was treated similar to group 1, however, received saline instead of PFE.
Groups 4 (n= 6) and 5 (n= 6) as negative control groups received 25 and 50 mg/kg/day of PFE alone for 9 consecutive days, respectively. Animal body weight was recorded on a daily basis. At the end of the experiment, the animals were anesthetized with ketamine (75 mg/kg, ip), blood samples were collected by heart puncture and the animals were sacrificed. The kidneys were removed immediately and weighed. Left kidney was fixed in 10% neutral formalin solution and right kidney was homogenized in 2 ml of saline for the measurements. Two additional groups of animals were also treated in this study. Group A (n= 6) received treatment the same as group 1 except PFE (100 mg/kg/day) and group B (n= 6) received 100 mg/kg/day PFE alone. The mortality rates in these two groups were above 70%. Therefore, their results were not considered in data analysis. The reason of this high mortality rate seems to be related to high dose of drug.
3.7. Measurements
Serum level of creatinine (Cr) was determined using quantitative diagnostic kits (Pars Azmoon, Iran). The serum and kidney levels of nitrite (stable NO metabolite) were measured using a colorimetric assay kit (Promega Corporation, USA) based on the Griess reaction. Serum and kidney levels of malondialdehyde (MDA) were quantified according to the manual method. Briefly, 500 µl of the sample was mixed with 1000 µl of 10% trichloroacetic acid (TCA). The mixture was centrifuged at 2000 g for 10 min; 500 µl of the supernatant was added to 500 µl of 0.67% thiobarbituric acid (TBA). Then, the solution was incubated for 10 min in warm water bath at the temperature of 100 °C. After cooling, the absorbance was measured at the wavelength of 532 nm.
3.8. Histopathological procedures
Left kidney was fixed in 10% neutral formalin solution and embedded in paraffin. Tissue sections were stained with hematoxylin and eosin method. The kidney tissue damage score (KTDS) was determined by a pathologist who was blind to the study. The samples were scored from 1 to 4, while score 0 was assigned to normal kidney tissue without damage. Parameters of tubular damage included tubular dilation, tubular cells swelling and necrosis, tubular casts, and intraluminal cell debris.
3.9. Statistical analysis
Data was expressed as mean ± SD and median, minimum and maximum. The levels of Cr, MDA, and nitrite, as well as body weight and kidney weight (KW) were analyzed by the one-way ANOVA followed by the Tukey’s test using SPSS 16 Software. The tissue damage scores were compared by the Kruskal-Wallis or Mann-Whitney tests.
4. Results
4.1. Serum level of Cr
The mean serum level of Cr in group 3 (treated with CP and placebo) increased significantly when compared with groups 4 and 5 (p<0.05). This finding verified CP-induced nephrotoxicity. The low dose of PFE when accompanied with CP (group 1) significantly decreased the serum level of Cr (p<0.05), while such observation was not detected in group 2 (Figure 1). Statistical analysis also indicated that no difference was observed between serum Cr levels in groups 1, 4, and 5. This findings indicated that low dose of PFE potentially could reduce the Cr level increased by CP administration.
Figure 1 .
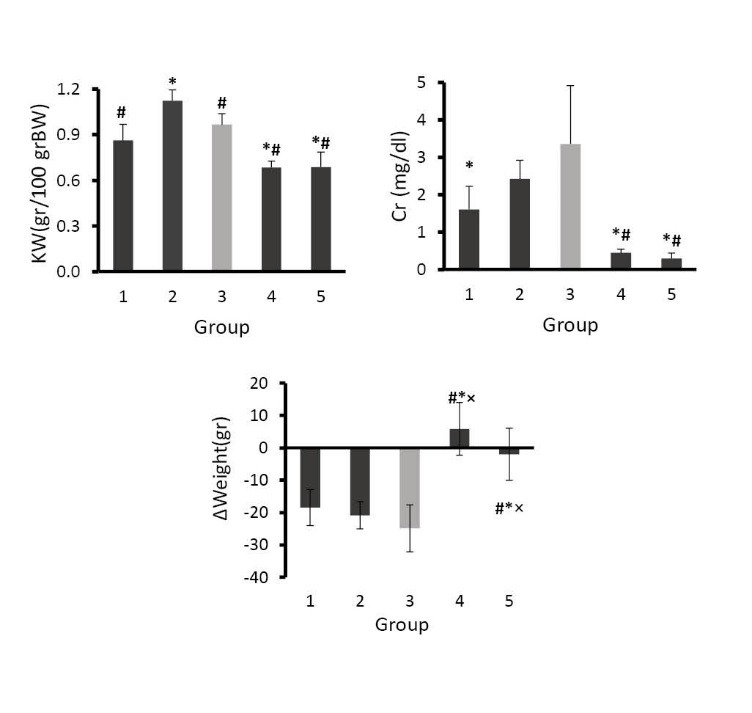
The data for serum level of creatinine (Cr, above right), total kidney weight (g)/100 (g) body weight (KW, above left), delta weight (∆W, weight difference, below), in five experimental groups (see text for group details). One-way ANOVA was applied for analyses. (*), (#) and (×) indicate significant difference (p<0.05) when compared with the positive control (group 3), group 2, and group 1, respectively.
4.2. Body weight, kidney weight, and KTDS
Both KW and KTDS increased significantly in the positive control group (group 3) when compared with the negative control groups (p<0.05). In addition, significant increase of KW was seen in group 2 compared with the positive control group (p<0.05). In all CP-treated animals, the weight loss were significant, and KTDS were also significantly higher when compared with the negative control groups (p<0.05;Table 2). However, the intensity of weight loss and KTDS in group 1 was lower than those in other CP-treated groups (Figure 1; Table 2). The images of kidney tissue samples are demonstrated in Figure 2.
Table 2 . The minimum and maximum range, median and mean± SD of kidney damage score in five experimental groups .
| Group | Minimum | Maximum | Median | Mean |
| CP +PFE 25 (mg/kg) | 2 | 3 | 2 | 2.2±0.45 |
| CP + PFE 50 (mg/kg) | 2 | 3 | 2.5 | 2.5±0.55 |
| CP | 2 | 3 | 2 | 2.38±0.52 |
| PFE 25 (mg/kg) | 0 | 1 | 0 | 0.25±0.5#*× |
| PFE 50 (mg/kg) | 0 | 0 | 0 | 0±0.00#*× |
(*), (#) and (×) indicate significant difference (P<0.05) when compared with the positive control (group 3), group 2, and group 1, respectively.
Figure 2 .
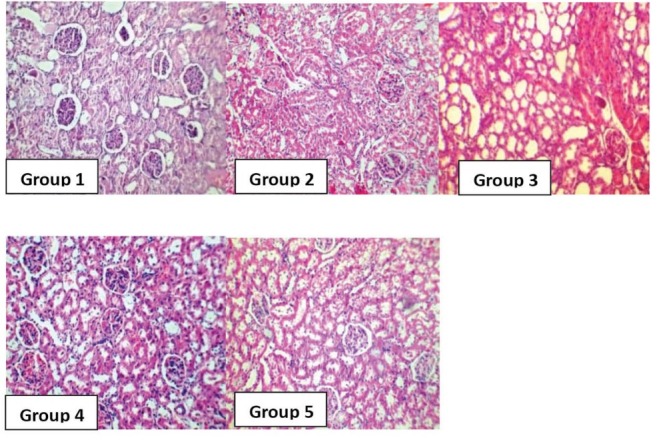
Micrographs of kidney tissue damage score in five experimental groups (see text for groups details). No damage was seen in groups 4 and 5. The tissue damage in group 1 is less than that in groups 2 and 3.
4.3. Serum and kidney nitrite and MDA
The serum nitrite level in group 2 was lower than that in other groups. However, no significant changes were observed in serum levels of nitrite and MDA and kidney tissue MDA in all experimental groups. Tissue levels of nitrite significantly decreased in the positive control and high dose of PFE plus CP-treated groups (p<0.05). Among CP-treated groups, low dose of PFE significantly improved kidney nitrite level (p<0.05; Table 1).
Table 1 . Serum and kidney level of malondialdehyde (MDA) and nitrite (NO) .
| Group | Serum nitrite (µmole/L) | Kidney nitrite (µmole/g tissue) | Serum MDA (µmole/L) |
Kidney MDA
(nmole/g tissue) |
| CP +PFE 25 (mg/kg) | 16.60 ± 4.98 | 0.23 ± 0.07*# | 12.07 ± 8.65 | 4.53 ± 1.64 |
| CP + PFE 50 (mg/kg) | 8.23 ± 2.75 | 0.13 ±0.03 | 15.40 ± 8.21 | 3.46 ± 2.25 |
| CP | 18.58 ± 12.08 | 0.12 ± 0.05 | 11.58 ± 10.59 | 4.40± 0.74 |
| PFE 25 (mg/kg) | 17.55 ± 6.99 | 0.23 ± 0.11* | 11.47 ± 9.21 | 3.52 ± 1.77 |
| PFE 50 (mg/kg) | 14.83 ± 3.68 | 0.26 ± 0.08*# | 14.11 ± 5.30 | 5.41 ± 0.91 |
(*) and (#) indicate significant difference (p<0.05) when compared with the positive control and the PFE 50+CP groups, respectively
5. Discussion
The aim of this study was to evaluate the protective effect of PFE against CP-induced renal toxicity. We found that low dose of PFE could ameliorate CP-induced kidney toxicity. CP injection induces kidney tissue damage in animals (15,16), and also increases the serum levels of BUN and Cr, and KW (17,18). CP also affects the serum level of MDA (19). Body weight loss in CP-treated group occurs following the gastrointestinal disturbance (20,21). It has been reported that administration of high dose of pomegranate fruit extract decreased body weight (22). This possibly indicates that tannins in pomegranate juice interacts with proteins, inhibit protein digestion (23). In contrast, the KW gain was observed in CP-treated groups. It is reported that KW increased in CP-treated animal, and it has a direct relationship with intensity of tissue damage (6). The low dose of PFE plus CP led to reduction in KW and KTDS whereas high dose of PFE with CP increased KW. Possibly the protective effect of PFE in low dose was attributed to its antioxidant properties (24). CP also decreased the level of nitrite in kidney tissue. NO, as a marker of endothelial function, plays a critical role in endothelial and NO is synthesized from the amino acid L-arginine by the endothelial NO synthase (eNOS) in endothelium. CP can disturb endothelium and endothelial function followed by reducing NO (3,25). Accordingly, the increase of kidney nitrite level by low dose of PFE has improved kidney NO to protect the kidney from endothelial dysfunction. Administration of PFE in low dose also ameliorated KTDS induced by CP. It has been shown that hydroalcoholic extract of pomegranate flowers can potentially improve recovery of kidney function that may be related to NO-dependent signaling pathway (26). We did not observed protective role of PFE in 50 mg/kg against CP-induced toxicity in the current animal model. It seems that it was related to the antioxidant dose, because high doses of some antioxidants do not have a protective effect, and can exacerbate tissue damage (27,28), while, other antioxidants such as Morus alba L. leaf extracts had protective role against CP induced nephrotoxicty (6). Although we did not observed significant difference in serum MDA levels between the groups, but according to other parameters such as Cr, KW and KTDS, the PFE in 25 mg/kg act as antioxidant against CP-induced nephrotoxicity. Therefore, similar study to investigate PFE in doses lower than 25 mg/kg is suggested.
6. Conclusions
The low dose of PFE (25 mg/kg) showed protective effects and ameliorated CP-induced nephrotoxicity through its antioxidant effects.
Authors’ contributions
All authors wrote the manuscript equally.
Conflict of interests
The authors declared no competing interests.
Funding/Support
This research was supported by Isfahan University of Medical Sciences.
Please cite this paper as: Motamedi F, Nematbakhsh M, Monajemi R, Pezeshki Z, Talebi A, Zolfaghari B, et al. Effect of pomegranate flower extract on cisplatin-induced nephrotoxicity in rats. J Nephropathol 2014; 3(4):133-138. DOI: 10.12860/jnp.2014.26
References
- 1.Antunes LM, Darin JD, Bianchi MD. Protective effects of vitamin c against cisplatin-induced nephrotoxicity and lipid peroxidation in adult rats: a dose-dependent study. Pharmacol Res. 2000;41(4):405–11. doi: 10.1006/phrs.1999.0600. [DOI] [PubMed] [Google Scholar]
- 2.Miller RP, Tadagavadi RK, Ramesh G, Reeves WB. Mechanisms of Cisplatin nephrotoxicity. Toxins (Basel) 2010;2(11):2490–518. doi: 10.3390/toxins2112490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eshraghi-Jazi F, Nematbakhsh M, Nasri H, Talebi A, Haghighi M, Pezeshki Z. et al. The protective role of endogenous nitric oxide donor (L-arginine) in cisplatin-induced nephrotoxicity: Gender related differences in rat model. J Res Med Sci. 2011;16(11):1389–96. [PMC free article] [PubMed] [Google Scholar]
- 4.Haghighi M, Nematbakhsh M, Talebi A, Nasri H, Ashrafi F, Roshanaei K. et al. The role of angiotensin II receptor 1 (AT1) blockade in cisplatin-induced nephrotoxicity in rats: gender-related differences. Ren Fail. 2012;34(8):1046–51. doi: 10.3109/0886022X.2012.700886. [DOI] [PubMed] [Google Scholar]
- 5.Pezeshki Z, Nematbakhsh M, Mazaheri S, Eshraghi-Jazi F, Talebi A, Nasri H. et al. Estrogen Abolishes Protective Effect of Erythropoietin against Cisplatin-Induced Nephrotoxicity in Ovariectomized Rats. ISRN Oncol. 2012;2012:890310. doi: 10.5402/2012/890310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nematbakhsh M, Hajhashemi V, Ghannadi A, Talebi A, Nikahd M. Protective effects of the Morus alba Lleaf extracts on cisplatin-induced nephrotoxicity in rat. Res Pharm Sci. 2013;8(2):71–7. [PMC free article] [PubMed] [Google Scholar]
- 7.Jadon G, Nainwani R, Singh D, Soni PK, Diwaker A. Antioxidant activity of various parts of punica granatum: a review. Journal of Drug Delivery and Therapeutics. 2012;2(6):138–41. [Google Scholar]
- 8.Sahu SC, Washington MC. Effects of antioxidants on quercetin-induced nuclear DNA damage and lipid peroxidation. Cancer Lett. 1991;60(3):259–64. doi: 10.1016/0304-3835(91)90122-x. [DOI] [PubMed] [Google Scholar]
- 9.Dekanski D, Janićijević-Hudomal S, Tadić V, Marković G, Arsić I, Mitrović DM. Phytochemical analysis and gastroprotective activity of an olive leaf extract. Journal of the Serbian Chemical Society. 2009;74(4):367–77. [Google Scholar]
- 10. Heber D, Schulman RN, Seeram NP. Pomegranates: ancient roots to modern medicine. CRC press; 2006.
- 11.Hollebeeck S, Winand J, Herent MF, During A, Leclercq J, Larondelle Y. et al. Anti-inflammatory effects of pomegranate (Punica granatum L) husk ellagitannins in Caco-2 cells, an in vitro model of human intestine. Food Funct. 2012;3(8):875–85. doi: 10.1039/c2fo10258g. [DOI] [PubMed] [Google Scholar]
- 12.Asgary S, Sahebkar A, Afshani M, Keshvari M, Haghjooyjavanmard S, Rafieian-Kopaei M. Clinical evaluation of blood pressure lowering, endothelial function improving, hypolipidemic and anti-inflammatory effects of pomegranate juice in hypertensive subjects. Phytother Res. 2014;28(2):193–9. doi: 10.1002/ptr.4977. [DOI] [PubMed] [Google Scholar]
- 13.Sadeeshkumar V, Arul D, Ravichandran S. Protective effects of gallic acid in the renal markers, histopathology and immunohistochemical studies on vancomycin induced nephrotoxic rats. International Journal of Advanced Life Sciences. 2013;6(3):356–65. [Google Scholar]
- 14.Cekmen M, Otunctemur A, Ozbek E, Cakir SS, Dursun M, Polat EC. et al. Pomegranate extract attenuates gentamicin-induced nephrotoxicity in rats by reducing oxidative stress. Ren Fail. 2013;35(2):268–74. doi: 10.3109/0886022X.2012.743859. [DOI] [PubMed] [Google Scholar]
- 15.Stakisaitis D, Dudeniene G, Jankūnas RJ, Grazeliene G, Didziapetriene J, Pundziene B. Cisplatin increases urinary sodium excretion in rats: gender-related differences. Medicina (Kaunas) 2009;46(1):45–50. [PubMed] [Google Scholar]
- 16.Deegan PM, Nolan C, Ryan MP, Basinger MA, Jones MM, Hande KR. The role of the renin-angiotensin system in cisplatin nephrotoxicity. Ren Fail. 1995;17(6):665–74. doi: 10.3109/08860229509037634. [DOI] [PubMed] [Google Scholar]
- 17.Nematbakhsh M, Ashrafi F, Nasri H, Talebi A, Pezeshki Z, Eshraghi F. et al. A model for prediction of cisplatin induced nephrotoxicity by kidney weight in experimental rats. J Res Med Sci. 2013;18(5):370–3. [PMC free article] [PubMed] [Google Scholar]
- 18.Saleh S, El-Demerdash E. Protective effects of L-arginine against cisplatin-induced renal oxidative stress and toxicity: role of nitric oxide. Basic Clin Pharmacol Toxicol. 2005;97(2):91–7. doi: 10.1111/j.1742-7843.2005.pto_114.x. [DOI] [PubMed] [Google Scholar]
- 19.Yildirim Z, Sogut S, Odaci E, Iraz M, Ozyurt H, Kotuk M. et al. Oral erdosteine administration attenuates cisplatin-induced renal tubular damage in rats. Pharmacol Res. 2003;47(2):149–56. doi: 10.1016/s1043-6618(02)00282-7. [DOI] [PubMed] [Google Scholar]
- 20.Chang B, Nishikawa M, Sato E, Utsumi K, Inoue M. L-Carnitine inhibits cisplatin-induced injury of the kidney and small intestine. Arch Biochem Biophys. 2002;405(1):55–64. doi: 10.1016/s0003-9861(02)00342-9. [DOI] [PubMed] [Google Scholar]
- 21.Bearcroft CP, Domizio P, Mourad FH, Andre EA, Farthing MJ. Cisplatin impairs fluid and electrolyte absorption in rat small intestine: a role for 5-hydroxytryptamine. Gut. 1999;44(2):174–9. doi: 10.1136/gut.44.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel C, Dadhaniya P, Hingorani L, Soni M. Safety assessment of pomegranate fruit extract: Acute and subchronic toxicity studies. Food Chem Toxicol. 2008;46(8):2728–35. doi: 10.1016/j.fct.2008.04.035. [DOI] [PubMed] [Google Scholar]
- 23. Butler LG, Rogler JC, editors. Biochemical mechanisms of the antinutritional effects of tannins. ACS Symposium series; 1992: ACS Publications.
- 24.Kaur G, Jabbar Z, Athar M, Alam MS. Punica granatum (pomegranate) flower extract possesses potent antioxidant activity and abrogates Fe-NTA induced hepatotoxicity in mice. Food Chem Toxicol. 2006;44(7):984–93. doi: 10.1016/j.fct.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 25.Dursun B, He Z, Somerset H, Oh DJ, Faubel S, Edelstein CL. Caspases and calpain are independent mediators of cisplatin-induced endothelial cell necrosis. Am J Physiol Renal Physiol. 2006;291(3):F578–87. doi: 10.1152/ajprenal.00455.2005. [DOI] [PubMed] [Google Scholar]
- 26.Singh AP, Singh AJ, Singh N. Pharmacological investigations of Punica granatum in glycerol-induced acute renal failure in rats. Indian J Pharmacol. 2011;43(5):551. doi: 10.4103/0253-7613.84971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Azarkish F, Nematbakhsh M, Fazilati M, Talebi A, Pilehvarian AA, Pezeshki Z. et al. N-acetylcysteine Prevents Kidney and Lung Disturbances in Renal Ischemia/Reperfusion Injury in Rat. Int J Prev Med. 2013;4(10):1139. [PMC free article] [PubMed] [Google Scholar]
- 28.Aruoma OI, Halliwell B, Hoey BM, Butler J. The antioxidant action of N-acetylcysteine: its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic Biol Med. 1989;6(6):593–7. doi: 10.1016/0891-5849(89)90066-x. [DOI] [PubMed] [Google Scholar]


