Abstract
BACKGROUND
American Indians/Alaskan Natives (AI/ANs) have the worst 5-year cancer survival of all racial/ethnic groups in the United States. Causes for this disparity are unknown. The authors of this report examined the receipt of cancer treatment among AI/AN patients compared with white patients.
METHODS
This was a retrospective cohort study of 338,204 patients who were diagnosed at age ≥65 years with breast, colon, lung, or prostate cancer between 1996 and 2005 in the Surveillance, Epidemiology, and End Results-Medicare database. Nationally accepted guidelines for surgical and adjuvant therapy and surveillance were selected as metrics of optimal, guideline-concordant care. Treatment analyses compared AI/ANs with matched whites.
RESULTS
Across cancer types, AI/ANs were less likely to receive optimal cancer treatment and were less likely to undergo surgery (P ≤ .025 for all cancers). Adjuvant therapy rates were significantly lower for AI/AN patients with breast cancer (P <.001) and colon cancer (P = .001). Rates of post-treatment surveillance also were lower among AI/ANs and were statistically significantly lower for AI/AN patients with breast cancer (P = .002) and prostate cancer (P <.001). Nonreceipt of optimal cancer treatment was associated with significantly worse survival across cancer types. Disease-specific survival for those who did not undergo surgery was significantly lower for patients with breast cancer (hazard ratio [HR], 0.62), colon cancer (HR, 0.74), prostate cancer (HR, 0.52), and lung cancer (HR, 0.36). Survival rates also were significantly lower for those patients who did not receive adjuvant therapy for breast cancer (HR, 0.56), colon cancer (HR, 0.59), or prostate cancer (HR, 0.81; all 95% confidence intervals were <1.0).
CONCLUSIONS
Fewer AI/AN patients than white patients received guideline-concordant cancer treatment across the 4 most common cancers. Efforts to explain these differences are critical to improving cancer care and survival for AI/AN patients.
Keywords: American Indian, Alaskan Native, cancer, guidelines, treatment
INTRODUCTION
American Indian/Alaskan Native (AI/AN) patients with cancer tend to present at more advanced stages of cancer and exhibit the worst 5-year cancer survival of all racial/ethnic groups in the United States.1 The Centers for Disease Control and Prevention’s analysis of cancer death rates from 1975 to 2004 demonstrated declining cancer death rates for all racial/ethnic groups except for AI/ANs.2 Previous research on AI/AN patients with cancer is limited, because this group has largely been evaluated only as part of a broader minority cohort. Therefore, factors underlying the lower cancer survival rates of AI/ANs have not been well explained.
Worse survival among AI/ANs may stem from nonreceipt of recommended cancer treatments. A previous study that assessed concordance with cancer treatment guidelines among Medicare beneficiaries indicated that receipt of guideline-concordant cancer care varied depending on patient age, race, cancer type, and geographic region.3 Accepted practice guidelines for most cancers are regularly updated by the National Comprehensive Cancer Network (NCCN), the National Quality Forum (NQF), and the National Cancer Institute (NCI). In this study, our group, the Collaborative to Improve Native Cancer Outcomes (CINCO) identified 22 nationally endorsed practice guidelines for the 4 most common cancers (breast, colon, prostate, and lung).
We used the Surveillance, Epidemiology, and End Results (SEER)–Medicare-linked registry, a population-based cancer data set that collects data on all incident cancer cases in various areas across the United States, capturing nearly 26% of the US population, including 42% of the AI/AN population.4 Although the AI/AN population represented in SEER is concentrated in New Mexico, the Pacific Northwest, and California, the SEER data set offers the most comprehensive examination of cancer incidence and treatment in AI/AN populations across the United States.5,6 Another benefit of the SEER-Medicare data set is its linkage to Indian Health Service (HIS) beneficiary records, which help to optimize the correct race classification of AI/ANs.7
We hypothesized that AI/AN patients would be less likely to receive optimal (or guideline-concordant) surgical and adjuvant cancer treatment and were less likely to undergo recommended post-therapy surveillance. We also hypothesized that nonreceipt of optimal care would be associated with worse survival. To test these hypotheses, we assessed rates of adherence to each guideline for eligible AI/AN patients and white patients. Our objective was to determine whether disparities exist in the receipt of guideline-concordant treatment for 4 major cancers among AI/AN patients. In addition, we examined the association between receipt of optimal cancer care and disease-specific survival.
MATERIALS AND METHODS
Data Source
We used the National Cancer Institute (NCI) SEER cancer registry linked to Medicare enrollment and claims data for this study. Individuals are classified as AI/AN if they have a reservation residence, if they have evidence of medical coverage through the IHS, or if their medical record indicates AI/AN race. Data collected in the registries include patient demographics, primary tumor site, stage at diagnosis, first course of treatment, and follow-up for vital status. For those aged ≥65 years, 97% are eligible for Medicare, and 93% of patients in the SEER files are matched to the Medicare enrollment file.8 This study included all Medicare-eligible patients with incident cases in the SEER database from 1996 through 2005 and Medicare claims follow-up through 2007.
Patient Eligibility
Patients were included in this study if they were diagnosed with 1 of the following 4 primary cancers: breast, colon, lung, or prostate. In total, 1032,605 patients were identified with these incident cancer diagnoses. Patients were excluded for the following reasons: cancer diagnosed before age 65 years or before the year 1996 (n = 267,423), in situ disease (Tis) or metastatic disease (TxNxM1) at diagnosis (n = 268,453), and information abstracted from autopsy/death certificates or nursing/hospice records (n = 28,881). To ensure complete claims data, we required continuous enrollment in both Medicare Parts A and B with no health maintenance organization coverage during the study period defined for each metric (excluded, n = 120,830). Charlson comorbidity index (CCI) scores were calculated using the methods outlined by Deyo et al.9 After exclusion criteria were applied, there were 338,204 patients who formed our study cohort. After diagnosis, patients were followed until death or until the last date for which Medicare claims data were available.
Defining Optimal Care
We selected nationally recognized, cancer-specific guidelines that address surgical therapy, adjuvant therapy, and post-treatment surveillance as metrics for defining optimal care for breast, lung, prostate, and colon cancer. Each was selected on the basis of it being a recommendation in the NCCN Clinical Practice Guidelines in Oncology categorized as either level 1(high-level evidence with uniform expert consensus), level 2a (lower level evidence with uniform expert consensus), level 2b (lower level evidence with expert consensus), or a guideline of the NCI or NQF. These 22 measures and their referenced sources are detailed in Table 1: Metric 1 includes surgical guidelines, Metric 2 includes adjuvant therapy guidelines, and Metric 3 includes post-therapy surveillance guidelines. Flow diagrams detailing the exclusionary and inclusionary criteria for each metric are presented elsewhere (see the online supporting information). Current (as opposed to historic) guidelines were used because they were publicly and readily available. In addition, all of the NCCN guidelines we selected for study, with the exception of androgen-deprivation therapy for prostate cancer, mirrored NCCN treatment guidelines published during the years of our study. Medicare claims codes, including International Classification of Diseases ninth edition (ICD-9), Current Procedure Terminology (CPT), and SEER codes, are provided in the online supporting information.
TABLE 1.
Definitions of Optimal Treatment
| Metric | Definition |
|---|---|
| Breast cancer | |
| Metric 1 | Axillary lymph node evaluation (sentinel lymph node biopsy or axillary dissection) for patients with stage 1–3 cancer who underwent any breast surgeries (breast conserving or mastectomy) within 12 mo after diagnosis (National Comprehensive Cancer Network [NCCN] 3.2013, BINV-D) |
| Metric 2a | Receipt of radiation (RT) after breast-conserving surgery for stage 1–3 invasive breast cancer (excluding patients who underwent mastectomy within 6 m after breast-conserving surgery; NQF 0219; NCCN 3.2013) |
| Metric 2b | Receipt of chemotherapy after any breast surgery for stage 1–3 invasive breast cancer, tumor >1 cm, and estrogen receptor-negative, applying only to patients aged <70 years (NQF 0559; NCCN 3.2013, BINV-8) |
| Metric 3 | Mammogram every 12 mo for 5 y after the first breast-conserving surgery (NCCN 3.2013, BINV-16) |
| Colon cancer | |
| Metric 1 | Colectomy with lymphadenectomy for stage 1–3 (resectable cancer; NCCN 3.2013, COL-2) |
| Metric 2a | Adjuvant chemotherapy for Stage 3 colon cancer (NQF, 0385; NCCN 3.2013, COL-4) |
| Metric 2b | Initiation of adjuvant chemotherapy within 3 mo of surgery date (NQF, 0223; for patients aged <80 y) |
| Metric 3 | Colonoscopy within 12 mo after resection for stage I–III cancer (NCCN 3.2013, COL-3 and COL-4) |
| Prostate cancer | |
| Metric 1 | Primary therapies are prostatectomy, EBRT, brachytherapy (alone or combined with EBRT), and active surveillance |
| Metric 1a | Stage 1–2, low grade (SEER grade 1 [well differentiated]): Active surveillance, surgery, EBRT, brachytherapy (NCCN 4.2013, PROS-2) |
| Metric 1b | Stage 1–2, intermediate grade (SEER grade 2 [moderately differentiated]): Surgery, EBRT, EBRT plus brachytherapy, EBRT plus ADT, EBRT with brachytherapy plus ADT (NCCN 4.2013, PROS-3) |
| Metric 1c | Stage 1–2, high grade: Surgery, EBRT plus ADT, EBRT with brachytherapy plus ADT (NCCN 4.2013, PROS-4) |
| Metric 1d | Stage 3, any grade: Surgery, EBRT plus ADT, EBRT with brachytherapy plus ADT (NCCN 4.2013, PROS-4) |
| Metric 2 | Any ADT or primary EBRT for high-grade tumors (SEER grade 3 and 4 [poorly differentiated, or undifferentiated, or anaplastic), during/near RT (NCCN 4.2013, PROS-4) |
| Metric 3 | At least 1 PSA every 12 mo for 5 y after the first primary treatment (surgeries, or EBRT, or brachytherapy) or after the first active surveillance period (from diagnosis to 12 mo) if no treatment (NCCN 4.2013, PROS-5) |
| Lung cancer | |
| Metric 1a | Nonsmall cell lung cancer stage 1–2: Surgery (lobectomy with mediastinal lymph node dissection; NCCN 2.2013, NSCL-3; NCI PDQ) |
| Metric 1b | Nonsmall cell lung cancer stage 3a: Neoadjuvant chemoradiation therapy followed by surgery (lobectomy plus mediastinal lymph node dissection; NCCN 2.2013, NSCL-7) |
| Metric 1c | Small cell lung cancer: No role for surgery; stage 3 other than 3a (such as stages 3b, 3c, 3 NOS, …): no role for surgery (NCCN 1.2014, SCL-A) |
| Metric 2a | No adjuvant therapy role for stage 1 disease (NCI PDQ) |
| Metric 2b | Adjuvant chemotherapy or RT for nonsmall cell lung cancer stage 2 (NCCN 2.2013, NSCL-3) |
| Metric 2c | Adjuvant RT for nonsmall cell lung cancer stage 3a (NCCN 2.2013, NSCL-3) |
| Metric 3 | Surveillance starts from 2 mo after completion of first treatment (treatments include surgeries for patients who undergo surgeries or first chemotherapy or RT for chemoradiation-only patients); the surveillance will be CT scans of the chest every 12 mo for 5 y (NCCN 2.2013, NSCL-13) |
Abbreviations: ADT, androgen-deprivation therapy; BINV, NCCN invasive breast cancer guideline; CT, computed tomography; EBRT, external-beam radiation therapy; NCCN 2.2013, NCCN guidelines, version 2.2013; NCCN 4.2013, NCCN guidelines, version 4.2013; NCI PDQ, National Cancer Institute Physician Data Query; NOS, not otherwise specified; NQF, National Quality Forum; NSCL, NCCN nonsmall cell lung cancer guideline; PROS, NCCN prostate cancer guideline; PSA, prostate-specific antigen; SCL, NCCN small cell lung cancer guideline; SEER, Surveillance, Epidemiology, and End Results Program.
Unadjusted rates for the receipt of surgical and adjuvant therapies (Metrics 1 and 2) were calculated as the proportion of patients that received a therapy among those who were eligible. The rate for cancer surveillance (Metric 3) was calculated for each patient as the ratio of the number of surveilled 12-month post-therapy periods (defined as receipt of at least 1 screening test during the 12-month period post-therapy) divided by the number of 12-month post-therapy periods available for analysis. Analyses ended as soon as 1 of the following events occurred: the date of death; the follow-up cutoff date of December 31, 2006; or at 5 years postoperatively.
Statistical Analysis
A direct comparison of optimal care receipt by AI/ANs and whites was not appropriate because of the extremely unbalanced sizes of the groups (eg, colon cancer: 194 AI/ANs vs 55,268 whites). Because of this imbalance and to mitigate the confounding effects of the demographic and clinical differences between AI/ANs and whites, as presented in Table 2, we developed a cohort of white patients who were matched to AI/AN patients based on sex, age at diagnosis, CCI, SEER registry geographic site, diagnosis year, and cancer stage at diagnosis. A random sample of matched whites was then drawn at a ratio of 10:1 for each matching pair with AI/ANs. When comparing sampled whites with AI/ANs, the chi-square test was used to test the significance of associations between race and categorical metric outcomes, and the Student t test was used to evaluate associations between race and continuous metric outcomes. To reduce bias because of the results from a single random sample, a subsampling method (m of n without replacement bootstrap) was applied.10,11 One thousand iterations (resampling plus testing) were run for each cancer metric. P values presented in Table 3 were the optimal results summarized from all iterations.10 To compare the likelihood of receiving optimal care by race using adjusted odds ratios, we conducted standard multivariate logistic regression modeling for Metrics 1 and 2 on the cohort of AI/ANs and matched whites. Covariates adjusted for in our analyses included race, age, SEER registry site/geographic region, diagnosis year, cancer stage, and CCI.
TABLE 2.
Cohort Demographics and Diagnoses
| Race | Sample Size: No. (%) | Women, % | Age at Diagnosis, %
|
Disease Stage, %
|
CCI Score 6 Mo Before Diagnosis, %
|
|||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 65–69 Y | 70–74 Y | 75–79 Y | ≥80 Years | I | II | III | 0 | 1 | ≥2 | |||
| Breast cancer | ||||||||||||
| AI/AN | 216 (0.24) | 100 | 25.9 | 25.9 | 24.1 | 24.1 | 50.9 | 39.8 | 9.3 | 61.5 | 23.5 | 15 |
| White | 76,185 (86.1) | 99.1 | 23.1 | 24.1 | 23.9 | 29 | 57.2 | 35.8 | 7 | 74.5 | 18.3 | 7.2 |
| Colon cancer | ||||||||||||
| AI/AN | 211 (0.3) | 61.6 | 22.8 | 27.5 | 20.9 | 28.9 | 25.6 | 42.2 | 32.2 | 53.1 | 26.6 | 20.3 |
| White | 58,318 (83) | 55.2 | 16.3 | 20.4 | 23.5 | 39.8 | 30.2 | 40.4 | 29.4 | 57 | 26.8 | 16.2 |
| Prostate cancer | ||||||||||||
| AI/AN | 279 (0.27) | 0 | 42.3 | 26.5 | 20.4 | 10.8 | 40.1 | 44.1 | 15.8 | 68.4 | 20.5 | 11.1 |
| White | 80,654 (78.1) | 0 | 31.5 | 30.2 | 22.8 | 15.5 | 48.3 | 41.5 | 10.2 | 76.7 | 16.4 | 6.9 |
| Lung cancer | ||||||||||||
| AI/AN | 211 (0.28) | 44.1 | 29.4 | 24.6 | 23.7 | 22.3 | 36 | 9.5 | 54.5 | 33 | 39.6 | 27.4 |
| White | 64,240 (84.4) | 48 | 23.5 | 26.7 | 25.3 | 24.6 | 41.2 | 6.7 | 52.1 | 40.4 | 37.3 | 22.4 |
Abbreviations: AI/AN, American Indian/Alaskan Native; CCI, Charlson comorbidity index.
TABLE 3.
Adjusted Associations of Optimal Care Measures With Race
| Race | Metric 1: Surgery/Primary Care
|
Metric 2: Adjuvant Therapy
|
Metric 3: Post-Therapy Surveillance
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Matched Cohort
|
Pa | Matched Cohort
|
Pa | Matched Cohort
|
Pa | ||||
| No. | Treatment Rate,% | No. | Treatment Rate,% | No. | Treatment Rate,% | ||||
| Breast cancer | |||||||||
| AI/AN | 206 | 79.1 | .010 | 102 | 61.8 | < .001 | 92 | 64 | .002 |
| White | 8052 | 85.7 | 5038 | 82.2 | 4647 | 76.4 | |||
| Colon cancer | |||||||||
| AI/AN | 194 | 87.1 | .002 | 42 | 45.2 | .001 | 164 | 55.5 | .341 |
| White | 2012 | 94.2 | 306 | 72.9 | 1875 | 57.1 | |||
| Prostate cancerb | |||||||||
| AI/AN | 250 | 39.2 | <.001 | NAc | 10.4 | .756 | 213 | 57.8 | <.001 |
| White | 11,119 | 60 | 1264 | 12.1 | 10,131 | 81.4 | |||
| Lung cancer | |||||||||
| AI/AN | 95 | 26.3 | .025 | NAc | 41.7 | .576 | 129 | 49.6 | .223 |
| White | 1128 | 40 | 132 | 30.3 | 1735 | 55 | |||
Abbreviations: AI/AN, American Indian/Alaskan Native; NA, not available.
P values were based on 1000 bootstrapping iterations.
No sex variable was used for prostate cancer.
Information in this cell is masked to comply with privacy regulations.
To analyze the association between receipt of optimal care and cancer-specific survival, and because of the nonproportional hazards indication by the Schoenfeld residuals12 of Metric1 for all 4 cancers and Metric2 for breast cancer and colon cancer, we performed the extended Cox regression modeling, which could incorporate the time-dependent covariates and allowed nonproportional hazards.12,13 Patients who died from other causes were excluded. Survival was calculated from the date of diagnosis to the date of either death or last follow-up. Metrics 1 and 2 were set as the time-dependent covariates in their corresponding extended Cox models, because their values changed over the course of observation after the diagnosis date. The models were fitted on the entire unmatched cohort, and the estimates of the overall adjusted hazard ratios associated with receipt of Metrics 1 and 2 were obtained.13,14 The models adjusted for age at diagnosis, race, diagnosis year, cancer stage, CCI, and SEER registry site.
Because analyses comparing AI/ANs and whites were based on stringent matching criteria with resultant smaller white populations, we conducted a sensitivity analysis using a less stringent matching strategy (broader categorization by SEER geographic region vs individual registry site and broader categories of diagnosis years). In this way, we expanded the matched white population to ensure that our comparisons remained valid. All analyses in this study were conducted using the statistical software package SAS 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
Demographic and Clinical Characteristics
Demographics
Across all 4 cancers, fewer AI/ANs presented with stage I disease and higher percentages presented with locally advanced, or stage III, disease compared with white patients (Table 2). In addition, AI/AN patients tended to present at younger ages (eg 22.8% of AI/ANs vs 16.3% of whites were diagnosed with colon cancer at ages 65–69 years). AI/AN patients also tended to have a greater burden of comorbid disease, reflected by a higher CCI at the time of diagnosis.
Receipt of optimal care
Across the entire unmatched cohort, AI/ANs were less likely than whites to receive primary surgical therapy or to undergo recommended post-therapy surveillance across all cancer types (data not shown). AI/AN patients were also less likely than whites to receive adjuvant chemotherapy or radiation therapy for breast, colon, or prostate cancer. However, AI/ANs appeared to be more likely to receive adjuvant therapy for lung cancer.
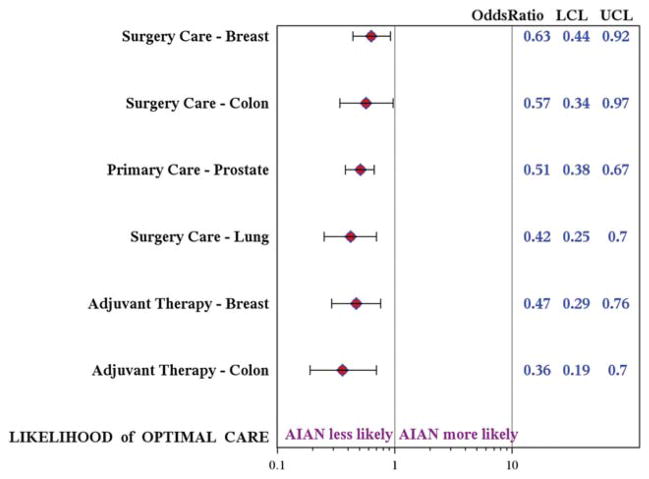
In adjusted analyses across all 4 cancers, AI/AN patients were significantly less likely than white patients to receive guideline-concordant care (Table 3). AI/AN patients were consistently less likely to undergo curative surgical resection (P ≤ .025 for all cancers) and were less likely to receive recommended adjuvant therapies for breast cancer (P <.001) and colon cancer (P = .001). AI/AN patients had 37% to 58% lower odds of undergoing surgery (Fig. 1), a disparity that was significant across cancer types. AI/AN patients also had 53% and 64% lower odds of receiving adjuvant therapy for breast cancer (odds ratio [OR], 0.47; 95% confidence interval [CI], 0.29–0.76) and colon cancer (OR, 0.36; 95%CI, 0.19–0.70), respectively. The rates at which patients received adjuvant therapy for prostate and lung cancer did not differ significantly, which may be attributable to the very small population eligible for this metric. Rates of post-therapy surveillance also were lower among AI/AN patients across cancer types, although the difference was significant only for breast cancer (P = .002) and prostate cancer (P <.001). Similar significant results were obtained from our sensitivity analysis.
Figure 1.
The likelihood of receiving optimal care among American Indians/Alaskan Natives (AI/ANs) compared with whites is illustrated. Multivariate logistic regression modeling is shown for Metric 1 (surgery/primary care) and Metric 2 (adjuvant therapy) for each cancer. Covariates included race; age at diagnosis; Surveillance, Epidemiology, and End Results registry site; year of diagnosis; cancer stage; and Charlson comorbidity index. LCL indicates lower control limit; UCL, upper control limit.
Survival and Receipt of Optimal Care
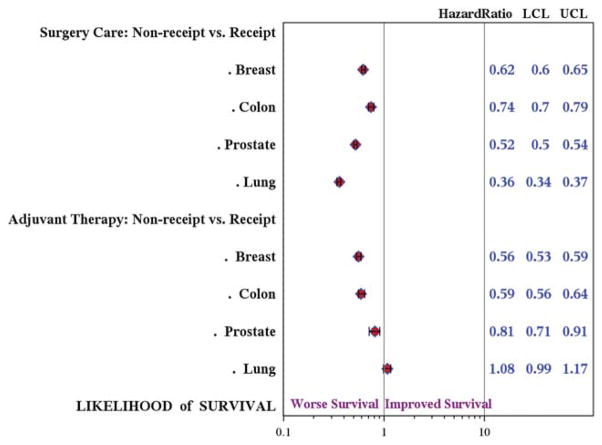
The median follow-up was 41 to 58 months for breast, colon, and prostate cancers and 14 months for lung cancer. Race was not significantly associated with survival once other demographic and disease variables were controlled for. Therefore, hazard ratios (HRs) for cancer-specific survival associated with nonreceipt of Metrics 1 and 2 were calculated using the entire cohort of AI/ANs and (unmatched/all) whites combined (Fig. 2). The overall likelihood of survival was 38%, 26%, 48%, and 64% lower among the patients who did not receive Metric 1 therapy for breast, colon, prostate, and lung cancer, respectively, compared with those who did undergo surgery (upper 95% CI, all <1.0). The overall likelihood of survival also was significantly lower for the patients with breast cancer (HR, 0.56; 95% CI, 0.53–0.59), colon cancer (HR, 0.59; 95% CI, 0.56–0.64), and prostate cancer (HR, 0.81; 95% CI, 0.71–0.91) who did not receive Metric 2 therapy. No significant association was observed between receipt of adjuvant therapy and lung cancer survival.
Figure 2.
The association between receipt of optimal care and survival is illustrated. Cox proportional hazards regression modeling was used to estimate the adjusted hazard ratios associated with receipt of Metric 1 (surgery/primary care) and Metric 2 (adjuvant therapy). The models were adjusted for race; age at diagnosis; Surveillance, Epidemiology, and End Results registry site; year of diagnosis; cancer stage; and Charlson Comorbidity Index. LCL indicates lower control limit; UCL, upper control limit.
Next, we assessed whether the interaction between race and optimal care had an important impact on the likelihood of survival for Metrics 1 (surgery) and 2 (adjuvant therapy) in the matched cohort. The interaction coefficient was not significant at the test criterion α = .05 level, which indicates that AI/ANs and whites have a similar likelihood of survival after controlling for receipt of treatment, demographics, and disease features.
DISCUSSION
Ours is the first study to explore lower survival rates among AI/AN patients with cancer by assessing whether disparities exist in the receipt of guideline-concordant cancer treatment. We observed that AI/AN patients were less likely to receive optimal, guideline-concordant care compared with whites. Rates of primary surgical therapy for the 4 most common cancers (breast, colon, prostate, and lung) were significantly lower among AI/ANs compared with matched white patients. Rates of adjuvant therapy also were lower among AI/AN patients with breast cancer and colon cancer. Given the very small sample size of patients evaluable for receipt of adjuvant therapy for prostate cancer and lung cancer, the lack of significant differences between racial groups is not surprising. Surveillance test rates after treatment for breast or prostate cancer (ie, screening mammogram, prostate-specific antigen) among AI/ANs lagged far behind whites. However, rates of surveillance with screening colonoscopy after colon cancer treatment were low across racial groups.
The entire AI/AN population comprises only about 1.7% of the US population, making it a challenge to conduct rigorous studies of either processes or outcomes of care among AI/ANs, particularly using single data sets.15 The NCI-funded SEER registry includes data on 42% of AI/ANs living in the United States. Prior studies of disparities in cancer treatment have been limited by AI/AN race misclassification and small sample size. Consequently, to date, AI/ANs have been categorized broadly in the context of “other” minorities, and data specific to AI/ANs are scarce. To mitigate the problem of race misclassification, the IHS, NCI, Centers for Disease Control and Prevention, and all SEER registries collaborated in 1999 to link IHS beneficiary records with cancer registry databases, which has led to a greatly improved process for identifying AI/AN race in the SEER registry.7,16–19
Among racial groups, AI/ANs have the lowest survival rates for cancers of the breast, lung, and prostate and for all cancers combined.20 Higher cancer mortality rates among AI/AN patients may relate to a more advanced stage of cancer at presentation, which we observed in this study. An earlier study by Espey et al also demonstrated that AI/ANs present with more advanced stages of cancer compared with white patients who have breast, prostate, and colon cancers.2 A more advanced stage of cancer at diagnosis and reduced rates of surgical resection and chemotherapy have been linked to survival disparities among black and Hispanic patients with lung and colorectal cancers.21–23 Another SEER-based study conducted between 1988 and 2006 indicated that AI/ANs with stage I through III nonsmall cell lung cancer had rates of surgical resection that were significantly lower than the rates among whites.24 In addition, 5-year lung cancer-specific survival was 47% for AI/ANs versus 56% for whites (P <.0001). Not surprisingly, we demonstrated that survival was lower among those patients with cancer who did not receive guideline-concordant therapy. Importantly, we demonstrated that if optimal treatment was received, and disease stage and demographics controlled for, disease-specific survival of AI/ANs and whites did not significantly differ.
Potential etiologies for lower rates of guideline-concordant cancer therapy and surveillance among AI/ANs likely include systems-based, provider-based, and patient-based factors. A larger percentage of AI/ANs (42% vs 23% whites) reside in rural areas with presumably lesser access to cancer specialists.15 The IHS hospitals and clinics are based on reservations and provide care to approximately 1.6 of the 4 million individuals in the United States who identify themselves as Native American.25,26 However, even for individuals who are eligible for the IHS, access to specialty care is limited. Cancer care is provided through Contract Health Services, because the IHS does not employ any oncologists.27 Specialist referrals made through the Contract Health Services depend on sufficient funding from Congress, which introduces additional potential delays in or barriers to care. The disparity we observed in receipt of optimal care cannot be explained by variance in insurance coverage, because all patients who were included in our study population had complete Medicare coverage.
Cultural factors also may play a role in lower rates of receipt of cancer treatments, because some AI/ANs may place greater trust in nontraditional health care providers. Others have reported that mistrust of the health care system and providers contributes to observed disparities in the treatment of AI/ANs.28,29 A novel survey study evaluating the beliefs of AIs and whites about cancer treatment revealed that AIs are significantly more likely to hold negative perceptions of how cancer treatment will affect them.29 For instance, 38% of AIs versus 1% of whites believed that cancer treatments always cause a patient’s hair to fall out. Twenty-nine percent of AIs versus 1% of whites believed that cancer treatments always make individuals so sick that they are unable to go on about their daily lives. Such misconceptions of the impact of cancer therapy on daily life may play a large role in AI patients’ reluctance to agree to standard therapies. Culturally focused education programs at the community level to inform AI/AN patients about such therapies could potentially increase their acceptance. Such an educational intervention targeted toward AI women succeeded in increasing rates of mammographic screening for breast cancer.30 Currently, the CINCO group is working within AI/AN communities to conduct key informant interviews and patient-provider dyad surveys that will elucidate the barriers to receiving cancer treatment and will provide appropriate and collaborative targets for intervention.
Our study had limitations that should be noted. First, definitions of optimal cancer care were based on current disease-specific clinical practice guidelines and could yield biased results, particularly for those guidelines that were modified in the later years of the study. To mitigate this, we intentionally selected less controversial, longstanding treatment guidelines. Upon review of archived 1996, 2000, and 2005 NCCN guidelines attained with NCCN permission, all of our selected NCCN breast, lung, and colon cancer metrics were similar to historic NCCN guidelines from the study period. The only significant variation was in the prostate cancer guidelines, which introduced a recommendation of adjuvant androgen-deprivation therapy for intermediate grade stage 1 and 2 prostate cancer subsequent to 2005. Because patients were concordant for this metric (Metric 1b) whether or not they received androgen-deprivation therapy, this variation in the guideline should not have had an impact on our results. For Metric 2 (breast cancer adjuvant therapy), the NCCN guideline for radiation therapy after breast-conserving surgery has changed in recent years to apply only to those patients aged <70 years who have estrogen receptor-positive cancers and are receiving tamoxifen. We performed an additional analysis examining rates of receipt of Metric 2 adjuvant therapy restricted to patients aged <70 years and observed that the significant disparity between AI/ANs (n = 30) and matched whites (n = 1633) persisted (P = .033). Given the small number of AI/AN patients to which this subset analysis was restricted, we opted to present the larger population of AI/ANs unrestricted by age, as indicated in Table 3. In addition, because we were interested in a comparison between AI/ANs and whites with regard to the receipt of optimal care, as opposed to the primary rates of concordance within each racial category, we did not believe it was necessary to adjust guidelines by individual year. In addition, we controlled for year of diagnosis in our multivariate analysis. Second, the SEER AI/AN population is largely comprised of AI/ANs in the West (New Mexico, Pacific Northwest, and California) and may not be generalizable to all AI/ANs in the United States. Third, the population size of AI/ANs in the SEER-Medicare data is still small, approaching only 0.3% that of whites and resulting in challenges to meaningful comparison for some quality metrics. However, the SEER-Medicare data set still provides 1 of the most robust tools publicly available to study AI/ANs.2
In summary, we demonstrated that AI/AN patients are less likely to receive guideline-concordant, or optimal, surgical and adjuvant therapy and post-therapy surveillance compared with white patients. In addition, nonreceipt of guideline-concordant cancer care was associated with worse cancer-specific survival for AI/ANs and whites alike. In light of these pervasive disparities and our confirmation that receipt of optimal cancer treatments is associated with improved cancer survival, efforts should be made to address root causes. In the CINCO group, we are currently examining both more distal or systems-related and access-related issues as well as more proximal, community-based and individual-based factors to clarify which interventions could most effectively target such disparities.
Supplementary Material
Acknowledgments
We acknowledge the efforts of the Applied Research Program, National Cancer Institute; the Office of Research, Development, and Information, Centers for Medicare and Medicaid Services; Information Management Services, Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
The Collaborative to Improve Native Cancer Outcomes includes: D. Buchwald, D.R. Flum, E.M. Garroutte, A.A. Gonzales, J.A. Henderson, P. Nez Henderson, D.L. Patrick, S.P. Tu, and R.L. Winer.
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The ideas and opinions expressed herein are those of the author(s), and endorsement by the State of California, the Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their contractors and subcontractors is not intended nor should be concluded.
FUNDING SUPPORT
This research was performed under the auspices of the Collaborative to Improve Native Cancer Outcomes, a P50 program project sponsored by the National Cancer Institute (grant no. 1P50CA148110). The collection of the California cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute’s Surveillance, Epidemiology and End Results Program under contract N01-PC-35,136 awarded to the Northern California Cancer Center, contract N01-PC-35,139 awarded to the University of Southern California, and contract N02-PC-15,105 awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement U55/CCR921930-02 awarded to the Public Health Institute.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
Dr. Flum reports a paid salary from the Patient Centered Outcomes Research Institute (PCORI) and travel expenses for meetings of the PCORI Methodology Committee; he is an owner of Surgical Consulting, LLC,; he is a paid consultant to Pacira Pharmaceuticals; he is a co-owner of Benchmarket, LLC; and, at the American College of Surgeons, he is former Chair for the Surgical Research Committee and current Chair for the Bi-Annual Outcomes Research Course, for which he receives travel expenses. He has received research funding from Nestle Health Sciences for the Strong for Surgery Initiative. He also reports the receipt of travel expenses for national and international meetings and symposiums from Covidien, the Australia New Zealand Hepato-Biliary Association, and Kenes International; and he reports honorarium and travel expenses for meetings and presentations from the American Academy of Orthopedic Surgeons and Nestle Health Sciences.
Additional Supporting Information may be found in the online version of this article.
References
- 1.US Department of Health and Human Services (DHHS) DHHS Publication No. 271-848-40085. 3. Washington, DC: US Government Printing Office; 1991. Health Status of Minorities and Low-Income Groups. [reprinted as: National Cancer Institute. NIH Publication No. 94-3603. Bethesda, MD; National Institutes of Health; 1994] [Google Scholar]
- 2.Espey DK, Wu XC, Swan J, et al. Annual report to the nation on the status of cancer, 1975–2004, featuring cancer in American Indians and Alaska Natives. Cancer. 2007;110:2119–2152. doi: 10.1002/cncr.23044. [DOI] [PubMed] [Google Scholar]
- 3.Greenberg CC, Lipsitz SR, Neville B, et al. Receipt of appropriate surgical care for Medicare beneficiaries with cancer. Arch Surg. 2011;146:1128–1134. doi: 10.1001/archsurg.2011.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cobb N, Paisano RE. Patterns of cancer mortality among Native Americans. Cancer. 1998;83:2377–2383. doi: 10.1002/(sici)1097-0142(19981201)83:11<2377::aid-cncr18>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 5.Cobb N, Wingo PA, Edwards BK. Introduction to the supplement on cancer in the American Indian and Alaska Native populations in the United States. Cancer. 2008;113(5 suppl):1113–1116. doi: 10.1002/cncr.23729. [DOI] [PubMed] [Google Scholar]
- 6.Wiggins CL, Espey DK, Wingo PA, et al. Cancer among American Indians and Alaska Natives in the United States, 1999–2004. Cancer. 2008;113(5 suppl):1142–1152. doi: 10.1002/cncr.23734. [DOI] [PubMed] [Google Scholar]
- 7.Espey DK, Wiggins CL, Jim MA, et al. Methods for improving cancer surveillance data in American Indian and Alaska Native populations. Cancer. 2008;113(5 suppl):1120–1130. doi: 10.1002/cncr.23724. [DOI] [PubMed] [Google Scholar]
- 8.Warren JL, Klabunde CN, Schrag D, Bach PD, Riley GF. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care. 2002;40:IV-3, IV-18. doi: 10.1097/01.MLR.0000020942.47004.03. [DOI] [PubMed] [Google Scholar]
- 9.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45:613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 10.Politis DN, Romano JP. Large sample confidence regions based on subsamples under minimal assumptions. Ann Stat. 1994;22:2031–2050. [Google Scholar]
- 11.Bickel PJ, Gotze F, van Zwet WR. Resampling fewer than n observations: gains, losses and remedies for losses. Stat Sinica. 1997;7:1–31. [Google Scholar]
- 12.Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model. New York: Springer-Verlag; 2000. [Google Scholar]
- 13.Hosmer DW, Lemeshow S. Applied Survival Analysis: Regression Modeling of Time to Event Data. New York: John Wiley & Sons, Inc; 1999. [Google Scholar]
- 14.Allison PD. Survival Analysis Using SAS: A Practical Guide. Cary, NC: SAS Institute Inc; 2010. [Google Scholar]
- 15.US Census Bureau. 2010 Census Briefs. Washington, DC: US Census Bureau; 2012. [Accessed February 4, 2014]. The American Indian and Alaska Native Population: 2010. Available at: http://www.census.gov/prod/cen2010/briefs/c2010br-10.pdf. [Google Scholar]
- 16.Zippin C, Lum D, Hankey BF. Completeness of hospital case reporting from the SEER Program of the National Cancer Institute. Cancer. 1995;76:2343–2350. doi: 10.1002/1097-0142(19951201)76:11<2343::aid-cncr2820761124>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 17.National Cancer Institute. Overview of the SEER Program. Bethesda, MD: National Cancer Institute; 2010. [Accessed March 20, 2012]. Available at: http://healthservices.cancer.gov/seermedicare/overview/linked.html. [Google Scholar]
- 18.Research Data Assistance Center (ResDac) CMS 301: Using SEER/Medicare Data for Research. Minneapolis, MN: ResDAC; 2008. [Google Scholar]
- 19.National Center for Health Statistics. [Accessed February 4, 2014];US Census Populations With Bridged Race Categories. Available at: ftp://ftp.cdc.gov/pub/Health_Statistics/NCHS/datasets/nvss/bridgepop/table3.xls.
- 20.Clegg LX, Li FP, Hankey BF, et al. Cancer survival among US whites and minorities: a SEER (Surveillance, Epidemiology, and End Results) Program population-based study. Arch Intern Med. 2002;162:1985–1993. doi: 10.1001/archinte.162.17.1985. [DOI] [PubMed] [Google Scholar]
- 21.Bach PB, Cramer LD, Warren JL, et al. Racial differences in the treatment of early stage lung cancer. N Engl J Med. 1999;341:1198–1205. doi: 10.1056/NEJM199910143411606. [DOI] [PubMed] [Google Scholar]
- 22.Morris AM, Billingsley KG, Hayanga AJ, et al. Residual treatment disparities after oncology referral for rectal cancer. J Natl Cancer Inst. 2008;100:738–744. doi: 10.1093/jnci/djn396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris AM, Rhoads KF, Stain SC, et al. Understanding racial disparities in cancer treatment and outcomes. J Am Coll Surg. 2010;211:105–113. doi: 10.1016/j.jamcollsurg.2010.02.051. [DOI] [PubMed] [Google Scholar]
- 24.Smith CB, Bonomi M, Packer S, et al. Disparities in lung cancer stage, treatment and survival among American Indians and Alaskan Natives. Lung Cancer. 2011;72:160–164. doi: 10.1016/j.lungcan.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 25.Rural Policy Research Institute. [Accessed February 4, 2014]; Available at: www.rupri.org.
- 26.US Census Bureau. [Accessed March 29, 2002];Database C90STF3C. Available at: http://fact-finder.census.gov/servlet/BasicFactsServlet.e.
- 27.Burhansstipanov L. Cancer Among Elder Native Americans. Denver, CO: Native Elder Health Care Resource Center; 1996. [Google Scholar]
- 28.Call KT, McAlpine DD, Johnson PJ, et al. Barriers to care among American Indians in public health care programs. Med Care. 2006;44:595–600. doi: 10.1097/01.mlr.0000215901.37144.94. [DOI] [PubMed] [Google Scholar]
- 29.Guadagnolo BA, Cina K, Helbig P, et al. Medical mistrust and less satisfaction with health care among Native Americans presenting for cancer treatment. J Health Care Poor Underserved. 2009;20:210–226. doi: 10.1353/hpu.0.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weiner D, Burhansstipanov L, Krebs LU, et al. From survivorship to thrivership: native peoples weaving a healthy life from cancer. J Cancer Educ. 2005;20(1 suppl):28–32. doi: 10.1207/s15430154jce2001s_07. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.