Abstract
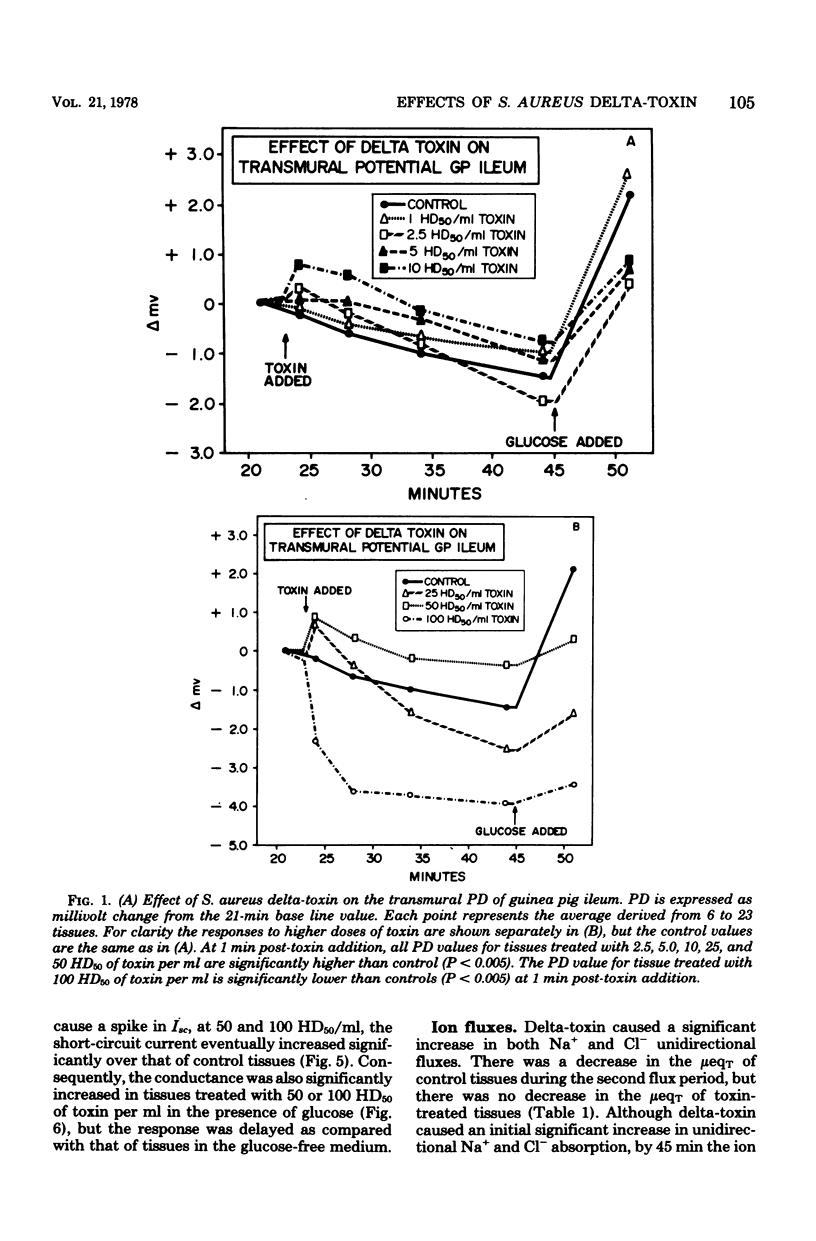
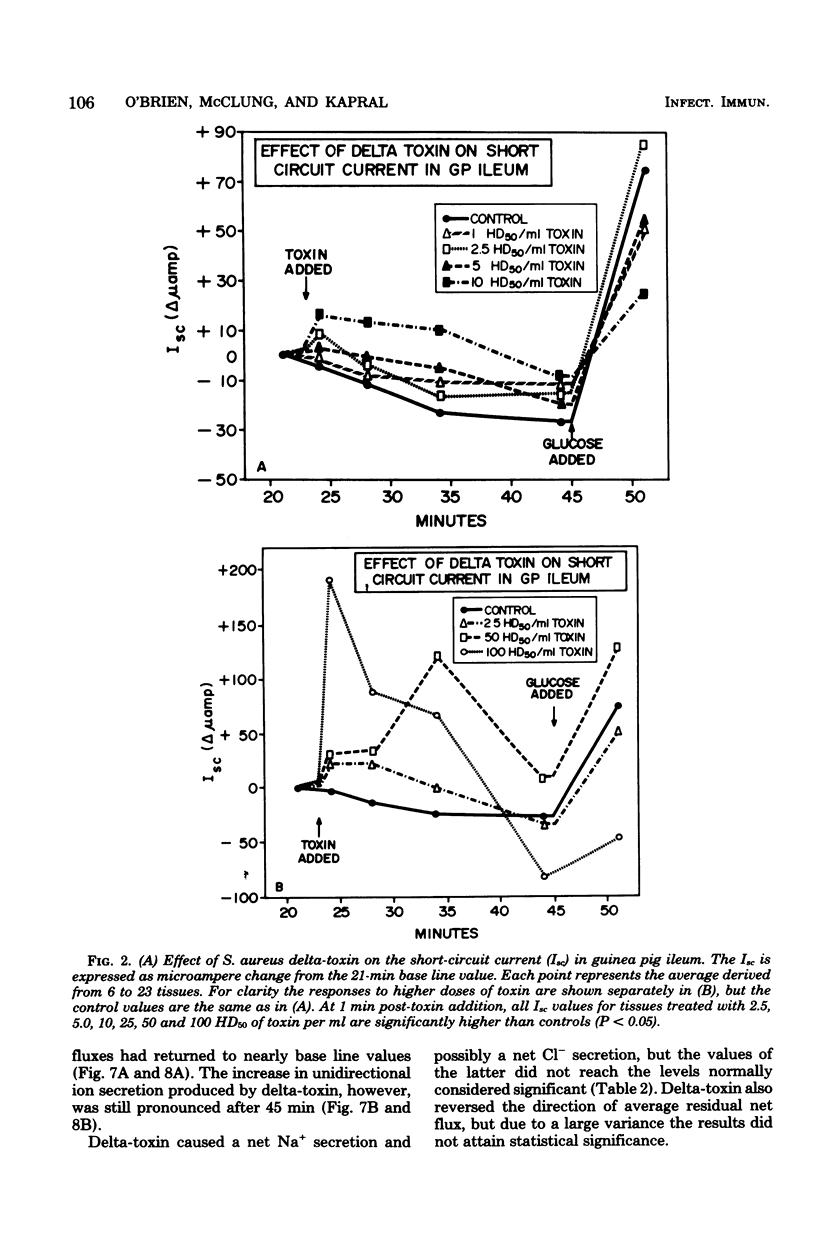
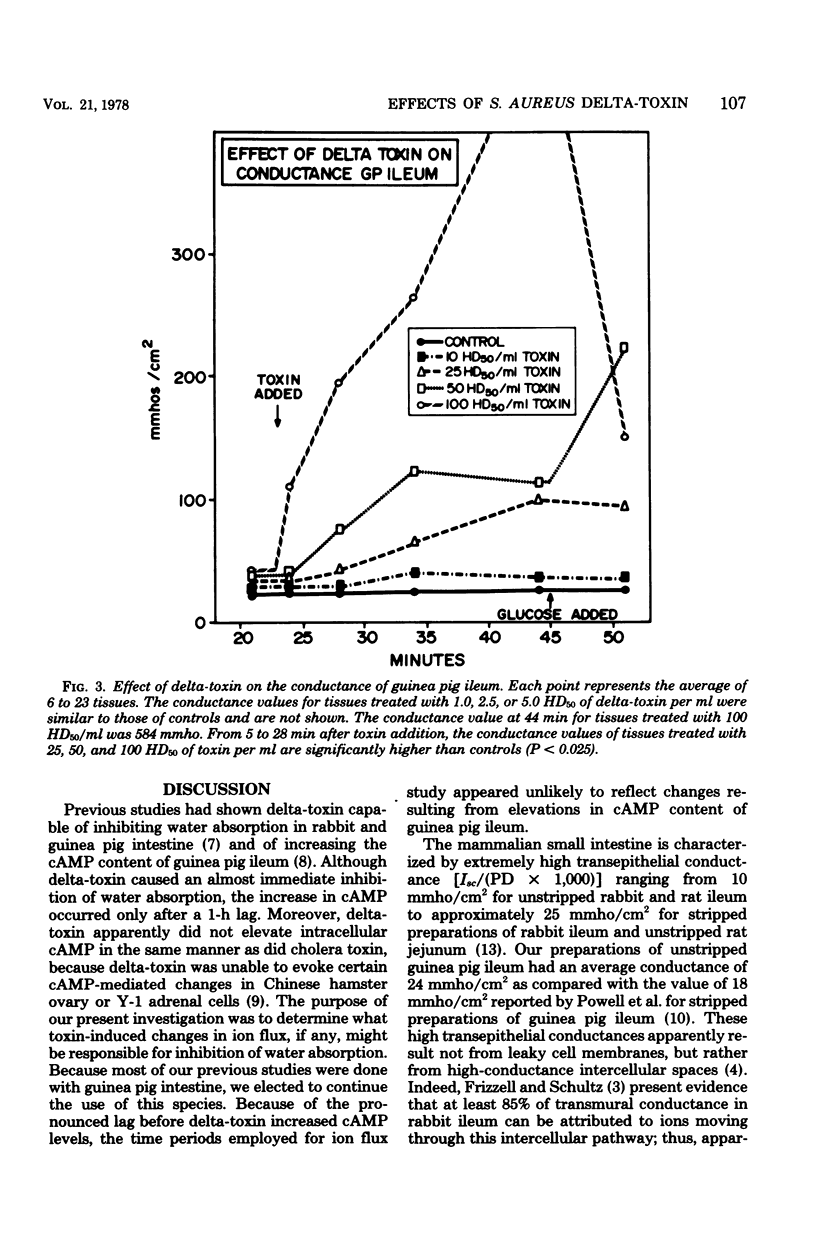
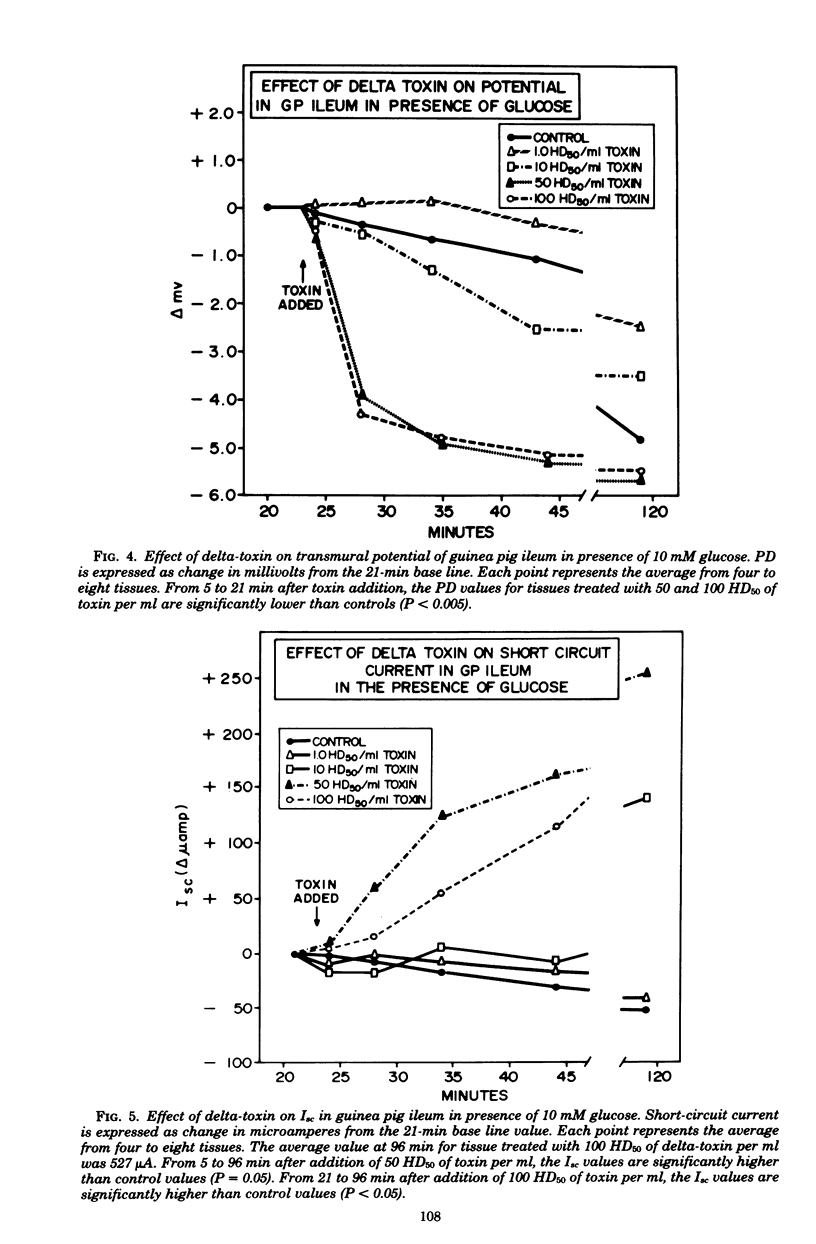
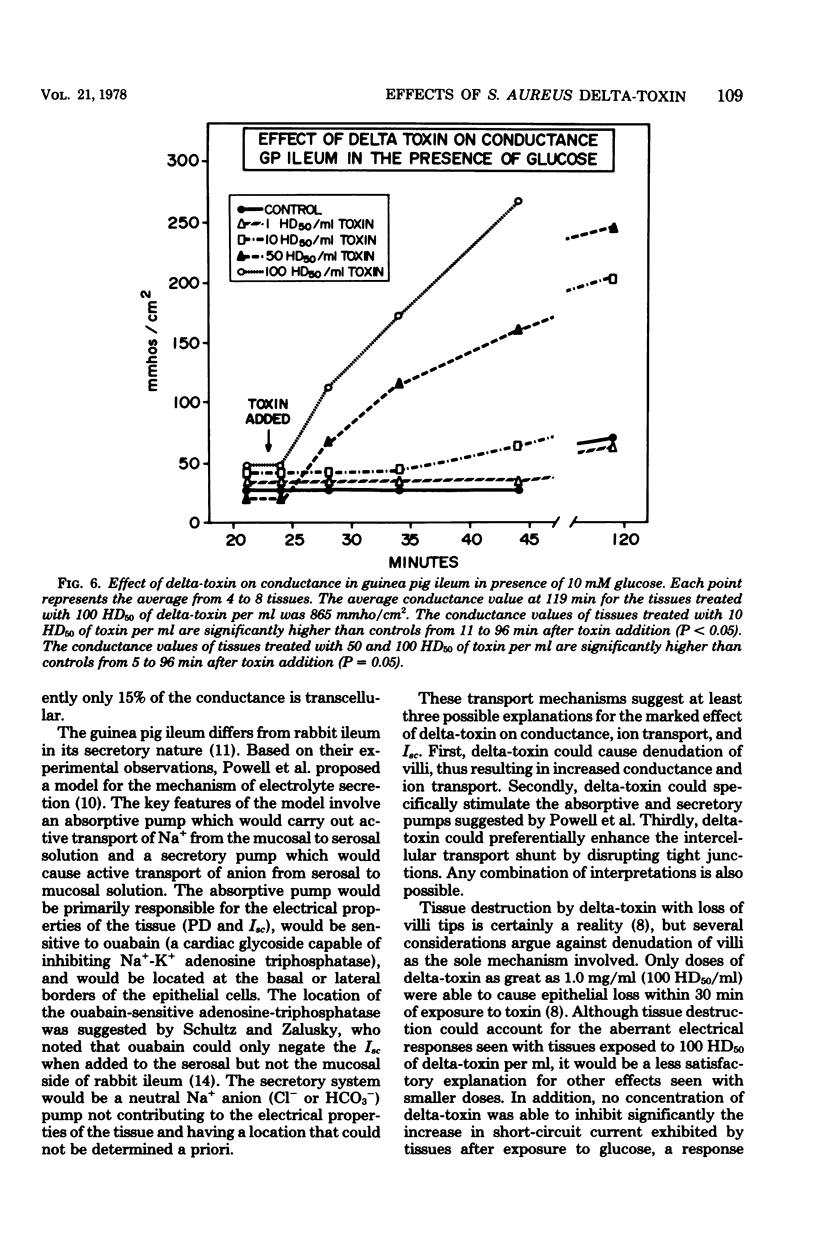
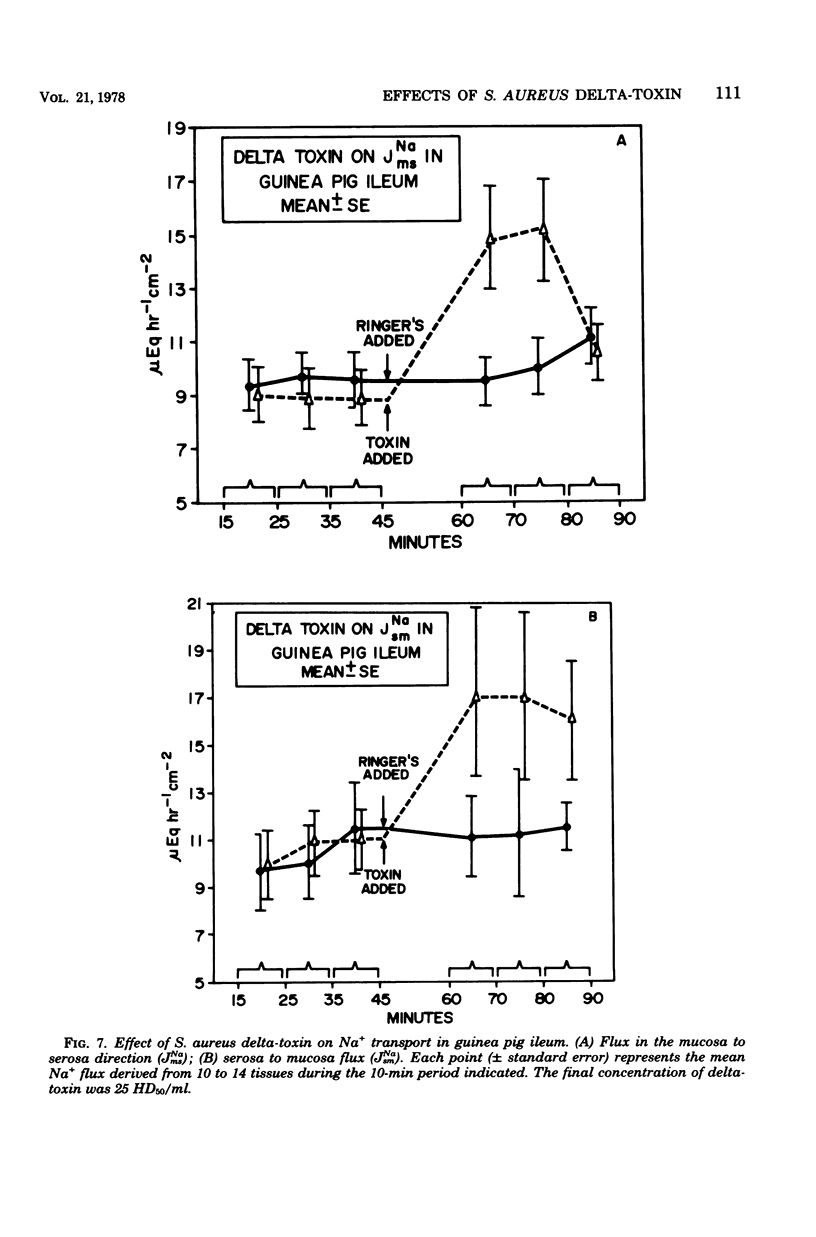
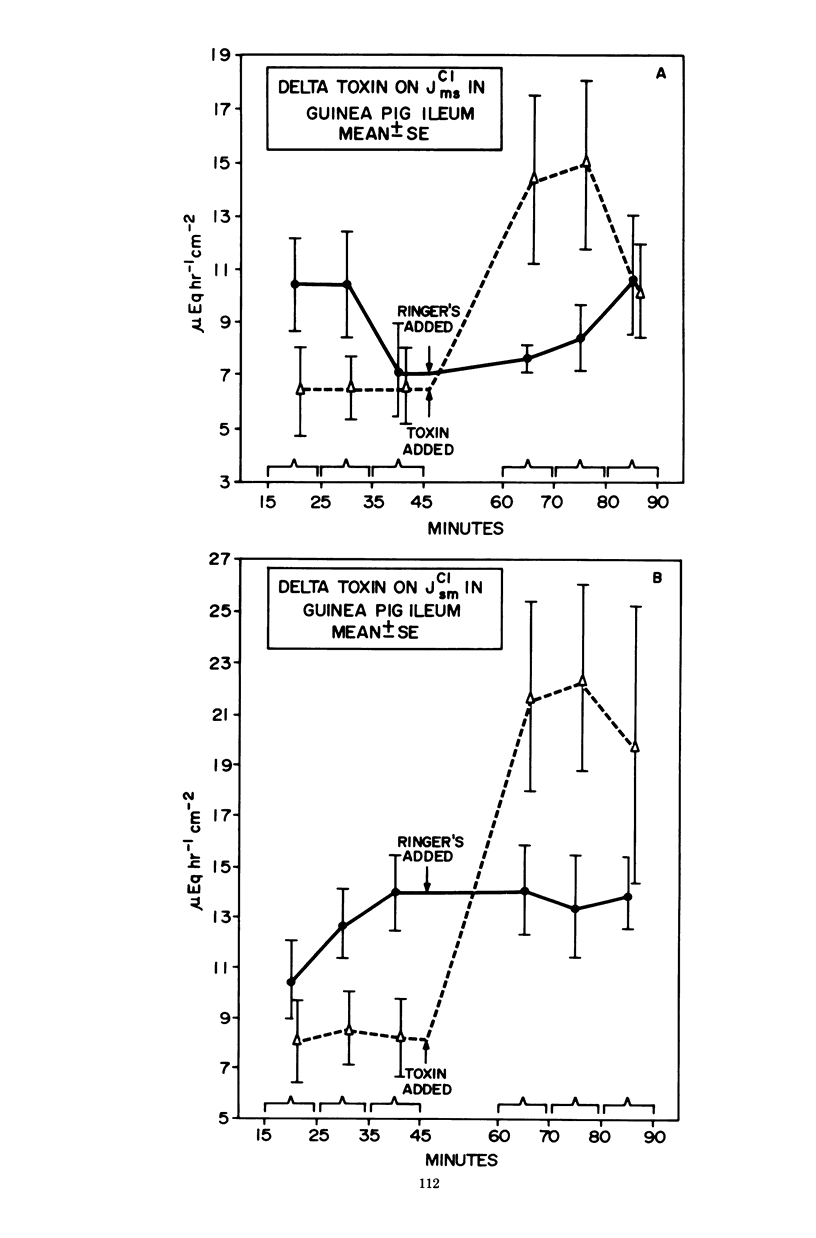
Prior studies had shown that Staphylococcus aureus delta-toxin was able to inhibit water absorption in guinea pig ileum and to elevate the cyclic AMP content of this tissue, but was unable to elicit certain cyclic AMP-mediated changes in Y-1 adrenal or Chinese hamster ovary cells. Because water movement passively follows the net movement of electrolytes in the gut, this study investigated the effect of delta-toxin on ion transport in guinea pig ileum maintained in vitro. The transmural potential difference (PD) of guinea pig ileum was measured and nullified with an automatic voltage clamp. The short circuit flowing under these conditions (Isc) was measured, and the conductance was calculated (Isc/PD). Unidirectional 22Na+ and 36Cl− fluxes were measured. In a glucose-free Ringer solution, delta-toxin caused an immediate spike in PD and Isc, and the extent and duration of the spike generally increased with increasing toxin concentration. The conductance of ileum was increased by delta-toxin, and this effect on conductance could be blocked by lecithin, a known inhibitor of delta-toxin. Tissue in the presence of glucose did not exhibit a spike in PD or Isc when exposed to delta-toxin. In a glucose-free medium, delta-toxin caused a 1.5- to 2.5-fold increase in both the unidirectional absorption and secretion of Na+ and Cl−, whereas the net secretion of Na+ increased above basal levels. The observation that delta-toxin causes a prompt increase in intestinal ion flux lends credence to the concept that the elevation in cellular cyclic AMP, which occurs later, is a secondary response to the toxin. The rapid increase in ion flux may reflect the ability of delta-toxin to augment intercellular movement of ions across the mucosa rather than the stimulation of transcellular processes.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binder H. J., Powell D. W., Curran P. F. Effect of hexoses on ion transport in guinea pig ileum. Am J Physiol. 1972 Sep;223(3):538–544. doi: 10.1152/ajplegacy.1972.223.3.538. [DOI] [PubMed] [Google Scholar]
- Field M., Fromm D., McColl I. Ion transport in rabbit ileal mucosa. I. Na and Cl fluxes and short-circuit current. Am J Physiol. 1971 May;220(5):1388–1396. doi: 10.1152/ajplegacy.1971.220.5.1388. [DOI] [PubMed] [Google Scholar]
- Frizzell R. A., Schultz S. G. Ionic conductances of extracellular shunt pathway in rabbit ileum. Influence of shunt on transmural sodium transport and electrical potential differences. J Gen Physiol. 1972 Mar;59(3):318–346. doi: 10.1085/jgp.59.3.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frömter E., Diamond J. Route of passive ion permeation in epithelia. Nat New Biol. 1972 Jan 5;235(53):9–13. doi: 10.1038/newbio235009a0. [DOI] [PubMed] [Google Scholar]
- Kapral F. A. Inhibition of Staphylococcus aureus delta hemolysin by phospholipids. Proc Soc Exp Biol Med. 1972 Nov;141(2):519–521. doi: 10.3181/00379727-141-36812. [DOI] [PubMed] [Google Scholar]
- Kapral F. A., Miller M. M. Product of Staphylococcus aureus responsible for the scalded-skin syndrome. Infect Immun. 1971 Nov;4(5):541–545. doi: 10.1128/iai.4.5.541-545.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapral F. A., O'Brien A. D., Ruff P. D., Drugan W. J., Jr Inhibition of water absorption in the intestine by Staphylococcus aureus delta-toxin. Infect Immun. 1976 Jan;13(1):140–145. doi: 10.1128/iai.13.1.140-145.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien A. D., Kapral F. A. Effect of Staphylococcus aureus delta toxin on Chinese hamster ovary cell morphology and Y-1 adrenal cell morphology and steroidogenesis. Infect Immun. 1977 Jun;16(3):812–816. doi: 10.1128/iai.16.3.812-816.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien A. D., Kapral F. A. Increased cyclic adenosine 3',5'-monophosphate content in guinea pig ileum after exposure to Staphylococcus aureus delta-toxin. Infect Immun. 1976 Jan;13(1):152–162. doi: 10.1128/iai.13.1.152-162.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell D. W., Binder H. J., Curran P. F. Electrolyte secretion by the guinea pig ileum in vitro. Am J Physiol. 1972 Sep;223(3):531–537. doi: 10.1152/ajplegacy.1972.223.3.531. [DOI] [PubMed] [Google Scholar]
- Powell D. W., Malawer S. J., Plotkin G. R. Secretion of electrolytes and water by the guinea pig small intestine in vivo. Am J Physiol. 1968 Nov;215(5):1226–1233. doi: 10.1152/ajplegacy.1968.215.5.1226. [DOI] [PubMed] [Google Scholar]
- Rothe C. F., Quay J. F., Armstrong W. M. Measurement of epithelial electrical characteristics with an automatic voltage clamp device with compensation for solution resistance. IEEE Trans Biomed Eng. 1969 Apr;16(2):160–164. doi: 10.1109/tbme.1969.4502631. [DOI] [PubMed] [Google Scholar]
- SCHULTZ S. G., ZALUSKY R. ION TRANSPORT IN ISOLATED RABBIT ILEUM. I. SHORT-CIRCUIT CURRENT AND NA FLUXES. J Gen Physiol. 1964 Jan;47:567–584. doi: 10.1085/jgp.47.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- USSING H. H., ZERAHN K. Active transport of sodium as the source of electric current in the short-circuited isolated frog skin. Acta Physiol Scand. 1951 Aug 25;23(2-3):110–127. doi: 10.1111/j.1748-1716.1951.tb00800.x. [DOI] [PubMed] [Google Scholar]



