Abstract
A suitable and standardized protein purification technique is essential to maintain consistency and to allow data comparison between proteomic studies for urine biomarker discovery. Ultimately, efforts should be made to standardize urine preparation protocols. The aim of this study was to develop an optimal analytical protocol to achieve maximal protein yield and to ensure that this method was applicable to examine urine protein patterns that distinguish disease and disease-free states. In this pilot study, we compared seven different urine sample preparation methods to remove salts, and to precipitate and isolate urinary proteins. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) profiles showed that the sequential preparation of urinary proteins by combining acetone and trichloroacetic acid (TCA) alongside high speed centrifugation (HSC) provided the best separation, and retained the most urinary proteins. Therefore, this approach is the preferred method for all further urine protein analysis.
Keywords: breast cancer, proteomics, SDS-PAGE, LC-MS/MS, urine, biomarker
Introduction
The identification of novel biomarkers for the early detection of cancer, monitoring cancer progression, and assessing response to therapy holds promise for improving clinical outcomes. Currently, a major obstacle in the early detection of breast cancer (BC) is the development of methods that efficiently and accurately identify potential proteomics biomarkers. In BC, most of the urinary markers identified to date are metabolomic markers.1,2
Urinary proteome analysis is attractive in clinical proteomics research as urine is relatively simple and easy to collect. It is commonly used for the diagnosis and classification of diseases.3 As a biological sample, human urine is abundant in proteins, which reflects the physiological and the pathological state of an individual.4 Because of its complex nature, compounds present in urine such as salts, peptides, oligosaccharides, and glycosaminoglycans (GAG) can interfere with the electrophoretic migration of other proteins.5 Thus, precipitation and concentration steps are essential to purify and isolate the proteins of interest. The precipitation of proteins occurs in solutions of extreme ionic strength, high concentrations of organic solvents, and low pH. Most proteomic researchers use different protocols, devised to suit the biological specimen or analytes of interest. These protocols include ultrafiltration,6 ethanol precipitation,7 various concentrations of acetone,8 acetone and trichloroacetic acid (TCA) combination,9,10 ultrafiltration, and combination of TCA and ultracentrifugation.11,12
Although there are numerous published urine preparation protocols, when applied to the metastatic BC specimens in our laboratory, these existing techniques could not efficiently desalt and concentrate the urine samples.
No group of investigators to date have published standardized technical information on the preparation of urine to achieve maximal protein yield for BC protein biomarkers. The validity of the BC biomarker discovery greatly relies on the handling of urine samples in a uniform manner, thus highlighting the importance of a standardized protocol. Here, we demonstrate an efficient and reliable technical method including the sequential preparation of urinary proteins by acetone, and TCA in combination with high speed centrifugation (HSC) for urine sample preparation. We found that this approach can maintain consistency and reproducibility to allow urinary data comparison.
Materials and Methods
Urine collection protocol
In this pilot study, urine samples were collected from female patients (ranging 35–60 years) with metastatic BC (n = 15) and age-matched healthy disease-free control group (n = 18). Prior ethical approval for this study was obtained from the Human Research Ethics Committee of South Eastern Area Health Service Ethics Committee (SEA HRCE #07/71Li). The study was conducted in accordance with the Declaration of Helsinki. All subjects provided their written, informed consent to participate. All urine samples were collected as a midstream, clean catch specimen into a sterile urine container and immediately transported on ice to prevent microbe contamination and proteolysis. The samples were then clarified and insoluble materials were removed by centrifugation at 2,000 × g (4,000 rpm) at 4°C for 10 minutes,5 within 30 minutes (min) of collection, to prevent protein release from these artifacts. The supernatants were carefully removed and frozen at −20°C in 2 mL aliquots to prevent freezing-and-thawing cycles (transferred to −80°C for long-term storage). The biological characteristics of urine samples were examined for color (pale yellow, no blood), turbidity (clear not cloudy), and pH (4.5–8.0) to ensure that there were no notable discrepancies. To prevent technical and analytical variation caused by handling, all samples were collected, processed, and stored following the same procedural conditions, and by the same laboratory personnel until a final protocol was established. Protease inhibitors were not used in this study.
Protein extraction and precipitation techniques
To achieve a representative urinary proteome that portrays the group pattern sample variability,13 the frozen aliquots were completely thawed, mixed well, and then equal volumes of each donor supernatant were pooled into the BC or control group.
The pooled urine supernatants were subjected to seven different urine protein extraction–precipitation methods, along with various combinations of these techniques (summarized in Table 1). The main precipitation techniques (methods 1–4) included acetone, TCA, ultrafiltration (UF), and acetone plus TCA combined. All precipitation procedures were performed at 4°C, and in methods 1–4, the samples were initially centrifuged at a low speed centrifugation (LSC), then repeated at HSC to attempt to further desalt and remove non-soluble materials. All supernatant washes for each technique were kept and analyzed for protein loss. The details of all the urine protein precipitation methods applied are as follows:
Table 1.
Summary of all the urine precipitation techniques applied.
| METHOD APPLIED | PRECIPITATION TECHNIQUE | SAMPLE VOLUME | PRECIPITATION TIME/TEMPERATURE | CENTRIFUGATION SPEED × (g) AT 4°C | TIME | PROTEIN PELLET EXTRACTED |
|---|---|---|---|---|---|---|
| 1 | Acetone | sample: acetone 1:8 | 1 hour at −20°C | Low speed centrifugation (LSC) | 30 min | Y |
| 2 | Trichloroacetic-Acid (TCA) | sample: TCA 4:1 | 1 hour at 4°C | LSC | 30 | Y |
| 3 | Ultra-filtration (UF) | 15 mL | LSC | 30 | Y | |
| 4 | Acetone/TCA | sample: acetone (1:8) sample: TCA (4:1) |
1 hour at −20°C 1 hour at 4°C |
LSC LSC |
30 30 |
Y |
| 5 | High speed centrifugation (HSC) | 11,000 | 30 | |||
| 1, 5 | Acetone-HSC | HSC | 30 | Y | ||
| 2, 5 | TCA-HSC | HSC | 30 | Y | ||
| 3, 5 | UF-HSC | HSC | 30 | Y | ||
| 4, 5 | Acetone/TCA-HSC | HSC | 30 | Y | ||
| 6 | Glyco-ammino glycan removal (GAG) | CPC: pellet (3:1) | 30 min at 26°C | 11,000 | 30 | |
| 1, 5, 6 | Acetone-HSC-GAG | HSC | 30 | N | ||
| 2, 5, 6 | TCA-HSC-GAG | HSC | 30 | N | ||
| 3, 5, 6 | UF-HSC-GAG | HSC | 30 | N | ||
| 4, 5, 6 | Acetone/TCA-HSC-GAG | HSC | 30 | Y | ||
| 7 | Cell shearing (CS) | 100 μL lysis buffer | Bench top 11,000 | 10 45 |
||
| 1, 5, 7 | Acetone-HSC-CS | Y | ||||
| 2, 5, 7 | TCA-HSC-CS | N | ||||
| 3, 5, 7 | UF-HSC-CS | N | ||||
| 4, 5, 7 | Acetone/TCA-CS | Y | ||||
Notes: The seven urine preparation methods (1–7) including 16 individual approaches are shown. The main precipitation techniques are methods 1–4; HSC (5) has four combination approaches with methods 1–4; GAG (6) has four combination approaches with methods 1–4; cell shearing (7) also has four combination approaches with methods 1–4. At the end of each extraction method, the ability to achieve a protein pellet was shown.
Abbreviations: CPC, cetyl pyridinium chloride solution; Y, protein pellet was extracted; N, no protein pellet was extracted.
(1) Acetone method: Eight parts of ice-cold acetone were combined with one part of urine sample (1:8 urine sample-to-solvent ratio), and the mixture was stored at −20°C for 1 hour. The pellets were air-dried to remove residual acetone; (2) TCA method: One part of fresh TCA solution (10 g TCA in 10 mL Milli-Q H2O) was added to four parts of urine (4:1 urine sample-to-solvent ratio), and the mixture was vortexed and then incubated for 1 hour at 4°C; (3) Ultrafiltration method: This approach was carried out according to the procedure provided with the device (Amicon® Ultra-15 Centrifugal Filter Units, Millipore, Darmstadt, Germany) in order to reduce the initial volume of urine to 500 μL; (4) Combined acetone/TCA method: Acetone precipitation was performed for 1 hour at −20°C (as per method 1), dried-off for 5 minutes at room temperature, followed by TCA precipitation (as per method 2), vortexing, and then incubation for 1 hour at 4°C. All protein pellets were initially collected at LSC (Optima LE-80K; Beckman Coulter, Inc., Fullerton, CA, USA) at 4,000 × g for 30 minutes; (5) HSC method: This approach includes the application of HSC, centrifugation at 11,000 × g for 30 minutes after each precipitation method 1–4 (see Table 1: method 5) to concentrate the protein samples.
Additional purification steps that included either GAG precipitation5,14 or sonication-“cell shearing” were examined separately with each single precipitation method (1–4); the combined techniques applied are listed in Table 1. The details of the double precipitation methods include (6); GAG precipitation method: Following precipitation with the single methods (1–4) at HSC (method 5), each protein pellet was incubated at 26°C for 30 minutes in a 5% cetyl pyridinium chloride (CPC) solution (CPC solution-to-protein pellet ratio 3:1), and then washed twice with 1 M NaCl (sodium chloride) solution to disrupt the CPC–GAG complex; and (7) sonication-“cell shearing” method. Following each of the methods (1–4) at HSC (method 5), the urine protein pellets were resuspended in 100 μL lysis buffer (7 M urea, 0.1 M CHAPS (3-[(3 cholamidopropyl)dimethylammonio]-1-propanesulfonate), 0.1 M dithioerythritol [DTT], and 35 mM Tris-base) and 0.1 g of zirconium beads (0.1 mm diameter). Samples were sheared at 5,000 rpm in a mini-bead beater (Biospec Product, Oklahoma, USA) for 90 seconds and then kept on ice for 5 minutes to limit heating. This procedure was repeated three times. After briefly centrifuging on a mini spin bench top, the samples were centrifuged at 10,000 × g for 10 minutes, and the supernatants were carefully collected for HSC for 45 minutes.
The precipitated protein pellets from all methods were resuspended and solubilized in 100 μL of rehydration buffer (RB), 7 M urea, 2 M thiourea, 1% CHAPS, 50 mM DTT, for protein assay analysis and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). The samples were subjected to protein quantitation using the 2-D Quant kit (GE Healthcare, USA), with bovine serum albumin (BSA) as a reference standard. Numerous protein assays were conducted and a standard curve was established for each assay. All samples were run in triplicate. The standard curve was used to create a trend line and a linear equation established was used to calculate the protein values with R2 > 0.99.
Protein separation and examination
SDS-PAGE was used to determine the best method for the extraction and precipitation for liquid-chromatography/tandem-mass spectrometry (LC-MS/MS). A volume of protein solution was taken to load 30 μg of protein, which was mixed with an equal volume of TruSep SDS sample buffer (NuSep, Homebush, NSW, Australia) and boiled for 5 minutes. The whole sample was run on 12%T-Tris/glycine precast mini gels at 180 V, 50 mA/gel for 1 hour with Tris-glycine running buffer (25 mM Tris, 192 mM glycine, and 0.1% SDS in Milli-Q H2O pH 8). Gels were stained using a solution of Coomassie Blue R-250, 0.1% w/v in 10% methanol (CB R-250).
Protein desalting was done with C18 Stage Tips (ThermoScientific, Waltham, MA, USA.) as recommended by the manufacturer. Peptide fractions were digested with trypsin (12.5 ng/μL trypsin proteomic grade, Sigma-Aldrich, St. Louis, MO, USA) to a final enzyme:protein ratio of 1:100 (w/w). All the samples were digested and prepared for LC-MS/MS analysis using equivalent fixed amounts of protein starting material of 10 μg. Analysis was conducted on the protein digests by LC-MS/MS using a LTQ-Velos Orbitrap ETD (Thermo Electron, Bremen, Germany) as described previously.15 Briefly, C18-LC elution was conducted over 60-minute linear gradients. The false discovery rate (FDR) was less than 2% at 95% confidence for peptides. Protein datasets (Peak lists) were generated using Mascot Daemon software (Matrix Science, London, England), and analyzed using Peak Integration with Progenesis LC-MS (Non-Linear Dynamics, UK).
Results
Urinary proteins examined on SDS-PAGE and identified with LC-MS/MS
Our results clearly indicate that the quality and the variability of the urinary protein recovery were greatly affected by the preparation protocols used. Initially, precipitation methods 1–4 were performed at LSC, and no MS data was detected in these protein sample extracts (Table 2: A1–2, C1–3). Additionally, the excessive drag in the gel results from the metastatic BC urine samples (Fig. 1: C1–3) demonstrated a difference between the metastatic BC and the control samples (Fig. 1: A2). Therefore, additional desalting steps to clarify the sample further were required. To observe the optimal method for urine sample preparation for metastatic BC and healthy control urine samples, seven different urine precipitation techniques were compared. This information was used to determine the method that could provide the highest resolution on SDS-PAGE and the greatest number of urinary proteins detected with LC-MS/MS.
Table 2.
Summary of the number of proteins identified with LC-MS/MS and total protein extracted with the different urine protein precipitation methods.
| SDS-PAGE ID | PROTEIN PRECIPITATION TECHNIQUE | METHOD N0 | TOTAL PROTEIN EXTRACTED (μG) | N0 OF PROTEINS ID WITH LC-MS/MS |
|---|---|---|---|---|
| Normal control urine samples—LSC and HSC | ||||
| M | Mass Marker | |||
| A1 | TCA LSC | 2 | 215 | ND |
| A2 | Acetone LSC | 1 | 576 | ND |
| A3 | TCA at HSC | 2, 5 | 345 | 73 |
| A4 | Acetone at HSC | 1, 5 | 905 | 113 |
| A5 | Ultra filtration | 3 | 187 | 47 |
| A6 | Acetone/TCA* at HSC | 4, 5 | 987 | 149* |
| Normal control urine samples, HSC | ||||
| B1 | Acetone | 1, 5 | 834 | 115 |
| B2 | Acetone and Cell shearing | 1, 5, 7 | 676 | 70 |
| B3 | TCA | 2, 5 | 476 | 79 |
| B4 | Acetone/TCA* | 4, 5 | 1184 | 154* |
| B5 | Acetone/TCA - CPC | 4, 5, 6 | 93, 55 | ND |
| B6 | Acetone/TCA and Cell shearing | 4, 5, 7 | 313 | 55 |
| Metastatic BC urine samples—LSC | ||||
| C1 | Acetone | 1 | 155 | ND |
| C2 | TCA | 2 | 146 | ND |
| C3 | Acetone/TCA | 4 | 202 | ND |
| C4 | Ultra filtration at HSC | 3, 5 | 108 | ND |
| C5 | All CPC washes | <30# | ||
| C6 | All Acetone washes | <30# | ||
| Metastatic BC urine samples—all HSC | ||||
| D1 | Acetone | 1, 5 | 865 | 117 |
| D2 | TCA | 2, 5 | 589 | ND |
| D3 | Acetone/TCA* | 4, 5 | 1023 | 165, 167* |
| D4 | All TCA washes | <20# | ||
| D5 | All acetone/TCA washes | <20# | ||
Notes: All urine precipitation methods are tabulated against the protein concentration, in both disease-free control (shown in A–B) and BC specimens (C–D). Corresponding gel images are shown in Figure 1. The majority of the techniques employed HSC centrifugation (11,000 × g) at 4°C for 30 minutes (except A1–2, A5, and C1–3 where LSC was applied).
Highlights the precipitation technique (acetone/TCA at HSC) with the highest total protein extract and number of proteins.
Indicates there was minimal loss of protein found in the washes.
Abbreviations: CPC, cetyl pyridinium chloride; HSC, high speed centrifugation; ID, identified; LSC, low speed centrifugation (4,000 × g); N0, number; ND, not detected; TCA, trichloroacetic acid.
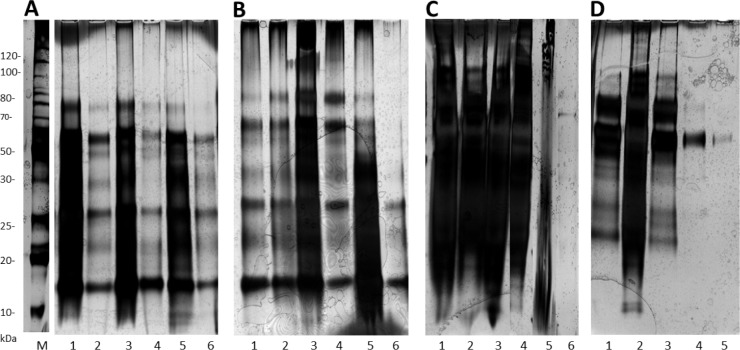
Figure 1.
The comparison of urinary protein precipitation methods on SDS-PAGE. The effects of precipitation techniques and centrifugation on urinary proteins were examined on healthy control (A–B, n = 18) and metastatic BC samples (C–D, n = 15). The details of the technique employed, total protein concentrations, and number of proteins identified by LC-MS/MS are summarized in Table 2.
A total of 20 gels were examined, which included each protein extraction method, where a pellet was extracted (see Table 1), being run at least five times across several different gels. The four representative gels demonstrate the protein extracts achieved following the application of the seven different precipitation techniques in various combinations (Fig. 1). The total protein extracted and LC-MS/MS data, which correspond to the SDS-PAGE results (Fig. 1), are summarized in Table 2.
Combination approach with acetone, then TCA at HSC
Overall, the greatest number of urinary proteins and total protein yield, representing both the high and low molecular weight (MW) proteins, as well as best gel resolution, were observed by combining three precipitation techniques (ie acetone, then TCA all using HSC, see methods 4 and 5) in both the control and BC samples (Fig. 1 and Table 2 in *A6, B4, and D3). The number of MS/MS spectra obtained from the peptide and protein identified (using Mudpit approach with SCX and C18 separation prior to MS, ~13-hour run) for both labeled and unlabeled analysis (Table 2) showed the increased number of proteins identified in both the BC (159, 167) and control samples (149, 154), compared to acetone alone (methods 1 and 5) with only 117 and 115 proteins identified in the same samples. The efficiency of the acetone/TCA at HSC technique (methods 4 and 5) was supported by the increase in the concentration of the total protein extracted for each sample (around 1,000 μg) and was also confirmed by minimal protein loss demonstrated in the washes (Fig. 1 and Table 1: D5). A Venn diagram was used to demonstrate that an increased number of proteins were identified by LC-MS/MS using the acetone/TCA protein precipitation at HSC technique in both the control (Fig. 2A) and metastatic BC (Fig. 2B) urine samples. Unfortunately, the application of double precipitation with cell shearing showed no additional benefit and instead led to the loss of proteins with each sequential extraction (Fig. 1: B2, B6).
Figure 2.
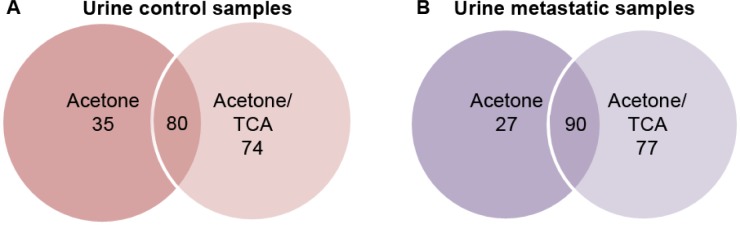
Venn diagram comparison of proteins identified by LC-MS/MS in the control and metastatic BC samples following acetone and acetone/TCA precipitation. The two major precipitation techniques, acetone and acetone/TCA, were compared for the urine samples from healthy controls (A) and metastatic BC patients (B). The MS data (Table 2) for the protein extracts indicated that an increased number of proteins were identified in the acetone/TCA protein precipitates in both the controls (154) and BC samples (167), compared to 115 and 117, respectively. The overlap represents the proteins in common.
Urinary proteins in BC and control samples at HSC
Comparing all the precipitation techniques (methods 1–4), our gel results clearly demonstrated that the HSC was essential to desalt and remove non-soluble materials, and isolate urinary proteins in the BC samples (Fig. 1: C1–3 LSC against D1–3 HSC). As mentioned above, the LC-MS/MS data confirmed that urine proteins extracted using methods 1–4 with LSC were either not detectable or low (Table 2: controls A1–2 and BC samples C1–4). However, the same methods with HSC could achieve a far superior extraction for both the control (Fig. 1: A3–4, B1–4) and BC samples (Fig. 1: D1–3).
Precipitation with organic solvent and acid alone
Although the application of HSC (method 5) has been shown to be more effective in desalting and concentrating the urine samples, acetone (method 1, see Fig. 1: A4, B1, and D1) was more successful than TCA (method 2) as shown on SDS-PAGE (Fig. 1: A3, B3, and D2). This finding was further confirmed with the LC-MS/MS data from acetone (Table 2: A4, B1, and D1) and TCA (Table 2: A3, B3, and D2). Our results also demonstrated that the combination of organic solvent and acid with HSC (method 4 and 5) provided a superior extract (Fig. 1 and Table 2: A6, B4, and D3).
Other precipitation methods
In addition to the above-mentioned methods, we also found that ultrafiltration of urine was not an effective protein precipitation technique (Fig. 1: A5 and C4), the protein extracts from this method had a low protein yield and were difficult to detect with MS (Table 2: A5 and C4).
To achieve an increased protein yield, a double precipitation method was applied, in which urinary proteins were first precipitated by one of the methods 1–4 at HSC (5), and then the pellet was re-precipitated with the CPC method to remove the GAG. Although this technique was attempted numerous times, it was ineffective in further purifying the samples. Instead, there was aggregation and high salt contamination on the gels (Fig. 1: B5). Despite clean-up attempts using SCX and C18 stage tips, this method showed the least total protein extract, and proteins were not detectable using MS (Table 2: B5), even though the washes indicated no protein loss (Fig. 1: C5). This technique was very laborious with excessive sample handling and is not recommended.
Our results indicate that the approach of acetone/TCA at HSC is an optimal method for increased protein yield, number of protein detected with LC-MS/MS, ease of handling, and reproducibility compared to other approaches.
Discussion
In the current study, our objective was to determine the best method to achieve the highest yield from urine samples for future biomarker discovery of BC patients. To achieve a comprehensive comparison for the preparation of urinary proteins, seven different techniques were systematically investigated, which included 16 individual precipitation approaches. To optimize the removal of proteins, the techniques applied examined the effect of organic solvent, acid, and ultrafiltration. We found that a combination of organic solvent and acid all at LSC and then HSC along with two additional purification steps included eight combination approaches (Table 1). Although 16 approaches (single methods: 1–4; combination methods: 5–7) were investigated, only 11 of the techniques successfully achieved a protein pellet. The effectiveness of these different techniques was different as shown on SDS-PAGE and LC-MS/MS (Table 2). SDS-PAGE technique allowed for the identification of abundant proteins. The clarity, drag, aggregation, and number of distinct bands are good indicators of the downstream success in the identification of proteins related to the contaminants, salts, chemical degradation, and sampling degradation content within the samples.
LC-MS/MS is one of the most popular proteomic techniques and requires a very small volume of protein sample for analysis. Because of the low concentration of urinary proteins and high concentration of salt and metabolites, the purity of the urine protein pellet for proteomic analysis is critical. Therefore, the aim is to consistently achieve a high quality recovery, urinary proteins extract for downstream analysis. The data presented here clearly demonstrate the importance of the precipitation and concentration method applied to urine samples. Although other literature show a common theme for urine collection, processing, and downstream analysis,16 our current study is only a guide to the development of a standard method. The precipitation of urine proteins occurs in solutions of extreme ionic strength, high concentrations of organic solvents, and low pH. Our findings indicate that the techniques applied to urine preparation described in the literature were unsuccessful when applied to our metastatic BC urine samples. We found that aggregation and high salt concentration in most of the protein extracts from BC urine samples made it difficult to obtain sufficient MS spectra. Following a detailed review of urine sample preparation, using different techniques for proteomic biomarker studies in BC,17 we modified the urine preparation techniques to improve protein yield for MS analysis.
In this study, the most robust and reproducible techniques were acetone at HSC (methods 1 and 5) and acetone/TCA at HSC (methods 4 and 5). Overall, the best resolution on the gels and the highest number of proteins were achieved using the precipitation with acetone/TCA at HSC in both the metastatic BC and control urine samples. Our results demonstrate an optimized urine sample preparation method, which allows us to obtain the best urinary protein MS data. This recommended method can produce a protein-rich fraction and limit protein loss. In addition, we also found that this approach is a robust and reproducible analytical protocol that can separate the proteins from interfering compounds and achieve the maximum yield of protein precipitate with minimal sample handling. Furthermore, the protein fraction from this method can produce the highest resolution on SDS-PAGE and the greatest number of proteins by LC-MS/MS.
Urine sample preparation is a complicated process. There is no standardized urine sample preparation method currently available. The experimental design for urine sample preparation should also include sample collection and storage as sample collection and storage are crucial to the final protein analysis. The pooled urine specimens, within disease and non-disease states, can enhance the population proteins to estimate the prevalence of disease in the population and dilute the natural biological variance for proteomic studies.
In summary, although some progress has been made, challenges still remain in the development of an optimal sample preparation method for proteomic analysis of urine for gel-based electrophoresis and label-free LC-MS/MS analysis. This study provides optimal conditions for urinary protein precipitation for our metastatic BC samples. We demonstrate that the acetone/TCA at HSC is the most promising approach for urine preparation. Our current recommended urine preparation approach also includes the assessment of biological characteristics to ensure that there are no major underlying conditions that could affect urine stability and its analysis. The discovery of novel proteins and the validity of biomarker discovery greatly rely on the handling of urine samples in a uniform manner and thus highlight the need for a reproducible standardized protocol.
Acknowledgments
We appreciated the support received from Professor John Kearsley.
Glossary
Abbreviations
- BC
breast cancer
- BSA
bovine serum albumin
- CPC
cetyl pyridium chloride
- FDR
false discovery rate
- GAG
glyco amino glycan
- HSC
high speed centrifugation
- LC-MS/MS
liquid-chromatography/tandem-mass spectrometry
- MS
mass spectrometry
- RB
rehydration buffer
- SDS-PAGE
sodium dodecyl sulphate-polyacrylamide gel electrophoresis
- TCA
trichloracetic acid
- UF
ultrafiltration
Footnotes
Author Contributions
Conceived and designed the experiments: JB, VW. Analyzed the data: JB, VW. Wrote the first draft of the manuscript: JB. Contributed to the writing of the manuscript: JB, VW, YL. Agree with manuscript results and conclusions: JB, VW, PS, PG, YL. Jointly developed the structure and arguments for the paper: JB, VW, PS, PG, YL. Made critical revisions and approved final version: JB, VW, YL. All authors reviewed and approved of the final manuscript.
ACADEMIC EDITOR: Barbara Guinn, Editor in Chief
FUNDING: The authors would like to thank the St George Cancer Care Centre Research Trust Fund for supporting this study. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: Authors disclose no potential conflicts of interest.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Upon submission manuscript was subject to anti-plagiarism scanning. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties. This journal is a member of the Committee on Publication Ethics (COPE).
REFERENCES
- 1.Cho SH, Jung BH, Lee SH, Lee WY, Kong G, Chung BC. Direct determination of nucleosides in the urine of patients with breast cancer using column-switching liquid chromatography-tandem mass spectrometry. Biomed Chromatogr. 2006;20:1229–1236. doi: 10.1002/bmc.689. [DOI] [PubMed] [Google Scholar]
- 2.Gaikwad NW, Yang L, Muti P, et al. The molecular etiology of breast cancer: evidence from biomarkers of risk. Int J Cancer. 2008;122:1949–1957. doi: 10.1002/ijc.23329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adachi J, Kumar C, Zhang Y, Olsen JV, Mann M. The human urinary proteome contains more than 1500 proteins, including a large proportion of membrane proteins. Genome Biol. 2006;7:R80. doi: 10.1186/gb-2006-7-9-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Said J. Biomarker discovery in urogenital cancer. Biomarkers. 2005;10(suppl 1):S83–S86. doi: 10.1080/13547500500215050. [DOI] [PubMed] [Google Scholar]
- 5.Verdier JM, Dussol B, Dupuy P, Berland Y, Dagorn JC. Preliminary treatment of urinary proteins improves electrophoretic analysis and immunodetection. Clin Chem. 1992;38:860–863. [PubMed] [Google Scholar]
- 6.Pieper R. Preparation of urine samples for proteomic analysis. Methods Mol Biol. 2008;425:89–99. doi: 10.1007/978-1-60327-210-0_8. [DOI] [PubMed] [Google Scholar]
- 7.Thongboonkerd V, Mungdee S, Chiangjong W. Should urine pH be adjusted prior to gel-based proteome analysis? J Proteome Res. 2009;8:3206–3211. doi: 10.1021/pr900127x. [DOI] [PubMed] [Google Scholar]
- 8.Sun W, Li F, Wu S, et al. Human urine proteome analysis by three separation approaches. Proteomics. 2005;5:4994–5001. doi: 10.1002/pmic.200401334. [DOI] [PubMed] [Google Scholar]
- 9.Tyan YC, Guo HR, Liu CY, Liao PC. Proteomic profiling of human urinary proteome using nano-high performance liquid chromatography/electrospray ionization tandem mass spectrometry. Anal Chim Acta. 2006;579:158–176. doi: 10.1016/j.aca.2006.07.033. [DOI] [PubMed] [Google Scholar]
- 10.Kentsis A, Monigatti F, Dorff K, Campagne F, Bachur R, Steen H. Urine proteomics for profiling of human disease using high accuracy mass spectrometry. Proteomics Clin Appl. 2009;3:1052–1061. doi: 10.1002/prca.200900008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci U S A. 2004;101:13368–13373. doi: 10.1073/pnas.0403453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fung ET, Yip TT, Lomas L, et al. Classification of cancer types by measuring variants of host response proteins using SELDI serum assays. Int J Cancer. 2005;115:783–789. doi: 10.1002/ijc.20928. [DOI] [PubMed] [Google Scholar]
- 13.Liu X, Shao C, Wei L, et al. An individual urinary proteome analysis in normal human beings to define the minimal sample number to represent the normal urinary proteome. Proteome Sci. 2012;10:70. doi: 10.1186/1477-5956-10-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Catterall JB, Rowan AD, Sarsfield S, Saklatvala J, Wait R, Cawston TE. Development of a novel 2D proteomics approach for the identification of proteins secreted by primary chondrocytes after stimulation by IL-1 and oncostatin M. Rheumatology. 2006;45:1101–1109. doi: 10.1093/rheumatology/kel060. [DOI] [PubMed] [Google Scholar]
- 15.Coumans JV, Gau D, Poljak A, Wasinger V, Roy P, Moens P. Green fluorescent protein expression triggers proteome changes in breast cancer cells. Exp Cell Res. 2014;320:33–45. doi: 10.1016/j.yexcr.2013.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thomas CE, Sexton W, Benson K, Sutphen R, Koomen J. Urine collection and processing for protein biomarker discovery and quantification. Cancer Epidemiol Biomarkers Prev. 2010;19:953–959. doi: 10.1158/1055-9965.EPI-10-0069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beretov J, Wasinger VC, Graham PH, Millar EK, Kearsley JH, Li Y. Proteomics for breast cancer urine biomarkers. Adv Clin Chem. 2014;63:123–167. doi: 10.1016/b978-0-12-800094-6.00004-2. [DOI] [PubMed] [Google Scholar]