With improving expertise and supportive care, cord blood (CB) transplants are now associated with outcomes comparable to unrelated and sibling donor transplants.1 There is a need to increase the size and diversity of international CB inventories and to retain cryopreserved CB units for as long as safely possible. There are in vitro data supporting >90% recovery of hematopoietic progenitor cells from frozen CB cells stored for up to 12 years,2 and data showing that progenitor cell recoveries from short-term freeze samples ranging from 2 to 8 weeks are comparable to those cryopreserved for 10–15 years.3,4 Broxmeyer et al.5 reported adequate recovery (80–100%) of granulocyte–macrophage and multi-potential hematopoietic progenitors from functional CB units cryopreserved for up to 23.5 years as well as recovery of viable progenitor cells from CBs frozen for 15 years, which were able to generate CD45+ human cell engraftment when infused into sublethally irradiated NOD-SCID mice and were comparable to that reported with fresh CB.6 Despite this in vitro evidence, transplant physicians still remain concerned about the possible loss of integrity of frozen CB units associated with longer durations of storage.7 In one report, the incidence of bag breaks over a 6.5-year period was 3.5%, where 75% of the breaches occurred in units that had been cryopreserved for >2 years.7 Therefore, it is important to determine whether prolonged time of cryopreservation and storage may adversely affect clinical transplant outcomes, and with the new US Food and Drug Administration licensing regulations for CB units this question has become even more relevant: ‘is there an expiration date for CB unit in storage?’.
We reviewed 86 consecutive single CB transplant recipients in the period from March 1996 to June 2011, where 15 patients received CB units older than 5 years (CB age 12.2 years = 1; >8 years = 3; >7 years = 1; >6 years = 2; >5 years = 8). The vast majority of the CB units were obtained from NMDP (National Marrow Donor Program) or Netcord Banks (American Red Cross Portland = 1; AUNCB = 1; Australia = 1; Barcelona = 5; Belgium = 5; Bergan Paramus, NJ, USA = 1; Caitlin Raymond, UK = 1; NC, USA = 1; Colorado = 9; Dusseldorf = 10; France = 1; JP McCarthy, USA = 1; London = 5; MD Anderson = 2; Italy = 9; NYCB = 16; Puget Sound, OR, USA = 1; Spain = 2; St Louis = 13; Tokyo = 1). All CB units underwent washing and RBC depletion before freezing. The median length of storage of CB units was 2 years (range, 0.03–12 years). For the purpose of analysis, duration of CB unit storage was divided into four equal quartiles as well as an arbitrary cutoff of 5 years based on the common practice adopted by some public and private CB banks. Standard thawing procedures were followed at the MD Anderson Cell Therapy Laboratory.8 The pre-infusion viabilities were >90%. Patient and CB unit characteristics are described in Table 1.
Table 1.
Patient and CB characteristics
| Quartiles
|
Old vs new CBU
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Q1 (n = 21) | Q2 (n = 22) | Q3 (n = 22) | Q4 (n = 21) | P-value | Old CBU (n = 15) | New CBU (n = 71) | P-value | |
| Weight, kg | 25 (5–76) | 24 (8–76) | 55 (10–78) | 24 (9–93) | 0.1 | 24 (9–80) | 31 (5–93) | 0.3 |
| Age, years | 8.6 (0.3–41) | 7.3 (0.8–57) | 18.4 (1.7–54) | 7.3 (1–53) | 0.08 | 7.3 (1.5–52.6) | 8.8 (0.4–56.5) | 0.3 |
| Diagnosis | 0.2 | 0.03 | ||||||
| ALL | 9 (43%) | 9 (41%) | 10 (45%) | 8 (38%) | 5 (33%) | 31 (44%) | ||
| AML/MDS | 5 (24%) | 10 (45%) | 8 (36%) | 3 (14%) | 3 (20%) | 23 (32%) | ||
| CML | 1 (5%) | 0 | 1 (5%) | 2 (10%) | 1 (7%) | 3 (4%) | ||
| Others | 6 (28%) | 3 (14%) | 3 (14%) | 8 (38%) | 6 (40%) | 14 (20%) | ||
| Bone marrow blasts at time of transplant (%) | 4 (0–90) | 2 (0–90) | 3 (0–38) | 3 (0–73) | 1.0 | 3 (1–73) | 2 (0–90) | 0.8 |
| Race | 0.05 | <0.0001 | ||||||
| Caucasian | 16 (76%) | 13 (59%) | 14(64%) | 8 (38%) | 6 (40%) | 45 (63%) | ||
| Black | 1 (5%) | 1 (5%) | 2 (9%) | 4 (19%) | 3 (20%) | 5 (7%) | ||
| Others | 4 (19%) | 8 (36%) | 6 (27%) | 9 (43%) | 6 (40%) | 21 (30%) | ||
| Gender (female) | 11 (52%) | 9 (41%) | 9 (41%) | 11 (52%) | 0.8 | 8 (53%) | 32 (45%) | 0.6 |
| Myeloablative regimena | 20 (95%) | 20 (91%) | 15 (68%) | 11 (52%) | 0.002 | 7 (47%) | 59 (83%) | 0.005 |
| ATG use | 3 (14%) | 4 (18%) | 7 (32%) | 5 (24%) | 0.6 | 5 (33%) | 14 (20%) | 0.5 |
| Duration of storage (years), median (range) | 0.07 (0–0.7) | 1.3 (0.71–1.96) | 2.8 (1.97–3.87) | 5.3 (3.88–12.2) | 0.1 | 5.7 (5–12) | 1.5 (0.03–4.8) | <0.0001 |
| Cryo_vol (mL) | 30 (25–170) | 32.6 (25–351) | 44.4 (11–300) | 37 (24–340) | 0.2 | 37 (24–205) | 34 (11–351) | 0.8 |
| TNC (×106) | 1200 (42.5–6192) | 1720 (220–3432) | 1539 (305.8–2979.2) | 1175 (360–2720) | 0.6 | 1107 (260–2331) | 1380 (42.5–6192) | 0.1 |
| TNC (×106)/kg | 40.1 (2.36–564) | 37.7 (6.5–174.7) | 35.6 (4.5–146.4) | 45.2 (17.6–107) | 0.5 | 41.9 (17.6–107) | 40.2 (2.4–564) | 0.6 |
| CD34 (×106)/kg | 0.14 (0–2.4) | 0.15 (0–1.1) | 0.08 (0–0.6) | 0.14 (0–1.1) | 0.7 | 0.16 (0.08–0.68) | 0.1 (0–2.4) | 0.9 |
| Processing | 0.1 | 0.3 | ||||||
| None | 19 (91%) | 14 (64%) | 16 (73%) | 19 (91%) | 14 (93%) | 54 (76%) | ||
| RBC depletion | 1 (5%) | 3 (14%) | 2 (9%) | 1 (5%) | 1 (7%) | 6 (8%) | ||
| Sex mismatch | 0.9 | 0.5 | ||||||
| No mismatch | 10 (48%) | 9 (41%) | 12 (55%) | 11 (52%) | 7 (47%) | 35 (49%) | ||
| Male to female | 5 (24%) | 4 (18%) | 3 (14%) | 5 (24%) | 5 (33%) | 12 (17%) | ||
| Female to male | 5 (24%) | 3 (73%) | 6 (27%) | 5 (24%) | 3 (20%) | 16 (23%) | ||
| HLA match | 0.6 | 0.7 | ||||||
| ≥2 Ag MM | 6 (29%) | 10 (45%) | 10 (46% | 10 (48%) | 5 (33%) | 31 (44%) | ||
| 1 Ag MM | 10 (47%) | 7 (32%) | 10 (46%) | 7 (33%) | 6 (40%) | 28 (40%) | ||
| No mismatcha | 5 (24%) | 3 (14%) | 1 (5%) | 3 (14%) | 3 (20%) | 9 (13%) | ||
Abbreviations: ATG = anti-thymocyte globulin; CB =cord blood; CBU =CB unit; Cryo =cryopreservation; MDS = myelodysplastic syndrome; MM =mismatch; TNC =total nucleated count. The patients and CB units were divided into four equal quartiles and arbitrary distribution based on 5-year cutoff. Q = quartiles Q1: age <0.7 years (median: 0.07; n =21); Q2: 0.71–1.96 years (median: 1.3 years; n =22); Q3: 1.97–3.87 years (median: 2.8 years; n =22) and Q4: 3.88–12.2 years (median: 5.3 years; n = 21). ‘Old’ CB units (age ≥ 5 years; n = 15, median = 5.7 years (5–12.2 years); ‘new’ CB units (age <5 years; n =71; median =2 years (0.3–4.8 years). One-way analysis of variance test was used to compare means for the cell product doses between different CB quartiles. Independent t-test was utilized to compare means between ‘old’ vs ‘new’ CB units. All analyses were performed using SPSS software (IBM Software, Chicago, IL, USA).
Conditioning regimens included: BU/clofarabine/thiotepa =3; BU/cytoxan = 5; cytoxan/etoposide/TBI =1; cytoxan/fludarabine =5; cytoxan/TBI =1; cytoxan/TBI/thiotepa =1; fludarabine/BU =4; fludarabine/cytoxan/TBI =1; fludarabine/melphalan = 3; fludarabine/melphalan/TBI =23; fludarabine/TBI =10; melphalan/etoposide/TBI =1; melphalan/thiotepa/fludarabine =1; thiotepa/BU/cytoxan =27.
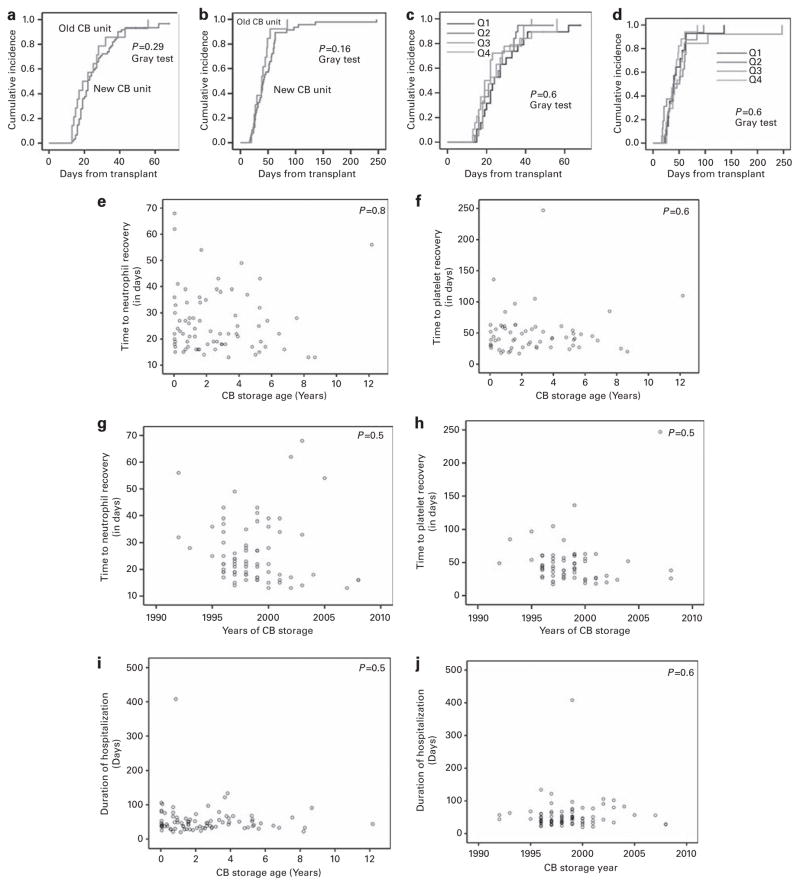
Seventy-six (89%) patients achieved engraftment (early death = 4; graft failure = 11). In univariate analysis, only the degree of HLA mismatch was significantly associated with engraftment (P = 0.04; χ2-test). For the whole cohort, median time to neutrophil recovery was 22 days (range 13–68 days) and platelet recovery was 43 days (range 20–247 days). Median time to neutrophil and platelet recovery specifically for ‘old’ CB units was as follows: CB age >5 years: 24 and 40 days; age >6 years: 19 and 42 days; age >7 years: 28 and 85 days; age >8 years: 13 and 25 days; age >12 years: 56 and 109 days, respectively. No significant differences were observed in the time to neutrophil or platelet recovery when comparing ‘old’ vs ‘new’ CB unit recipients (Figures 1a and b), among CB quartiles (Figures 1c and d), CB storage duration (Figures 1e and f) or the year of CB storage (Figures 1g and h). Significant factors affecting the time to neutrophil recovery included recipient age <8 years (20 vs 23 days; P = 0.05); weight <30 kg (19 vs 24 days; P = 0.011); total nucleated count (TNC; ×106/kg) count >47 (18 vs 25 days; P = 0.034); and CD34+ (×106/kg) cell count >0.235 (18 vs 23 days; P = 0.002).
Figure 1.
CB transplant clinical outcomes. The CB units were divided into quartiles: Q =quartiles Q1: age <0.7 years (median: 0.07; n =21); Q2: 0.71–1.96 years (median: 1.3 years; n =22); Q3: 1.97–3.87 years (median: 2.8 years; n =22) and Q4: 3.88–12.2 years (median: 5.3 years; n =21). An arbitrary cutoff of 5 years was used to distinguish CB units: ‘old’ CB units (age ≥5 years; n =15, median =5.7 years (5–12.2 years) and ‘new’ CB units (age <5 years; n =71); median =2 years (0.3–4.8 years). Graft failure was defined as failure to achieve three consecutive days of ANC ≥500/mm3 by post-transplant day 42 or subsequent loss of neutrophil recovery. Neutrophil recovery was defined as the first of the three days in which ANC >500/μL for three consecutive days; platelet recovery was defined as first of seven days when the platelet count >20 000/μL for seven consecutive days. Cumulative incidences (CIs) were calculated using NCSS software (Kaysville, UT, USA). Gray’s test was used to compare the CIs at significance level of 0.05. The CI of (a) time to neutrophil recovery and (b) time to platelet recovery for ‘old’ vs ‘new’ CB units; and (c) time to neutrophil recovery and (d) time to platelet recovery for four different CB unit quartiles. Dot plot graph of (e) time to neutrophil recovery and (f) time to platelet recovery as a function of CB storage age. Pearson’s correlation analysis is used to evaluate for significance. Dot plot graph of (g) time to neutrophil recovery and (h) time to platelet recovery as a function of duration of storage of CB units (in years). Pearson’s correlation analysis is used to evaluate for significance. Dot plot graph of duration of hospitalization as a function of (i) CB storage age and (j) year of CB storage. Pearson’s correlation analysis is used to evaluate for significance.
No differences were observed for duration of hospitalization and storage duration or storage year (Figures 1i and j). Significant factors influencing the duration of hospitalization to <45 days included: age <8 years (62% vs 39%; P = 0.04), engraftment status (55% vs 10%; P = 0.008) and HLA match (75% vs 45%; P = 0.09).
Median follow-up for survivors was 8 years. Day-100 and 1-year non-relapse mortality for the whole cohort was 19% and 35%, respectively. The Kaplan–Meir method was used to calculate survival from the date of transplant.9 The estimated 2-year, 5-year and 10-year survival for whole cohort were 43.5%, 40% and 35%, respectively. No survival differences were seen based on the CB storage age. The 3-, 6- and 12-month survival for ‘old’ vs ‘new’ CB units was 80%, 80% and 60% vs 79%, 62% and 50%, respectively. The 3-, 6- and 12-month survival breakdown for the four quartiles were 76%, 67% and 62% vs 73%, 59% and 41% vs 86%, 67% and 53% vs 81%, 67% and 52%, respectively. Significant factors affecting the survival in univariate analysis included myeloablative conditioning (P = 0.007) and degree of HLA mismatch (P = 0.05).
This is the first clinical report of human single CB transplants evaluating the effect of duration of CB storage. Although a majority of the CB transplant recipients in our analysis were children, which may have accounted for a higher TNC and CD34+ cell dose as compared with adults, the overall age distribution was comparable in all different cohorts. Even though a lower number of ‘old’ CB patients received ablative conditioning, which was an independent predictor of survival, no differences were seen in the survival outcomes when comparing old vs new CB recipients. Major limitations of our analyses include its retrospective nature as well as small number of cases. We conclude that, in our restricted data set, the cryopreserved storage time of CB units did not affect engraftment, hematopoietic recovery, duration of hospitalization or survival. CB units within this age range should be considered a viable source of hematopoietic stem cells for allo-SCTs. Larger studies are needed to confirm our findings as well as to examine longer durations of cell storage.
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
References
- 1.Laughlin MJ, Eapen M, Rubinstein P, Wagner JE, Zhang MJ, Champlin RE, et al. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N Engl J Med. 2004;351:2265–2275. doi: 10.1056/NEJMoa041276. [DOI] [PubMed] [Google Scholar]
- 2.Mugishima H, Harada K, Chin M, Suzuki T, Takagi K, Hayakawa S, et al. Effects of long-term cryopreservation on hematopoietic progenitor cells in umbilical cord blood. Bone Marrow Transplant. 1999;23:395–396. doi: 10.1038/sj.bmt.1701580. [DOI] [PubMed] [Google Scholar]
- 3.Kobylka P, Ivanyi P, Breur-Vriesendorp BS. Preservation of immunological and colony-forming capacities of long-term (15 years) cryopreserved cord blood cells. Transplantation. 1998;65:1275–1278. doi: 10.1097/00007890-199805150-00024. [DOI] [PubMed] [Google Scholar]
- 4.Yamamoto S, Ikeda H, Toyama D, Hayashi M, Akiyama K, Suzuki M, et al. Quality of long-term cryopreserved umbilical cord blood units for hematopoietic cell transplantation. Int J Hematol. 2011;93:99–105. doi: 10.1007/s12185-010-0755-x. [DOI] [PubMed] [Google Scholar]
- 5.Broxmeyer HE, Lee MR, Hangoc G, Cooper S, Prasain N, Kim YJ, et al. Hematopoietic stem/progenitor cells, generation of induced pluripotent stem cells, and isolation of endothelial progenitors from 21- to 23.5-year cryopreserved cord blood. Blood. 2011;117:4773–4777. doi: 10.1182/blood-2011-01-330514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Broxmeyer HE, Srour EF, Hangoc G, Cooper S, Anderson SA, Bodine DM. High-efficiency recovery of functional hematopoietic progenitor and stem cells from human cord blood cryopreserved for 15 years. Proc Natl Acad Sci USA. 2003;100:645–650. doi: 10.1073/pnas.0237086100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thyagarajan B, Berger M, Sumstad D, McKenna DH., Jr Loss of integrity of umbilical cord blood unit freezing bags: description and consequences. Transfusion. 2008;48:1138–1142. doi: 10.1111/j.1537-2995.2008.01688.x. [DOI] [PubMed] [Google Scholar]
- 8.Rubinstein P, Dobrila L, Rosenfield RE, Adamson JW, Migliaccio G, Migliaccio AR, et al. Processing and cryopreservation of placental/umbilical cord blood for unrelated bone marrow reconstitution. Proc Natl Acad Sci USA. 1995;92:10119–10122. doi: 10.1073/pnas.92.22.10119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Amer Statist Assoc. 1958;53:457–481. [Google Scholar]