Abstract
The survival of Staphylococcus aureus in the lungs of mice was studied under various conditions. Doses of 107 to 109 washed staphylococci were quantitatively introduced into the lungs after intratracheal inoculation in mice under either ether or sodium pentobarbital anesthesia. Mice were sacrificed at intervals, the lungs were excised and homogenized, and the cocci were enumerated by plate count. The 50% lethal dose was 6 × 108 cocci per mouse, and mice died within 24 h but without proliferation of the inoculum. Mice given 108 cocci intratracheally under pentobarbital anesthesia regularly survived and eliminated the organisms over a 48-h period. The use of ether anesthesia resulted in persistence of the inoculum for up to 48 h, but the organisms were then eliminated. Inability to proliferate did not appear to result from a lack of iron because pretreatment of the mice with ferric ammonium citrate or Imferon did not alter inoculum survival. Staphylococci inoculated intratracheally in mice infected with influenza virus 3 to 21 days previously showed no enhanced persistence or multiplication. Cocci preclumped with fibrinogen, inocula mixed with 10 times the number of Formalin-killed staphylococci, or inocula of the encapsulated Smith strain did not survive any better than conventional inocula, suggesting that phagocytosis might not be the sole mechanism for elimination. However, a sedimentable fraction from normal or infected lung homogenates proved either inhibitory or cidal for staphylococci in vitro.
Full text
PDF

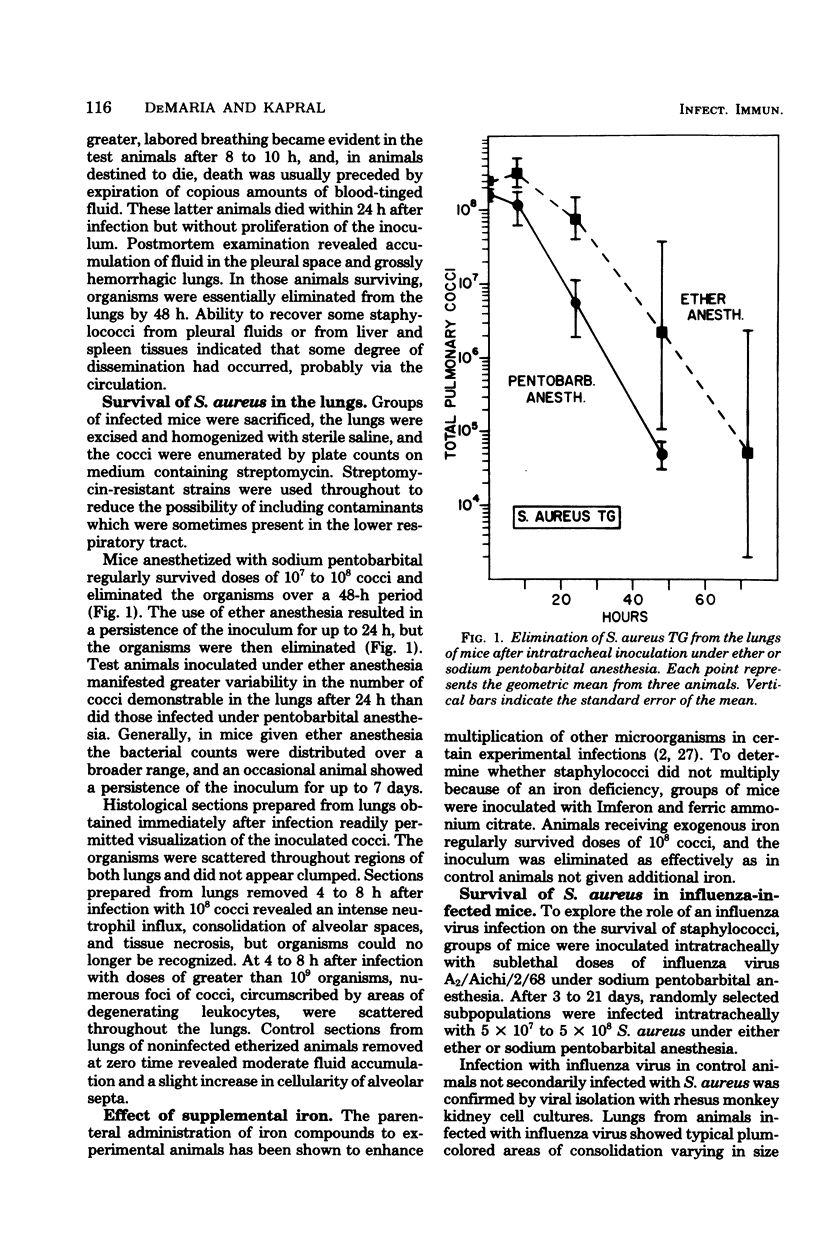
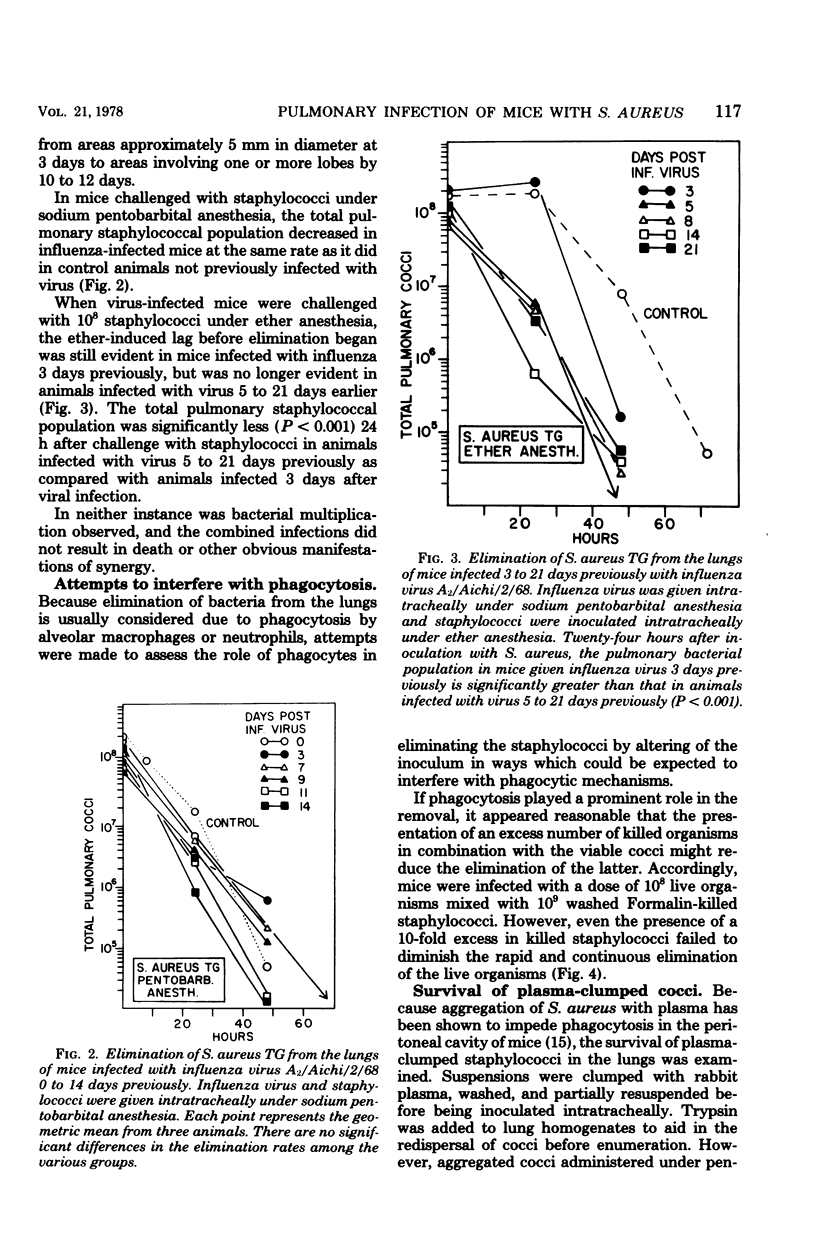
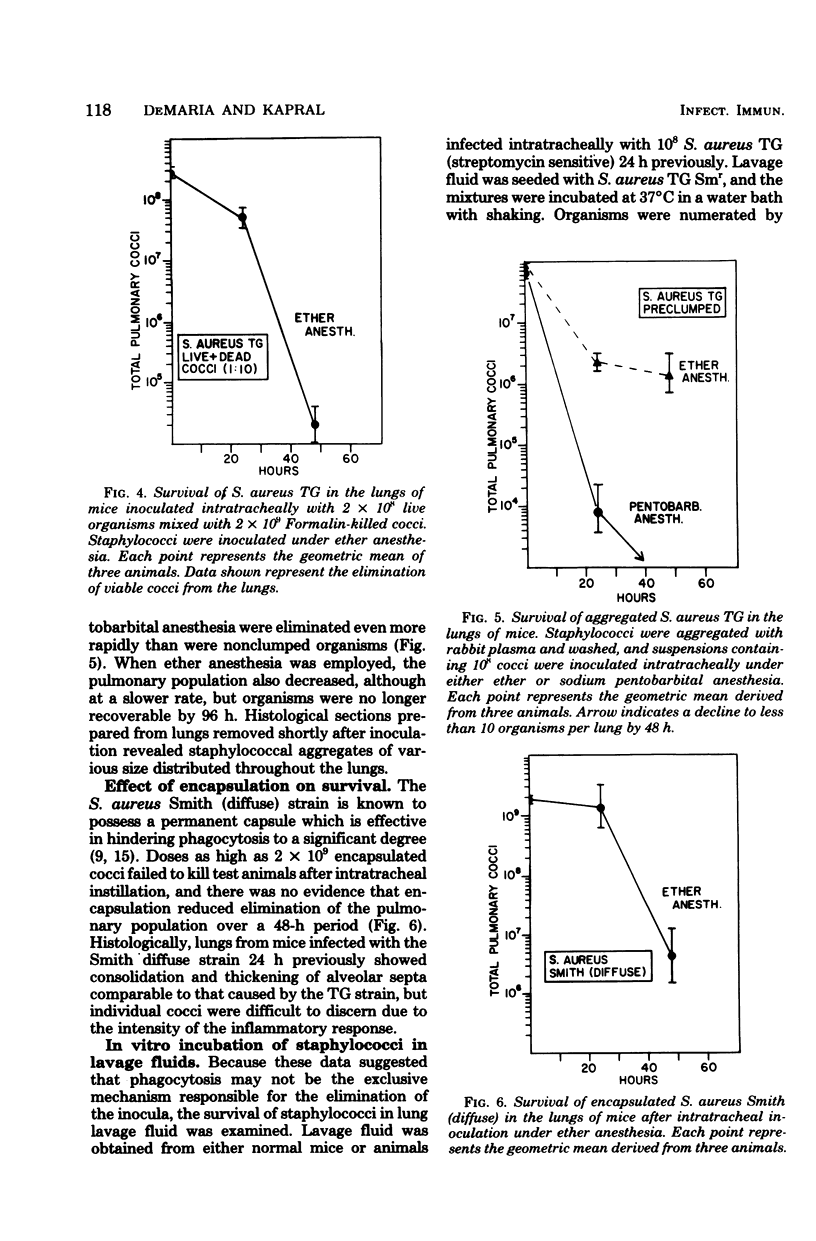
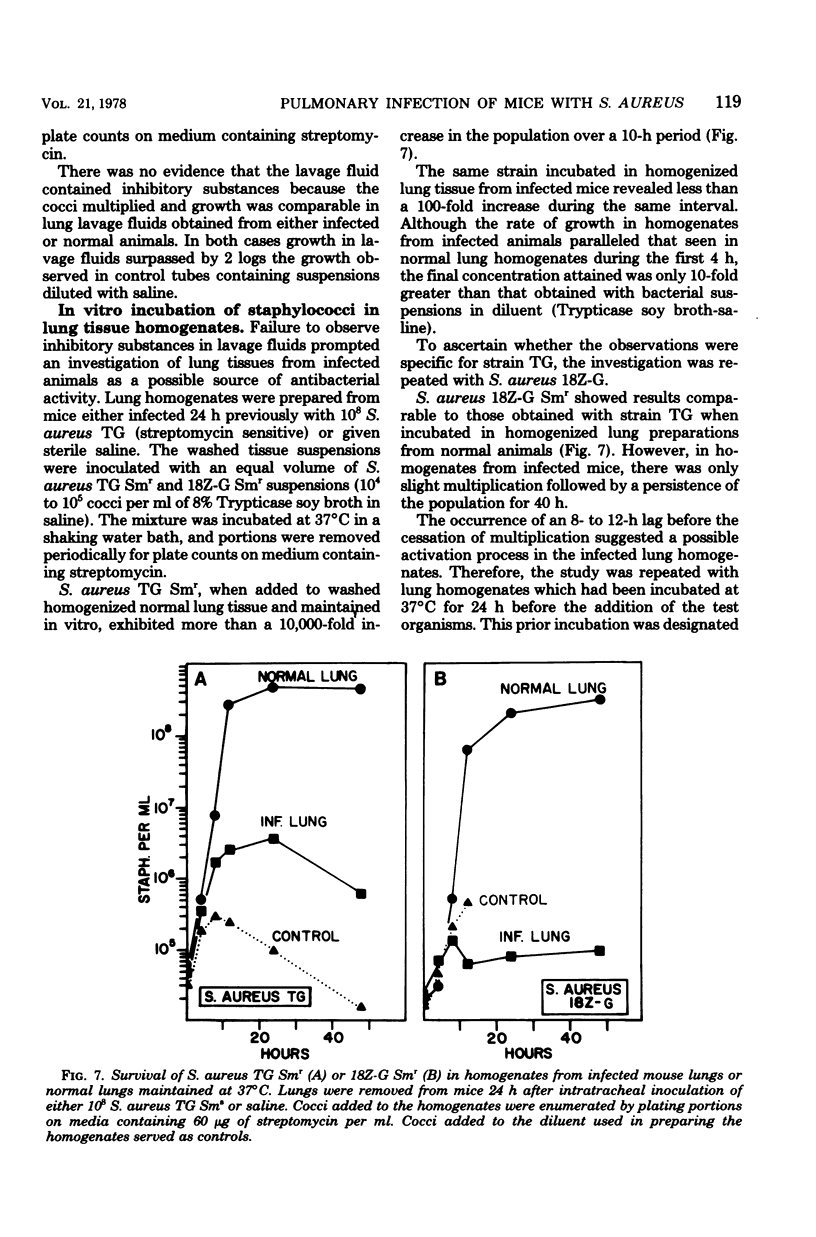



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruce D. L., Wingard D. W. Anesthesia and the immune response. Anesthesiology. 1971 Mar;34(3):271–282. doi: 10.1097/00000542-197103000-00017. [DOI] [PubMed] [Google Scholar]
- Bullen J. J., Ward C. G., Wallis S. N. Virulence and the role of iron in Pseudomonas aeruginosa infection. Infect Immun. 1974 Sep;10(3):443–450. doi: 10.1128/iai.10.3.443-450.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN G. M., KASS E. H. FACTORS INFLUENCING THE CLEARANCE OF BACTERIA BY THE LUNG. J Clin Invest. 1964 Apr;43:769–776. doi: 10.1172/JCI104961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN G. M., KASS E. H. THE ROLE OF THE ALVEOLAR MACROPHAGE IN THE CLEARANCE OF BACTERIA FROM THE LUNG. J Exp Med. 1964 Jan 1;119:167–176. doi: 10.1084/jem.119.1.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein E., Akers T., Prato C. Role of immunity in viral-induced bacterial superinfections of the lung. Infect Immun. 1973 Nov;8(5):757–761. doi: 10.1128/iai.8.5.757-761.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green G. M., Goldstein E. A method for quantitating intrapulmonary bacterial inactivation in individual animals. J Lab Clin Med. 1966 Oct;68(4):669–677. [PubMed] [Google Scholar]
- JANSSEN R. J., CHAPPELL W. A., GERONE P. J. SYNERGISTIC ACTIVITY BETWEEN PR8 INFLUENZA VIRUS AND STAPHYLOCOCCUS AUREUS IN THE GUINEA PIG. Am J Hyg. 1963 Nov;78:275–284. [PubMed] [Google Scholar]
- Jakab G. J., Green G. M. Defect in intracellular killing of Staphylococcus aureus within alveolar macrophages in Sendai virus-infected murine lungs. J Clin Invest. 1976 Jun;57(6):1533–1539. doi: 10.1172/JCI108423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab G. J., Green G. M. Effect of hypersensitivity pneumonitis on the pulmonary defense mechanisms of guinea pig lungs. Infect Immun. 1973 Jan;7(1):39–45. doi: 10.1128/iai.7.1.39-45.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakab G. J., Green G. M. Variations in pulmonary antibacterial defenses among experimental animals. Infect Immun. 1975 Mar;11(3):601–602. doi: 10.1128/iai.11.3.601-602.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. P., Taylor-Robinson D., Slavin G. Pneumonia in mice produced by Neisseria gonorrhoeae. Br J Vener Dis. 1977 Feb;53(1):26–30. doi: 10.1136/sti.53.1.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAPRAL F. A., LI I. W. Virulence and coagulases of Staphylococcus aureus. Proc Soc Exp Biol Med. 1960 May;104:151–153. doi: 10.3181/00379727-104-25761. [DOI] [PubMed] [Google Scholar]
- Kapral F. A. Clumping of Staphylococcus aureus in the peritoneal cavity of mice. J Bacteriol. 1966 Oct;92(4):1188–1195. doi: 10.1128/jb.92.4.1188-1195.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapral F. A., Miller M. M. Product of Staphylococcus aureus responsible for the scalded-skin syndrome. Infect Immun. 1971 Nov;4(5):541–545. doi: 10.1128/iai.4.5.541-545.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapral F. A. Staphylococcus aureus: some host-parasite interactions. Ann N Y Acad Sci. 1974 Jul 31;236(0):267–276. doi: 10.1111/j.1749-6632.1974.tb41497.x. [DOI] [PubMed] [Google Scholar]
- Kapral F. A. Subcutaneous multiplication of exfoliatin-producing staphylococci. Infect Immun. 1976 Mar;13(3):682–687. doi: 10.1128/iai.13.3.682-687.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass E. H., Green G. M., Goldstein E. Mechanisms of antibacterial action in the respiratory system. Bacteriol Rev. 1966 Sep;30(3):488–497. doi: 10.1128/br.30.3.488-497.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaForce F. M. Effect of alveolar lining material on phagocytic and bactericidal activity of lung macrophages against Staphylococcus aureau. J Lab Clin Med. 1976 Nov;88(5):691–699. [PubMed] [Google Scholar]
- LaForce F. M., Kelly W. J., Huber G. L. Inactivation of staphylococci by alveolar macrophages with preliminary observations on the importance of alveolar lining material. Am Rev Respir Dis. 1973 Oct;108(4):784–790. doi: 10.1164/arrd.1973.108.4.784. [DOI] [PubMed] [Google Scholar]
- Melish M. E., Glasgow L. A. The staphylococcal scalded-skin syndrome. N Engl J Med. 1970 May 14;282(20):1114–1119. doi: 10.1056/NEJM197005142822002. [DOI] [PubMed] [Google Scholar]
- Morgan T. E. Pulmonary surfactant. N Engl J Med. 1971 May 27;284(21):1185–1193. doi: 10.1056/NEJM197105272842105. [DOI] [PubMed] [Google Scholar]
- Newhouse M., Sanchis J., Bienenstock J. Lung defense mechanisms (second of two parts). N Engl J Med. 1976 Nov 4;295(19):1045–1052. doi: 10.1056/NEJM197611042951905. [DOI] [PubMed] [Google Scholar]
- Payne S. M., Finkelstein R. A. Pathogenesis and immunology of experimental gonococcal infection: role of iron in virulence. Infect Immun. 1975 Dec;12(6):1313–1318. doi: 10.1128/iai.12.6.1313-1318.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rehder K., Sessler A. D., Marsh H. M. General anesthesia and the lung. Am Rev Respir Dis. 1975 Oct;112(4):541–563. doi: 10.1164/arrd.1975.112.4.541. [DOI] [PubMed] [Google Scholar]
- SELLERS T. F., Jr, SCHULMAN J., BOUVIER C., McCUNE R., KILBOURNE E. D. The influence of influenza virus infection on exogenous staphylococcal and endogenous murine bacterial infection of the bronchopulmonary tissues of mice. J Exp Med. 1961 Aug 1;114:237–256. doi: 10.1084/jem.114.2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VERLINDE J. D., MAKSTENIEKS O. Experimental respiratory infection in monkeys produced by influenza A virus and Staphylococcus aureus. Arch Gesamte Virusforsch. 1954 Apr 12;5(4):345–360. doi: 10.1007/BF01243004. [DOI] [PubMed] [Google Scholar]



