Abstract
Many Gram-negative bacteria employ N-acyl homoserine lactones (AHLs) as signal molecules for quorum sensing. The binding of AHLs to their target LuxR-type receptor proteins can effect changes in growth, virulence, and other phenotypes. LuxR-type receptors therefore present attractive pharmaceutical targets for control of bacterial pathogenesis. Here, we present X-ray crystallographic and computational evidence that the conformation of free AHLs is biased away from the conformation observed when bound to their cognate receptor due to the influence of an n→π* interaction. In this n→π* interaction, the p-type lone pair (n) of the N-acyl oxygen overlaps with the π* orbital of the lactone carbonyl group. This overlap results in the release of approximately 0.64 kcal/mol of energy. We also show that this interaction can be attenuated by installing electron-withdrawing groups on the N-acyl chain. Modulating this previously unappreciated interaction could present a new avenue towards effective inhibitors of bacterial quorum sensing.
Despite being unicellular organisms, bacteria have evolved mechanisms of chemical communication that regulate various physiological processes in response to cell density, a phenomenon known as quorum sensing.1,2 This process is receiving much attention because of its influence on biofilm formation and virulence. The principle mediators of these communication events in Gram-negative bacteria are the N-acyl homoserine lactones (AHLs, Figure 1).3,4 AHLs act by binding to intracellular LuxR-type receptors, which are then activated as transcription factors.5,6 Physiological transitions in bacterial colonies are induced when AHL concentrations cross particular thresholds. The strong influence of AHLs on bacterial behavior has attracted the attention of chemical biologists, as modulators of AHL-binding could impart exquisite control of bacterial pathogenesis.7 Towards this end, we noticed that AHLs have proximal carbonyl groups (Figure 1). We have shown that the conformations of molecules with proximal carbonyl moieties can be influenced by an n→π* interaction (Figure 2).8,9 Here, we sought to determine if an n→π* interaction could influence the conformation of an AHL.
Figure 1.
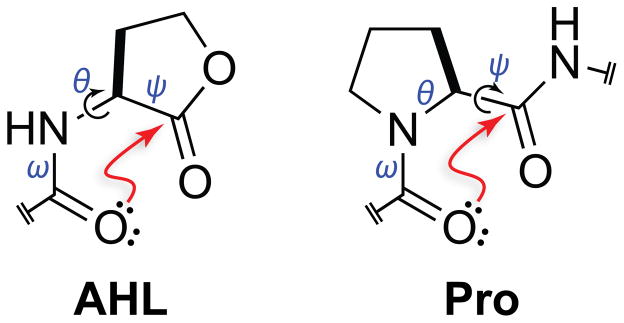
Structure of an N-acyl homoserine lactone (AHL) and a proline residue (Pro). Arrows indicate freely rotatable bonds (black) and putative n→π* interactions that would constrain those bonds (red).
Figure 2.
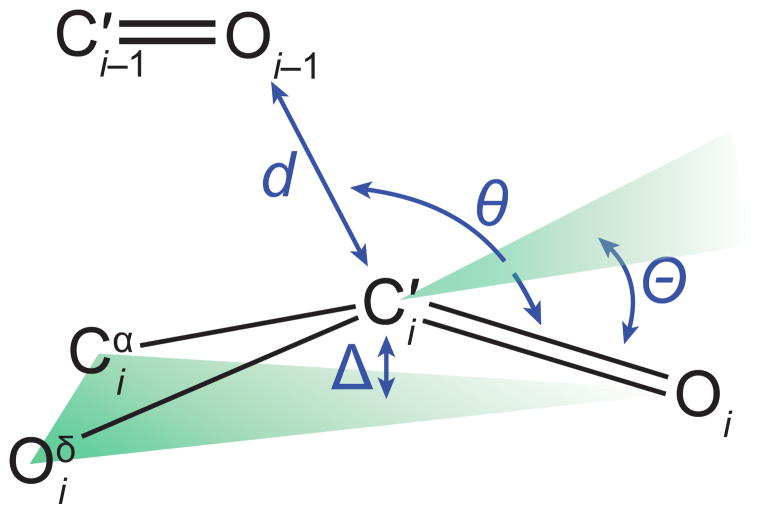
Structural parameters characterizing an n→π* interaction.
In an n→π* interaction, the filled lone pair (n) of one carbonyl group interpenetrates the empty π* orbital of another. The mixing of these orbitals releases energy, thereby causing attraction between the two groups. This overlap is most effective when the oxygen of the electron-pair donor forms a sub-van der Waals contact (d < 3.22 Å) with the carbon of the acceptor carbonyl group along the Bürgi–Dunitz trajectory for nucleophilic addition (95° < θ < 125°).10 We have estimated that such an interaction between adjacent amides in a polypeptide contributes 0.27 kcal/mol of stabilizing energy per occurrence.11 As these are relatively weak interactions, their influence is often only realized in systems in which carbonyl groups are in close proximity, as they are in proteins,12–15 peptides,16 peptoids,17–20 polyesters,21 and some small molecules.22,23
The preorganization of two carbonyl groups due to the constraint of an intervening ring can enhance an n→π* interaction.8,9,12,14,22–33 We realized that the γ-lactone of an AHL restricts its ψ dihedral angle (Ni–Cαi–C′i–Ni+1) and that amidic resonance restricts its ω dihedral angle (Cαi−1–C′i−1–Ni–Cαi),34,35 leaving only a single unconstrained bond between the two carbonyl groups. In this sense, an AHL is analogous to a proline residue, which has a restricted φ dihedral angle (C′i−1–Ni–Cαi–C′i) and has a strong tendency to form an Oi−1···C′i=Oi n→π* interaction (Figure 1). Thus, we suspected that AHLs, like proline residues, could be predisposed to form an n→π* interaction.
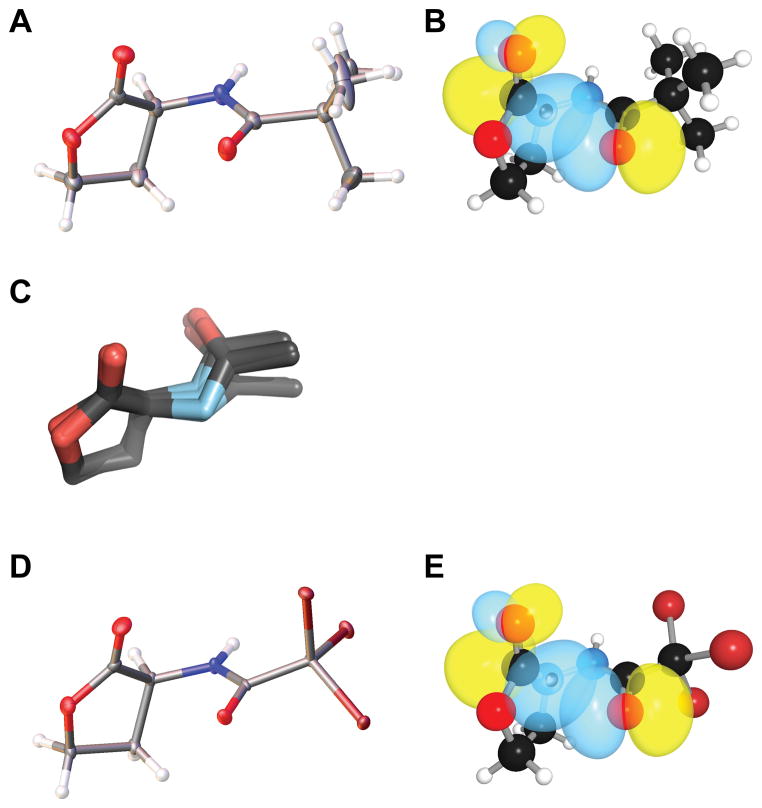
To begin, we sought evidence for a putative n→π* interaction by using X-ray diffraction analysis. Natural AHLs can have long alkyl chains that are resistant to crystallization, so we began by examining N-acetyl homoserine lactone. When this molecule did not afford crystals of sufficient quality for diffraction, we appended minimal precipitating groups to the N-acyl chain in attempts to drive crystal formation. Gratifyingly, we found the N-trimethylacetyl appendage to be suitable, affording crystals that diffracted to high resolution (Figure 3A). In this molecular structure, we noted the presence of a short contact between the N-acyl carbonyl oxygen and the lactone carbonyl carbon (d = 2.73 Å), which is 15% below the sum of the van der Waals radii (Table 1). We also found the angle of approach of the donor oxygen to the acceptor carbonyl (θ = 90.6°) to be consistent with an n→π* interaction.12 As these structural features were strongly suggestive of an n→π* interaction, we were motivated to search for characteristic structural deviations that result from n→π* donation. In particular, we have shown that the presence of n→π* interaction engenders pyramidalization of the acceptor carbonyl carbon toward the donor oxygen, as measured by the distortion parameter Θ.9,12,14–16,22,28 In N-trimethylacetyl homoserine lactone, we observed substantial pyramidalization (Θ = 2.7°) of the acceptor carbonyl toward the donor, in accord with that observed for other molecules with confirmed n→π* interactions. Distortion of the carbonyl carbon toward the n→π* is strong evidence of an attractive interaction; otherwise, distortion would likely occur away from the short contact so as to reduce unfavorable Pauli repulsion.27
Figure 3.
Structures of AHLs. (A) Crystal structure of N-trimethylacetyl homoserine lactone drawn with 50% probability ellipsoids. (B) Rendering of the n and π* orbitals of N-trimethylacetyl homoserine lactone in its optimized geometry. (C) Overlap of the crystal structures of the acetyl homoserine lactone moieties in the 14 ligands listed in Table 2. (D) Crystal structure of N-tribromoacetyl homoserine lactone drawn with 50% probability ellipsoids. (E) Rendering of the n and π* orbitals of N-tribromoacetyl homoserine lactone in its optimized geometry.
Table 1.
Conformational parameters of N-acyl homoserine lactones used in this work.
Confident that AHLs have a preference for forming an n→π* interaction, we wished to evaluate the energy of this interaction. To do so, we performed natural bond orbital (NBO) analysis of the N-trimethylacetyl homoserine lactone structure optimized by density functional theory (DFT) calculations in vacuo at the B3LYP/6-311+G(2d,p) level of theory.36 Using second-order perturbation theory, as implemented by NBO 5.9,37,38 we observed significant overlap of the n and π* orbitals (Figure 3B), with an estimated energy of En→π* = 0.64 kcal/mol. This value is larger than that observed with a proline residue,11 consistent with the carbonyl group of an ester being a better acceptor than that of an amide (Figure 1).
As the biological activity of an AHL relies upon its binding to its target LuxR-type receptor, we sought to compare the structure of a free AHL to that observed in a receptor·AHL complex. The Protein Data Bank (PDB)39 currently houses the atomic coordinates of ten LuxR-type receptor structures with bound AHLs, reflecting four distinct receptors as well as four structures of two different AHL-lactonases with bound AHLs. Remarkably, the conformation of the bound AHL ligand is nearly identical in all of these complexes (Table 2; Figure 3C), and that conformation differs dramatically from the conformation in the unbound state. In particular, each of these proteins prefers to bind the AHL ligand with a φ dihedral angle between −100° and −160°, a nearly 180°-reorientation from that observed in the unbound state (φ ~50°; Table 1). The dichotomy in the conformation of the free and bound forms indicates that the receptor must reorganize the ligand for binding. The conformation of a bound AHL is enforced by hydrogen bonds with its receptor. In particular, the amide oxygen of a bound AHL forms a hydrogen bond with the phenolic hydroxyl group of a conserved tyrosine residue.6 This C′i−1=Oi−1···H hydrogen bond competes with the Oi−1···C′i=Oi n→π* interaction of the free ligand. Accordingly, attenuating the basal n→π* interaction could preorganize an AHL for binding to its receptor.
Table 2.
Conformational parameters of protein-bound N-acyl homoserine lactones.
| PDB entry | Resolution (Å) | Protein | N-Acyl group | φ (°) | ψ (°) |
|---|---|---|---|---|---|
| 4g8ba | 1.30 | AidH | butyryl | −110 | −136 |
| 3qp1 | 1.55 | CviR | hexanoyl | −103 | −139 |
| 3qp2 | 1.64 | CviR | octanoyl | −108 | −138 |
| 3qp4 | 1.55 | CviR | decanoyl | −106 | −139 |
| 3qp6 | 2.00 | CviR | hexanoyl | −102 | −140 |
| 3qp8b | 1.60 | CviR | decanoyl | −108 | −139 |
| 3ojg | 1.60 | GKL | butyryl | −159 | −137 |
| 4h9ta | 2.10 | GKL | butyryl | −139 | −135 |
| 4h9xa | 2.20 | GKL | butyryl | −100 | −145 |
| 2uv0b | 1.80 | LasR | 3-oxo-dodecanoyl | −114 | −144 |
| 3ix3a | 1.40 | LasR | 3-oxo-dodecanoyl | −110 | −145 |
| 3szta | 2.55 | QscR | 3-oxo-dodecanoyl | −109 | −125 |
| 1l3lb | 1.66 | traR | 3-oxo-octanoic | −107 | −129 |
| 2q0oa | 2.00 | traR | 3-oxo-octanoic | −108 | −128 |
Mean for two molecules in the asymmetric unit.
Mean for four molecules in the asymmetric unit.
We reasoned that electron-withdrawing groups in the N-acyl chain would reduce the nucleophilicity of the donor oxygen and thereby reduce the influence of the n→π* interaction.20,40 To control for the influence of sterics, we replaced the three methyl groups in N-trimethylacetyl homoserine lactone with nearly isosteric bromo groups. Again using X-ray diffraction analysis, we found N-tribromoacetyl homoserine lactone adopts a conformation nearly identical to that of N-trimethylacetyl homoserine lactone (Table 1; Figure 3D). Moreover, the N-tribromoacetyl compound has a longer oxygen–carbon distance and diminished acceptor pyramidalization than does its N-trimethylacetyl analogue. These attributes are indicative of a weaker n→π* interaction in the N-tribromoacetyl compound.9,14 We confirmed this conclusion with NBO analysis of the optimized geometry of this compound, which reported that the energy associated with this n→π* interaction was 0.55 kcal/mol (Table 1; Figure 3E), approximately 14% lower than that in the parent compound. Although the N-trimethylacetyl and N-tribromoacetyl AHLs per se might not be ideal quorum sensing inhibitors due to steric concerns, our observations demonstrate not only that the n→π* interaction contributes to the conformation of these important signal molecules, but also that the conformation can be modulated by an appropriate choice of N-acyl substituents.
We note that the presence of an n→π* interaction could have another important pharmacological implication. γ-Lactones are susceptible to hydrolysis,41 which eliminates the activity of an AHL.42 An n→π* interaction increases the energy of the acceptor π* orbital22,23 and thereby reduces the electrophilicity of the carbonyl group of the γ-lactone.29,43,44 Thus, the n→π* interaction of AHLs could protect their γ-lactones against hydrolysis.
We conclude that an Oi−1···C′i=Oi n→π* interaction plays a key role in the conformation and, potentially, the biological activity of AHLs. Modifications that weaken this n→π* interaction should increase the affinity of AHLs to their cognate receptors. Indeed, the propensity of electron-withdrawing substituents to attenuate the n→π* interaction in AHLs could be contributing to the efficacy observed for certain synthetic AHLs as modulators of quorum sensing.45 Conversely, modifications that strengthen this interaction should decrease the rate of hydrolysis and endow AHLs with a longer biological half-life. We encourage exploration of this strategy for modulating bacterial quorum sensing with tailored small molecules.
Supplementary Material
Acknowledgments
We are grateful to H. E. Blackwell for valuable advice and discussions, and I. A. Guzei for assistance with X-ray diffraction analysis. R. W. N. was supported by NIH Biotechnology Training Grant T32 GM008349. This work was supported by grants R01 AR044276 (NIH) and CHE-1124944 (NSF). Nuclear magnetic resonance spectroscopy was supported by grants P41 RR002301 and P41 GM066326 (NIH). High-performance computing was supported by grant CHE-0840494 (NSF).
Footnotes
The authors declare no competing financial interest.
ASSOCIATED CONTENT
Procedures for the synthesis and conformational analysis of N-trimethylacetyl homoserine lactone and N-tribromoacetyl homoserine lactone, and data for their structural analysis with X-ray crystallography. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Hastings JW, Greenberg EP. Quorum sensing: The explanation of a curious phenomenon reveals a common characteristic of bacteria. J Bacteriol. 1999;181:2667–2668. doi: 10.1128/jb.181.9.2667-2668.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Waters CM, Bassler BL. Quorum sensing: Cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol. 2005;21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 3.Eberhard A, Burlingame AL, Eberhard C, Kenyon GL, Nealson KH, Oppenheimer NJ. Structural identification of autoinducer of Photobacterium fischeri luciferase. Biochemistry. 1981;20:2444–2449. doi: 10.1021/bi00512a013. [DOI] [PubMed] [Google Scholar]
- 4.Fuqua C, Parsek MR, Greenberg EP. Regulation of gene expression by cell-to-cell communication: Acyl-homoserine lactone quorum sensing. Annu Rev Genet. 2001;35:439–468. doi: 10.1146/annurev.genet.35.102401.090913. [DOI] [PubMed] [Google Scholar]
- 5.Ng WL, Bassler BL. Baterial quorum-sensing network architectures. Annu Rev Genet. 2009;43:197–222. doi: 10.1146/annurev-genet-102108-134304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Churchill MEA, Chen L. Structural basis of acyl-homoserine lactone-dependent signaling. Chem Rev. 2011;111:68–85. doi: 10.1021/cr1000817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rasmussen TB, Givskov M. Quorum-sensing inhibitors as anti-pathogenic drugs. Int J Med Microbiol. 2006;296:149–161. doi: 10.1016/j.ijmm.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 8.Hinderaker MP, Raines RT. An electronic effect on protein structure. Protein Sci. 2003;12:1188–1194. doi: 10.1110/ps.0241903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choudhary A, Gandla D, Krow GR, Raines RT. Nature of amide carbonyl–carbonyl interactions in proteins. J Am Chem Soc. 2009;131:7244–7246. doi: 10.1021/ja901188y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bürgi HD, Dunitz JD, Shefter E. hemical reaction paths. IV. Aspects of O···C=O interactions in crystals. Acta Crystallogr. 1974;B30:1517–1527. [Google Scholar]
- 11.Newberry RW, VanVeller B, Guzei IA, Raines RT. n→π* Interactions of amides and thioamides: Implications for protein stability. J Am Chem Soc. 2013;135:7843–7846. doi: 10.1021/ja4033583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bartlett GJ, Choudhary A, Raines RT, Woolfson DN. n→π* Interactions in proteins. Nat Chem Biol. 2010;6:615–620. doi: 10.1038/nchembio.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fufezan C. The role of Bürgi–Dunitz interactions in the structural stability of proteins. Proteins. 2010;78:2831–2838. doi: 10.1002/prot.22800. [DOI] [PubMed] [Google Scholar]
- 14.Bartlett GJ, Newberry RW, Vanveller B, Raines RT, Woolfson DN. Interplay of hydrogen bonds and n→π* interactions in proteins. J Am Chem Soc. 2013;135:18682–18688. doi: 10.1021/ja4106122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Newberry RW, Bartlett GJ, VanVeller B, Woolfson DN, Raines RT. Signatures of n→π* interactions in proteins. Protein Sci. 2014;23:xxx–xxx. doi: 10.1002/pro.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choudhary A, Raines RT. Signature of n→π* interactions in α-helices. Protein Sci. 2011;20:1077–1081. doi: 10.1002/pro.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gorske BC, Bastian BL, Geske GD, Blackwell HE. Local and tunable n→π* interactions regulate amide isomerism in the peptoid backbone. J Am Chem Soc. 2007;129:8928–8929. doi: 10.1021/ja071310l. [DOI] [PubMed] [Google Scholar]
- 18.Gorske BC, Stringer JR, Bastian BL, Fowler SA, Blackwell HE. New strategies for the design of folded peptoids revealed by a survey of noncovalent interactions in model systems. J Am Chem Soc. 2009;131:16555–16567. doi: 10.1021/ja907184g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stringer JR, Crapster JA, Guzei IA, Blackwell HE. Construction of peptoids with all trans-amide backbones and peptoid reverse turns via the tactical incorporation of N-aryl side chains capable of hydrogen bonding. J Org Chem. 2010;75:6068–6078. doi: 10.1021/jo101075a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laursen JS, Engel-Andreasen J, Fristrup P, Harris P, Olsen CA. Cis–trans amide bond rotamers in β-peptoids and peptoids: Evaluation of stereoelectronic effects in backbone and side chains. J Am Chem Soc. 2013;135:2835–2844. doi: 10.1021/ja312532x. [DOI] [PubMed] [Google Scholar]
- 21.Newberry RW, Raines RT. n→π* Interactions in poly(lactic acid) suggest a role in protein folding. Chem Commun. 2013;49:7699–7701. doi: 10.1039/c3cc44317e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choudhary A, Kamer KJ, Raines RT. An n→π* interaction in aspirin: Implications for structure and reactivity. J Org Chem. 2011;76:7933–7937. doi: 10.1021/jo201389d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamer KJ, Choudhary A, Raines RT. Intimate interactions with carbonyl groups: Dipole–dipole or n→π*? J Org Chem. 2013;78:2099–2103. doi: 10.1021/jo302265k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sonntag LS, Schweizer S, Ochsenfeld C, Wennemers H. The “azido gauche effect”—implications for the conformation of azidoprolines. J Am Chem Soc. 2006;128:14697–14703. doi: 10.1021/ja0654938. [DOI] [PubMed] [Google Scholar]
- 25.Chiang YC, Lin YJ, Horng JC. Stereoelectronic effects on the transition barrier of polyproline conformational interconversion. Protein Sci. 2009;18:1967–1977. doi: 10.1002/pro.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuemin M, Nagel YA, Schweizer S, Monnard FW, Ochsenfeld C, Wennemers H. Tuning the cis/trans conformer ratio of Xaa–Pro amide bonds by intramolecular hydrogen bonds: The effect on PPII helix stability. Angew Chem Int Ed. 2010;49:6324–6327. doi: 10.1002/anie.201001851. [DOI] [PubMed] [Google Scholar]
- 27.Jakobsche CE, Choudhary A, Miller SJ, Raines RT. n→π* Interaction and n)(π Pauli repulsion are antagonistic for protein stability. J Am Chem Soc. 2010;132:6651–6653. doi: 10.1021/ja100931y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Choudhary A, Pua KH, Raines RT. Quantum mechanical origin of the conformational preferences of 4-thiaproline and its S-oxides. Amino Acids. 2011;41:181–186. doi: 10.1007/s00726-010-0504-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pollock SB, Kent SBH. An investigation into the origin of the dramatically reduced reactivity of peptide-prolyl-thioesters in native chemical ligation. Chem Commun. 2011;47:2342–2344. doi: 10.1039/c0cc04120c. [DOI] [PubMed] [Google Scholar]
- 30.Erdmann RS, Wennemers H. Importance of ring puckering versus interstrand hydrogen bonds for the conformational stability of collagen. Angew Chem Int Ed. 2011;50:6835–6838. doi: 10.1002/anie.201008118. [DOI] [PubMed] [Google Scholar]
- 31.Liu P, Yang X, Birman VB, Houk KN. Origin of enantioselectivity in benzotetramisole-catalyzed dynamic kinetic resolution of azlactones. Org Lett. 2012;14:3288–3291. doi: 10.1021/ol301243f. [DOI] [PubMed] [Google Scholar]
- 32.Reddy DN, Thirupathi R, Tumminakatti S, Prabhakaran EN. A method for stabilizing the cis prolyl peptide bond: Influence of an unusual n→π* interaction in 1,3-oxazine and 1,3-thiazine containing peptidomimetics. Tetrahedron Lett. 2012;53:4413–4417. [Google Scholar]
- 33.Wang H, Kohler P, Overman LE, Houk KN. Origins of stereoselectivities of dihydroxylations of cis-bicyclo[3.3.0]octenes. J Am Chem Soc. 2012;134:16054–16058. doi: 10.1021/ja3075538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pauling L. The nature of the chemical bond. III. The transition from one extreme bond type to another. J Am Chem Soc. 1932;54:988–1003. [Google Scholar]
- 35.Pauling L. The Nature of the Chemical Bond. Cornell University Press; Ithaca, NY: 1939. pp. 186–193. [Google Scholar]
- 36.Reed AE, Curtiss LA, Weinhold F. Intermolecular interactions from a natural bond orbital, donor–acceptor viewpoint. Chem Rev. 1988;88:899–926. [Google Scholar]
- 37.Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, Scalmani G, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA, Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam JM, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas Ö, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ. Gaussian 09, Revision A.1. Gaussian, Inc; Wallingford, CT: 2009. [Google Scholar]
- 38.Glendening ED, Badenhoop JK, Reed AE, Carpenter JE, Bohmann JA, Morales CM, Weinhold F. NBO 5.9. Theoretical Chemistry Institute, University of Wisconsin-Madison; Madison, WI: 2012. [Google Scholar]
- 39.Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. The Protein Data Bank. Nucleic Acids Res. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choudhary A, Fry CG, Raines RT. Modulation of an n→π* interaction with α-fluoro groups. ARKIVOC. 2010:251–262. [PMC free article] [PubMed]
- 41.Rackham DM, Chakrabarti JK, Davies GLO. Study of hydrolysis of amido-substituted γ-butyrolactones by pH-stat titration and ultraviolet spectroscopic analyses. Talanta. 1981;28:329–331. doi: 10.1016/0039-9140(81)80171-3. [DOI] [PubMed] [Google Scholar]
- 42.Yates EA, Philipp B, Buckley C, Atkinson S, Chhabra SR, Sockett RE, Goldner M, Dessaux Y, Cámara M, Smith H, Williams P. N-Acylhomoserine lactones undergo lactonolysis in a pH-, temperature-, and acyl chain length-dependent manner during growth of Yersinia pseudotuberculosis and Pseudomonas aeruginos. Infect Immun. 2002;70:5635–5646. doi: 10.1128/IAI.70.10.5635-5646.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choudhary A, Kamer KJ, Powner MW, Sutherland JD, Raines RT. A stereoelectronic effect in prebiotic nucleotide synthesis. ACS Chem Biol. 2010;5:655–657. doi: 10.1021/cb100093g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Choudhary A, Fry CG, Kamer KJ, Raines RT. An n→π* interaction reduces the electrophilicity of the acceptor carbonyl group. Chem Commun. 2013;49:8166–8168. doi: 10.1039/c3cc44573a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Geske GD, O’Neill JC, Miller DM, Mattmann ME, Blackwell HE. Modulation of bacterial quorum sensing with synthetic ligands: Systematic evaluation of N-acylated homoserine lactones in multiple species and new insights into their mechanisms of action. J Am Chem Soc. 2007;129:13613–13625. doi: 10.1021/ja074135h. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.