Abstract
Background
To investigate the impact of common variants of FNDC5 on type 2 diabetes and clinical traits related to glucose metabolism in a large Chinese population sample.
Methods
Three tagging single nucleotide polymorphisms within the region of the FNDC5 gene were selected and genotyped in 6822 participants. Detailed clinical investigations and biochemistry measurements were carried out in all of the participants. Subjects without diabetes were classified into normal weight and overweight/obese subgroups according to body mass index (BMI).
Results
None of the SNPs were associated with either the risk of type 2 diabetes in all of the participants or with any of the clinical quantitative traits in the controls with normal glucose regulation. Subgroup analysis showed that in controls with normal weight (BMI <25 kg/m2), the rs16835198 major allele G was significantly associated with fasting insulin levels, and that each additional copy of the allele resulted in a 0.0178 mU/L increment of the values (p = 0.046). Moreover, after adjusting for confounding variables, there were trends towards correlation of rs16835198 with HOMA-insulin resistance (HOMA-IR) (p = 0.057) and low-density lipoprotein cholesterol (LDL-C) levels (p = 0.083). In overweight/obese subjects (BMI ≥25 Kg/m2), we noted rs16835198 showed trends towards association with fasting insulin (p = 0.057) and HOMA-IR levels (p = 0.091), both of which declined with additional copies of the major allele G. Moreover, rs16835198 was significantly associated with high-density lipoprotein cholesterol (HDL-C) levels (p = 0.013), and HOMA-β cell function (p = 0.028) in the overweight/obese subjects. Finally, we observed a significant interaction between BMI-rs16835198 and fasting insulin levels in the control group (p = 0.003).
Conclusions
Our data indicate that the effect of the common FNDC5 SNP rs16835198 on fasting insulin was significantly modified by BMI in the Chinese Han population.
Introduction
Diabetes is a major public health concern, and changes in diet and lifestyle have accelerated the rise in the incidence of this disease to pandemic proportions in many countries worldwide, including China. According to the national epidemiological survey conducted in 2008, China has the largest number of diabetics in any single country [1], of which 90% have type 2 diabetes. It is acknowledged that insulin resistance plays an important role in the pathogenesis of diabetes as well as obesity. In recent decades, with the global prevalence of obesity rising, the burden of chronic metabolic disorders has been boosted even further [2].
Physical exercise is recognized not only as an indispensable component of lifestyle intervention but also, increasingly, as a cornerstone for non-pharmacological therapy in ameliorating obesity and insulin resistance [3], [4]. As the largest organ in the body, skeletal muscle is increasingly considered an important endocrine organ that interacts with adipose tissue, pancreas, and liver to modulate metabolism through the beneficial effects of exercise [5]–[7]. In addition, skeletal muscle secretes metabolically active myokines such as irisin [8], which is encoded by the FNDC5 (FRCP2, PeP) gene in both mice and humans [9], [10]. Irisin undergoes proteolytic cleavage to yield a mature 112 aa polypeptide, which is secreted in response to physical exercise [8]. Irisin is expressed in fat, kidney, lung, and liver [11], with peak expression levels in skeletal muscle, where it is regulated by peroxisome proliferator-activated receptor γ (PPARγ) coactivator 1α (PGC1-α), an exercise-responsive mediator that is involved in energy metabolism [8]. Irisin has been shown in mice to induce the transformation of white adipose tissue to brown adipose tissue by induction of the Ucp1 and Cidea genes, implying its potential as therapeutic for combat obesity, diabetes and other disease could be ameliorate by exercise [8], [12], [13]. Up to now, two previous genetic studies have investigated the association of common SNPs in FNDC5 with insulin sensitivity and glucose metabolism [14], [15]. In addition, although irisin is a myokine known to be closely related with obesity, its impact on the relationship between glycolipid metabolism and BMI is not clearly defined. Given the lack of data implicating FNDC5 variants in human metabolic disease, we set out to examine the association between FNDC5 variants and a range of metabolic parameters in a large Chinese Han population.
Materials and Methods
Ethics statement
The study was approved by the Ethics Committee of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital and in accordance with the Declaration of Helsinki. Written informed consent was obtained from each participant.
Subjects recruitments
The overall study group totally comprised 6822 participants of Han Chinese ancestry residing in Shanghai. Detailed information regarding this study population was described in detail previously [16]–[18]. Among them, 3410 participants were unrelated individuals with type 2 diabetes, defined according to 1999 WHO criteria (fasting plasma glucose ≥7.0 mmol/l and/or 2-h postchallenge plasma glucose ≥11.1 mmol/l), and who were recruited from the clinical inpatient database of Shanghai Diabetes Institute [19]. The remaining 3412 participants were unrelated controls with normal glucose tolerance as assessed by 75 g oral glucose tolerant tests (OGTTs), and a negative family history of diabetes, and who were selected from community-based epidemiological studies of diabetes and related metabolic diseases. Using 1999 WHO criteria [20], BMI was used to divide individuals with normal blood glucose into two groups, namely: normal weight (BMI<25 Kg/m2), and overweight/obese (BMI≥25 Kg/m2). The clinical characteristics of all participants are shown in Table 1.
Table 1. Clinical characteristics of the study sample population.
| Cases of type2 diabetes | Controls | Controls | ||
| Normal weight | Overweight/obesity | |||
| Samples(n) | 3410 | 3412 | 2387 | 1022 |
| Male/female (n) | 1812/1597 | 1364/2048 | 920/1467 | 442/580 |
| Age (years) | 60.33±12.49 | 51.41±14.39 | 50.53±14.86 | 53.45±12.96 |
| BMI (kg/m2) | 24.20 (22.00,26.60) | 23.23 (21.27,27.68) | 22.07 (20.55,23.45) | 26.78 (25.81,28.23) |
| Fasting plasma glucose(mmol/L) | 12.78 (9.00,16.00) | 5.02 (4.70,5.40) | 5.00 (4.64,5.38) | 5.10 (4.73,5.46) |
| 2-h plasma glucose (mmol/L) | 17.00 (13.00,22.00) | 5.42 (4.60,6.30) | 5.30 (4.53,6.20) | 5.70 (4.80,6.60) |
| Total cholesterol (mmol/L) | 4.70 (4.00,5.50) | 4.70 (4.04,5.35) | 4.60 (3.97,5.30) | 4.86 (4.20,5.50) |
| Triglyceride(mmol/L) | 1.49 (0.99,2.18) | 1.25 (0.87,1.82) | 1.13 (0.80,1.64) | 1.61 (1.11,2.26) |
| HDL-C (mmol/L) | 1.11 (0.94,1.33) | 1.33 (1.13,1.51) | 1.36 (1.18,1.55) | 1.25 (1.05,1.43) |
| LDL -C (mmol/L) | 2.97 (2.42,3.57) | 3.04 (2.49,3.61) | 2.98 (2.42,3.53) | 3.20 (2.64,3.80) |
Data are shown as n or median (interquartile range).
BMI, body mass index; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol.
Clinical measurements
All participants underwent a detailed clinical investigation as described previously [21], [22]. In summary, anthropometric parameters such as height, weight, waist and hip circumference were measured. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Controls were subjected to standard OGTTs involving administration of 75 g glucose in the morning after an overnight fast. Blood samples were obtained at baseline and 2 h time points during the OGTT. Plasma glucose, serum insulin and serum lipid profiles were also measured. Insulin resistance index and β cell function were estimated using the homeostasis model assessment (HOMA), which was calculated using fasting plasma glucose and insulin levels [23]. In addition, Insulin sensitivity from the OGTT was also estimated according to the insulin sensitivity index (ISI) proposed by Gutt et al. [24]: ISI = [75000+ (fasting plasma glucose −2 h plasma glucose) × 0.19×weight]/120/mean plasma glucose/log10 mean insulin.
Single nucleotide polymorphism (SNP) selection and genotyping
Using the HapMap Phase III (release 27) Han Chinese database and a threshold of r2≥0.8, we selected three tagging SNPs in a region encompassing the FNDC5 gene and its 10 kb up- and downstream sequences. These three tagging SNPs tagged 100% of SNPs (5/5 SNPs in the HapMap Chinese Han sample) with a minor allele frequency (MAF) of >0.05. All SNPs were genotyped by primer extension of multiplex products with detection by matrix-assisted laser desorption ionization–time of flight mass spectroscopy, using a MassARRAY Compact Analyzer (Sequenom, San Diego, CA, USA) with an overall call rate of 99.6%.
Statistical analysis
For each variant, the Hardy-Weinberg equilibrium test was performed separately in the cases and controls prior to association analysis. SNPs failing this test (p<0.05 in the controls) were excluded. Pairwise linkage disequilibrium including |D’| and r2 was estimated using Haploview (version 4.2). Allele and genotype distributions between the cases and control subjects were compared with χ2 test or logistic regression [25], and odds ratios (ORs) with 95% confidence intervals (CIs) were calculated. All skewly distributed quantitative traits [including fasting plasma glucose, 2 h plasma glucose, fasting insulin, 2 h insulin, triglycerides, total cholesterol, low-density lipoprotein cholesterol (LDL-C), high-density lipoprotein cholesterol (HDL-C), estimated ISI, HOMA for β-cell function and insulin resistance] were logarithmically transformed to approximate univariate normality. Quantitative traits were analyzed in the control group by linear regression under an additive genetic model adjusted for age, gender and BMI as potential confounders. Both BMI-pooled analysis and BMI-specific analysis were conducted. All statistical analyses were performed using SAS for Windows (version 8.0; SAS Institute, Cary, NC, USA). The interaction analysis was accounted using PLINK (version 1.05). A two tailed p-value of 0.05 was considered statistically significant.
Quanto was used to calculate the statistical power [26]. Based on the assumption that the population risk was 9.6%, and a two-side α of 0.05, for SNPs in our sample with a minor allele frequency over 0.2, we had over 80% power to detect the minimum OR of 1.13 under the additive model.
Results
All the three SNPs were in Hardy-Weinberg equilibrium in the control population (p>0.05). Linkage disequilibrium analysis for these SNPs was shown in Figure 1. One haplotype block was constructed in this region. Single SNP association analysis failed to detect a significant association between any of the three SNP and type 2 diabetes, with a minimum p value of 0.265 for rs3480. Similarly, logistic regression analysis found no association between any SNP and type 2 diabetes (Table 2). To determine the effect of these SNPs on clinical characteristics in the control participants, we next performed genotype-phenotype analyses using an additive genetic model. As shown in the Supporting Information (Table S1 in File S1), none of the SNPs were significantly associated with quantitative traits related to glucose and lipid metabolism in the control population.
Figure 1. Linkage disequilibrium maps for SNPs genotyped in the region of the FNDC5 gene.
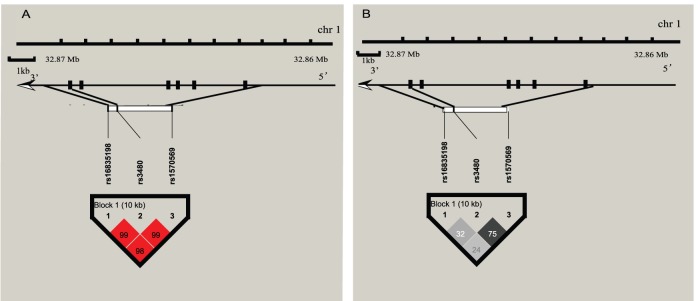
A. Shades of red demonstrate the strength of the pairwise linkage disequilibrium based on D’ and numbers represent the value of D’ expressed as a percentage. B. Shades of grey show the strength of the pairwise linkage disequilibrium based on r2 and numbers indicate the value of r2 expressed as a percentage.
Table 2. Associations of FNDC5 SNPs with type 2 diabetes.
| SNP | Chr. Position(Build 104) | Major/minorallele | Cases of type2 diabetes (n = 3410) | Controls (n = 3412) | OR for major allele(95%CI) | p value for majorallele | OR for genotype(95%CI) | p value forgenotype (p *) | ||
| Major allelefrequencies | Genotypecount 11/12/22# | Major allelefrequencies | Genotypecount 11/12/22# | |||||||
| rs16835198 | 33326681 | G/T | 0.518 | 929/1661/807 | 0.519 | 899/1735/768 | 0.995(0.930,1.064) | 0.880 | 0.995(0.930,1.064) | 0.880(0.929) |
| rs3480 | 33328165 | A/G | 0.734 | 1855/1275/267 | 0.742 | 1874/1306/225 | 0.957(0.887,1.034) | 0.265 | 0.958(0.888,1.034) | 0.269(0.205) |
| rs1570569 | 33336956 | G/T | 0.782 | 2088/1126/175 | 0.790 | 2121/1122/153 | 0.956(0.930,1.064) | 0.286 | 0.957(0.882,1.038) | 0.290(0.240) |
The additive model was used in the association analyses between genotype and type 2 diabetes.
11, major allele homozygotes; 12, heterozygotes; 22, minor allele homozygotes.
*Adjusted for age, gender and BMI.
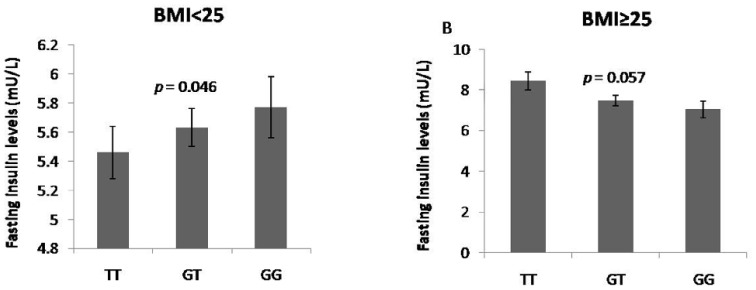
To examine the effects of FNDC5 SNPs on glycolipid metabolism parameters in subjects with different BMI, we then analyzed the distribution of these SNPs in two BMI-based subgroups of individuals with normal glucose regulation, namely, overweight/obese or normal weight. In the normal weight subgroup (BMI <25 Kg/m2), rs16835198 major allele G was significantly associated with a 0.0178 mU/L increment in fasting insulin levels for each additional allele copy (p = 0.046). Moreover, after adjustment for confounding variables, this SNP exhibited trends towards association with HOMA-IR (p = 0.057) and serum LDL-C levels (p = 0.083) in the same subgroup (Table 3). However, opposite association was observed in overweight/obese subgroup (BMI ≥25 Kg/m2) subgroup, with each additional copy of major allele (G) exhibiting lower values than TT homozygotes (p = 0.057 for fasting insulin, p = 0.091 for HOMA-IR, respectively). Moreover, rs16835198 was also significantly associated with HDL-C and HOMA-β cell function levels in the overweight/obese subgroup both before (p = 0.013, 0.028, respectively) and after (p = 0.001, 0.025, respectively) adjusting for these covariates (Table 4). Finally, we noted a conflict between the SNP rs16835198 and fasting insulin levels in the different subgroups (Figure 2). When we tested the SNP-BMI interaction in participants with normal glucose regulation, a significant interaction between rs16835198-BMI and fasting insulin levels was observed (p = 0.003). Results regarding the association between the other two SNPs and clinical traits in BMI-specific subgroups are shown in Supporting Information (Table S2 and S3 in File S1).
Table 3. Association analyses of rs16835198 with clinical characteristics in normal weight controls.
| rs16835198 | TT (n = 537) | GT (n = 1192) | GG(n = 650) | β | SE | p | p * |
| Age (years) | 50.36±15.25 | 50.51±14.42 | 50.66±15.39 | 0.1513 | 0.4327 | 0.727 | / |
| BMI (kg/m2) | 22.06 (20.52,23.34) | 22.10 (20.58,23.45) | 22.07(20.50,23.52) | 0.0014 | 0.0012 | 0.261 | / |
| Fasting plasma glucose(mmol/L) | 5.03 (4.70,5.40) | 5.00 (4.62,5.35) | 5.00 (4.65,5.35) | -0.0004 | 0.0013 | 0.739 | 0.572 |
| 2-h plasma glucose (mmol/L) | 5.49(4.54,6.21) | 5.30 (4.52,6.20) | 5.20 (4.55,6.12) | −0.0027 | 0.0028 | 0.328 | 0.211 |
| Fasting insulin (mU/L) | 5.46(3.94,7.65) | 5.63 (4.07,7.73) | 5.77 (3.98,7.94) | 0.0178 | 0.0089 | 0.046 | 0.059 |
| 2-h insulin (mU/L) | 24.21(15.45,43.63) | 25.79 (13.50,41.75) | 23.05 (14.08,41.29) | −0.0049 | 0.0124 | 0.691 | 0.498 |
| Total cholesterol (mmol/L) | 4.60(3.97,5.30) | 4.65 (4.00,5.30) | 4.55 (3.91,5.26) | −0.0027 | 0.0029 | 0.339 | 0.190 |
| Triglyceride (mmol/L) | 1.12 (0.83,1.64) | 1.13 (0.81,1.62) | 1.13(0.77,1.66) | −0.0027 | 0.0066 | 0.684 | 0.514 |
| HDL-C (mmol/L) | 1.37 (1.21,1.55) | 1.36 (1.19,1.55) | 1.35(1.15,1.56) | −0.0033 | 0.0028 | 0.238 | 0.198 |
| LDL-C (mmol/L) | 2.98(2.45,3.48) | 3.01 (2.46,3.58) | 2.91 (2.36,3.53) | −0.0060 | 0.0039 | 0.119 | 0.057 |
| HOMA-IR | 1.17 (0.85,1.71) | 1.23 (0.86,1.66) | 1.23 (0.83,1.72) | 0.0172 | 0.0093 | 0.064 | 0.083 |
| HOMA-B | 80.00 (54.90,121.33) | 84.81 (60.67,128.29) | 87.07 (61.36,126.40) | 0.0158 | 0.0102 | 0.124 | 0.128 |
| Gutt-ISI | 691.58(563.64,889.09) | 697.61(573.72,903.39) | 707.89(583.82,886.08) | 0.0013 | 0.0054 | 0.813 | 0.589 |
Data are shown as mean±SD or median (interquartile range).
BMI, body mass index; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; HOMA-IR, homeostasis assessment model of insulin resistance;
HOMA-B, homeostasis assessment model of β-cell function; Gutt-ISI, insulin sensitivity index proposed by Gutt.
p values <0.05 are shown in bold, p values <0.1 are shown in italics.
*Adjusted for age, gender and BMI.
Table 4. Association analyses of rs16835198 with clinical characteristics in overweight and obese controls.
| rs16835198 | TT (n = 229) | GT (n = 543) | GG(n = 248) | β | SE | p | p * |
| Age (years) | 53.69±13.01 | 53.66±12.78 | 52.68±13.34 | −0.5141 | 0.5938 | 0.387 | / |
| BMI (kg/m2) | 26.77 (25.73,28.38) | 26.76 (25.78,28.13) | 26.81(25.90,28.26) | −0.0563 | 0.0954 | 0.555 | / |
| Fasting plasma glucose(mmol/L) | 5.06 (4.70,5.40) | 5.10 (4.77,5.44) | 5.10 (4.73,5.48) | 0.0007 | 0.0020 | 0.706 | 0.613 |
| 2-h plasma glucose (mmol/L) | 5.70(4.90,6.57) | 5.80 (4.80,6.60) | 5.66 (4.75,6.70) | 0.0025 | 0.0042 | 0.549 | 0.334 |
| Fasting insulin (mU/L) | 8.43(5.83,11.38) | 7.47 (5.22,10.50) | 7.03 (5.04,10.18) | −0.0267 | 0.0140 | 0.057 | 0.054 |
| 2-h insulin (mU/L) | 31.34(18.99,60.36) | 37.22 (19.77,56.06) | 35.34 (19.52,60.18) | 0.0125 | 0.0197 | 0.528 | 0.437 |
| Total cholesterol (mmol/L) | 4.90(4.20,5.52) | 4.85 (4.18,5.54) | 4.81 (4.30,5.40) | 0.0018 | 0.0042 | 0.669 | 0.469 |
| Triglyceride (mmol/L) | 1.64 (1.09,2.26) | 1.58 (1.10,2.25) | 1.64(1.16,2.30) | −0.0029 | 0.0108 | 0.793 | 0.580 |
| HDL-C (mmol/L) | 1.22 (1.00,1.44) | 1.24 (1.06,1.41) | 1.29(1.10,1.46) | 0.0113 | 0.0045 | 0.013 | 0.001 |
| LDL-C (mmol/L) | 3.24(2.62,3.81) | 3.18 (2.63,3.80) | 3.20 (2.71,3.79) | 0.0022 | 0.0059 | 0.707 | 0.550 |
| HOMA-IR | 1.89 (1.26,2.46) | 1.67 (1.12,2.40) | 1.59 (1.12,2.24) | −0.0245 | 0.0145 | 0.091 | 0.089 |
| HOMA-B | 123.31 (75.64,181.55) | 104.67 (72.25,156.83) | 95.79 (74.31,147.13) | −0.0344 | 0.0157 | 0.028 | 0.025 |
| Gutt-ISI | 635.63(490.54,793.14) | 584.37(487.26,741.31) | 602.96(473.75,750.12) | −0.0079 | 0.0079 | 0.317 | 0.238 |
Data are shown as mean±SD or median (interquartile range).
BMI, body mass index; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; HOMA-IR, homeostasis assessment model of insulin resistance;
HOMA-B, homeostasis assessment model of β-cell function; Gutt-ISI, insulin sensitivity index proposed by Gutt.
p values <0.05 are shown in bold, p values <0.1 are shown in italics.
*Adjusted for age, gender and BMI.
Figure 2. Association between the SNP rs16835198 and fasting insulin levels in BMI-specific controls, under the additive genetic model.
The p value for interaction between rs16835198-BMI on fasting insulin was 0.003. A. Normal weight controls (BMI <25). B. Overweight/obesity controls (BMI ≥25).
Discussion
In the present study, three tagging SNPs in the region of the FNDC5 gene, which encodes the myokine irisin, were tested for their association with type 2 diabetes and related quantitative traits in a Chinese Han population. We found that the effect of the common SNP rs16835198 on fasting insulin was significantly modified by BMI.
Irisin was originally characterized as a regulator of brown-fat-like development in specific depots of white adipose tissue, whose overexpression in mice resulted in increased energy expenditure, improved glucose tolerance, and modest but significant weight loss [8]. Irisin has since been linked to a variety of disorders, including obesity, anorexia nervosa, insulin sensitivity, nonalcoholic fatty liver disease and chronic kidney disease [27]–[30]. Despite these reports, to the best of our knowledge, the relationship between variants of FNDC5 and the risk of type 2 diabetes and related clinical parameters in the Chinese population has not been previously evaluated. Here, we found that rs16835198 was significantly associated with the levels of fasting insulin in normal weight subjects without diabetes, and that the presence of the major allele G had an insulin-desensitizing effect on these individuals. Nevertheless, an opposite trend towards association of rs16835198 with fasting insulin and HOMA-IR levels was observed in overweight/obese controls. That is to say, the association of fasting insulin levels with this SNP in specific BMI-defined subgroups indicates that BMI contributes to the effect of this SNP on glycolipid metabolism. Moreover, we also detected that with each additional copy of the major allele G of rs16835198, HOMA-β cell function levels decreased significantly in overweight/obese controls, which means this SNP also influence β cell function in this part of subjects. However, so far, there is no unambiguous functional study focus on whether BMI modified the association between variants within FNDC5 and glycolipid metabolism. The exact mechanisms underlying the results should be worthy for further investigation.
In contrast, a previous study in Southern German Caucasians indicated that the effect of SNPs rs16835198 and rs726344 on insulin sensitivity and resistance was not BMI-dependent [14]. However, rs726344 was excluded from our study, due to its extremely low minor allele frequency (MAF = 0), as estimated from the HapMap Chinese database. Additionally, we also observed that rs3480 had a trend towards association with HDL and LDL levels in the overweight/obesity participants (Table S3 in File S1), rather than fasting insulin values, which is not consistent with the latest study performed in Japan [15]. These results reiterate the relationship of genetic architecture with ethnicity, and further highlight the importance of perform genetic analyses in multiple populations with distinct genetic backgrounds.
The present study has some limitations. Firstly, we didn’t detect the circulating irisin levels in our samples, which may enhance the conclusion and make the article more interesting. Secondly, as our patients with diabetes were not newly diagnosed and were treated with anti-diabetic drugs and/or insulin, we cannot perform the association between the SNPs in FNDC5 and clinical parameters in patients. Thirdly, due to lacking of functional study, the inner mechanism is still unclear. Therefore, it is imperative to extend studies of the influence of FNDC5 variants on type 2 diabetes and metabolic traits in other Chinese populations.
Conclusion
Our data suggest that the contribution of the common FNDC5 SNP rs16835198 to fasting insulin is significantly modified by BMI. Further investigations with larger East Asian origin populations are necessary to confirm and further characterize our observations.
Supporting Information
Table S1, association between SNPs in FNDC5 and clinical characteristics in the normal glucose tolerant group. Table S2, Association analyses of the rs3480 and rs1570569 genotypes with clinical characteristics in normal weight controls. Table S3, Association analyses of the rs3480 and rs1570569 genotypes with clinical characteristics in overweight and obese individuals.
(DOCX)
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was funded by grants from the Key Program of the Shanghai Municipality for Basic Research (11JC1409600), National 863 program (2012AA02A509), National Science Foundation of China (81322010, 81170735, 81200582 and 81300691), National Major Scientific and Technological Special Project for “Significant New Drugs Development” (2011ZX09307-001-02) and Key Discipline of Public Health of Shanghai (12GWZX0104), the Innovation Fund for PhD Students (BXJ201340) from the Shanghai Jiao Tong University School of Medicine, Excellent Young Medical Expert of Shanghai (XYQ2011041), Shanghai Talent Development Grant (2012041), and National Young Top Talent Supporting Program. The authors appreciate all the participants of this research study. The authors gratefully acknowledge the skillful technical support of all nursing and medical staff at the Shanghai Clinical Center for Diabetes. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Yang W, Lu J, Weng J, Jia W, Ji L, et al. (2010) Prevalence of diabetes among men and women in China. N Engl J Med 362: 1090–101. [DOI] [PubMed] [Google Scholar]
- 2. Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, et al. (2001) Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 344: 1343–50. [DOI] [PubMed] [Google Scholar]
- 3. Thompson D, Karpe F, Lafontan M, Frayn K (2012) Physical activity and exercise in the regulation of human adipose tissue physiology. Physiol Rev 92: 157–91. [DOI] [PubMed] [Google Scholar]
- 4. Roque FR, Hernanz R, Salaices M, Briones AM (2013) Exercise training and cardiometabolic diseases: focus on the vascular system. Curr Hypertens Rep 15: 204–14. [DOI] [PubMed] [Google Scholar]
- 5. Pedersen BK, Febbraio MA (2012) Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol 8: 457–65. [DOI] [PubMed] [Google Scholar]
- 6. Pedersen BK (2009) The diseasome of physical inactivity-and the role of myokines in muscle–fat cross talk. J Physiol 587: 5559–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Handschin C, Choi CS, Chin S, Kim S, Kawamori D, et al. (2007) Abnormal glucose homeostasis in skeletal muscle-specific PGC-1alpha knockout mice reveals skeletal muscle-pancreatic beta cell crosstalk. J Clin Invest 117: 3463–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bostrom P, Wu J, Jedrychowski MP, Korde A, Ye L, et al. (2012) A PGC1-alpha-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481: 463–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Teufel A, Malik N, Mukhopadhyay M, Westphal H (2002) Frcp1 and Frcp2, two novel fibronectin type III repeat containing genes. Gene 297: 79–83. [DOI] [PubMed] [Google Scholar]
- 10. Ferrer-Martinez A, Ruiz-Lozano P, Chien KR (2002) Mouse PeP: a novel peroxisomal protein linked to myoblast differentiation and development. Dev Dyn 224: 154–67. [DOI] [PubMed] [Google Scholar]
- 11. Huh JY, Panagiotou G, Mougios V, Brinkoetter M, Vamvini MT, et al. (2012) FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism 61: 1725–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Polyzos SA, Kountouras J, Shields K, Mantzoros CS (2013) Irisin: a renaissance in metabolism? Metabolism 62: 1037–44. [DOI] [PubMed] [Google Scholar]
- 13. Lee P, Linderman JD, Smith S, Brychta RJ, Wang J, et al. (2014) Irisin and FGF21 Are Cold-Induced Endocrine Activators of Brown Fat Function in Humans. Cell Metab 19: 302–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Staiger H, Bohm A, Scheler M, Berti L, Machann J, et al. (2013) Common genetic variation in the human FNDC5 locus, encoding the novel muscle-derived browning' factor irisin, determines insulin sensitivity. PLoS One 8: e61903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tanisawa K, Taniguchi H, Sun X, Ito T, Cao ZB, et al. (2014) Common single nucleotide polymorphisms in the FNDC5 gene are associated with glucose metabolism but do not affect serum irisin levels in Japanese men with low fitness levels. Metabolism 63: 574–83. [DOI] [PubMed] [Google Scholar]
- 16. Hu C, Zhang R, Wang C, Yu W, Lu J, et al. (2010) Effects of GCK, GCKR, G6PC2 and MTNR1B variants on glucose metabolism and insulin secretion. PLoS One 5: e11761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hu C, Wang C, Zhang R, Ng MC, Bao Y, et al. (2010) Association of genetic variants of NOS1AP with type 2 diabetes in a Chinese population. Diabetologia 53: 290–8. [DOI] [PubMed] [Google Scholar]
- 18. Hu C, Zhang R, Wang C, Wang J, Ma X, et al. (2010) Variants from GIPR, TCF7L2, DGKB, MADD, CRY2, GLIS3, PROX1, SLC30A8 and IGF1 are associated with glucose metabolism in the Chinese. PLoS One 5: e15542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alberti KG, Zimmet PZ (1998) Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med 15: 539–53. [DOI] [PubMed] [Google Scholar]
- 20.(2000) Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser 894: i–xii, 1–253. [PubMed]
- 21. Jia WP, Pang C, Chen L, Bao YQ, Lu JX, et al. (2007) Epidemiological characteristics of diabetes mellitus and impaired glucose regulation in a Chinese adult population: the Shanghai Diabetes Studies, a cross-sectional 3-year follow-up study in Shanghai urban communities. Diabetologia 50: 286–92. [DOI] [PubMed] [Google Scholar]
- 22. Jiang S, Fang Q, Yu W, Zhang R, Hu C, et al. (2012) Genetic variations in APPL2 are associated with overweight and obesity in a Chinese population with normal glucose tolerance. BMC Med Genet 13: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, et al. (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–9. [DOI] [PubMed] [Google Scholar]
- 24. Gutt M, Davis CL, Spitzer SB, Llabre MM, Kumar M, et al. (2000) Validation of the insulin sensitivity index (ISI(0,120)): comparison with other measures. Diabetes Res Clin Pract 47: 177–84. [DOI] [PubMed] [Google Scholar]
- 25. Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, et al. (2007) PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81: 559–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gauderman WJ (2002) Sample size requirements for matched case-control studies of gene-environment interaction. Stat Med 21: 35–50. [DOI] [PubMed] [Google Scholar]
- 27. Stengel A, Hofmann T, Goebel-Stengel M, Elbelt U, Kobelt P, et al. (2013) Circulating levels of irisin in patients with anorexia nervosa and different stages of obesity-correlation with body mass index. Peptides 39: 125–30. [DOI] [PubMed] [Google Scholar]
- 28. Liu JJ, Wong MD, Toy WC, Tan CS, Liu S, et al. (2013) Lower circulating irisin is associated with type 2 diabetes mellitus. J Diabetes Complications 27: 365–9. [DOI] [PubMed] [Google Scholar]
- 29. Polyzos SA, Kountouras J, Anastasilakis AD, Geladari EV, Mantzoros CS (2014) Irisin in patients with nonalcoholic fatty liver disease. Metabolism 63: 207–17. [DOI] [PubMed] [Google Scholar]
- 30. Wen MS, Wang CY, Lin SL, Hung KC (2013) Decrease in irisin in patients with chronic kidney disease. PLoS One 8: e64025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1, association between SNPs in FNDC5 and clinical characteristics in the normal glucose tolerant group. Table S2, Association analyses of the rs3480 and rs1570569 genotypes with clinical characteristics in normal weight controls. Table S3, Association analyses of the rs3480 and rs1570569 genotypes with clinical characteristics in overweight and obese individuals.
(DOCX)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.