Abstract
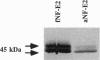
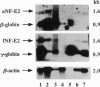
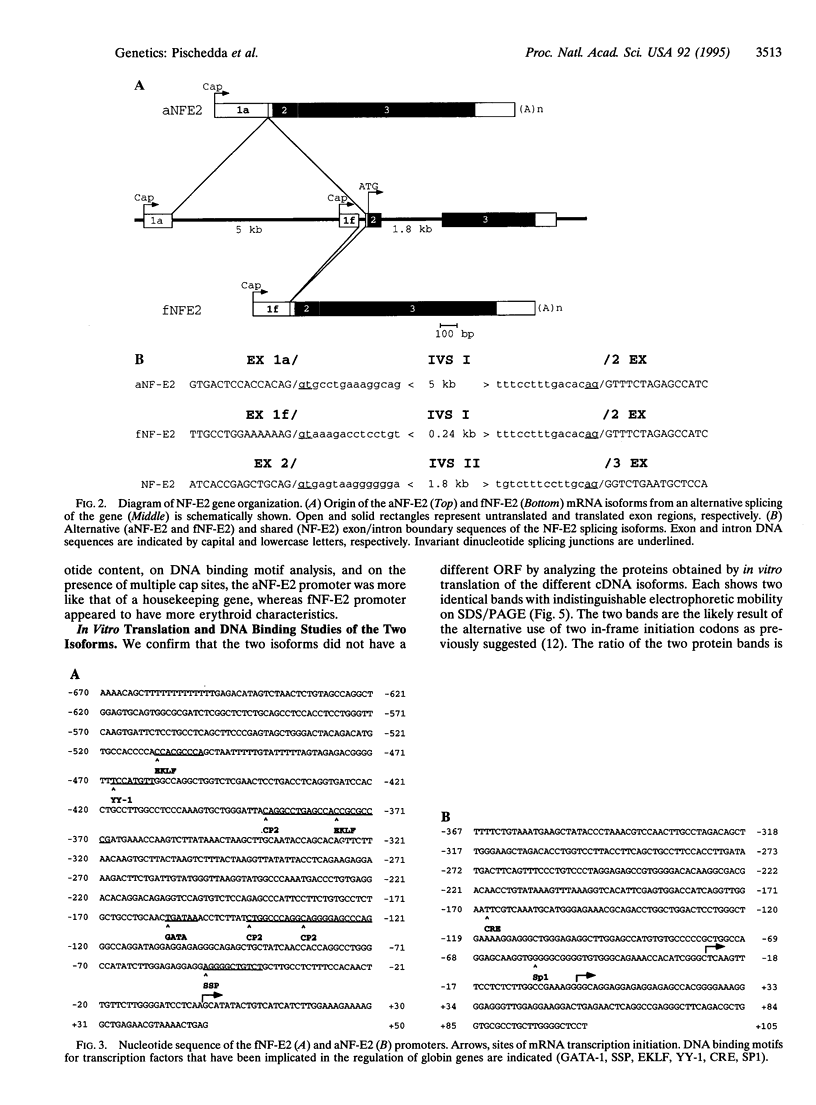
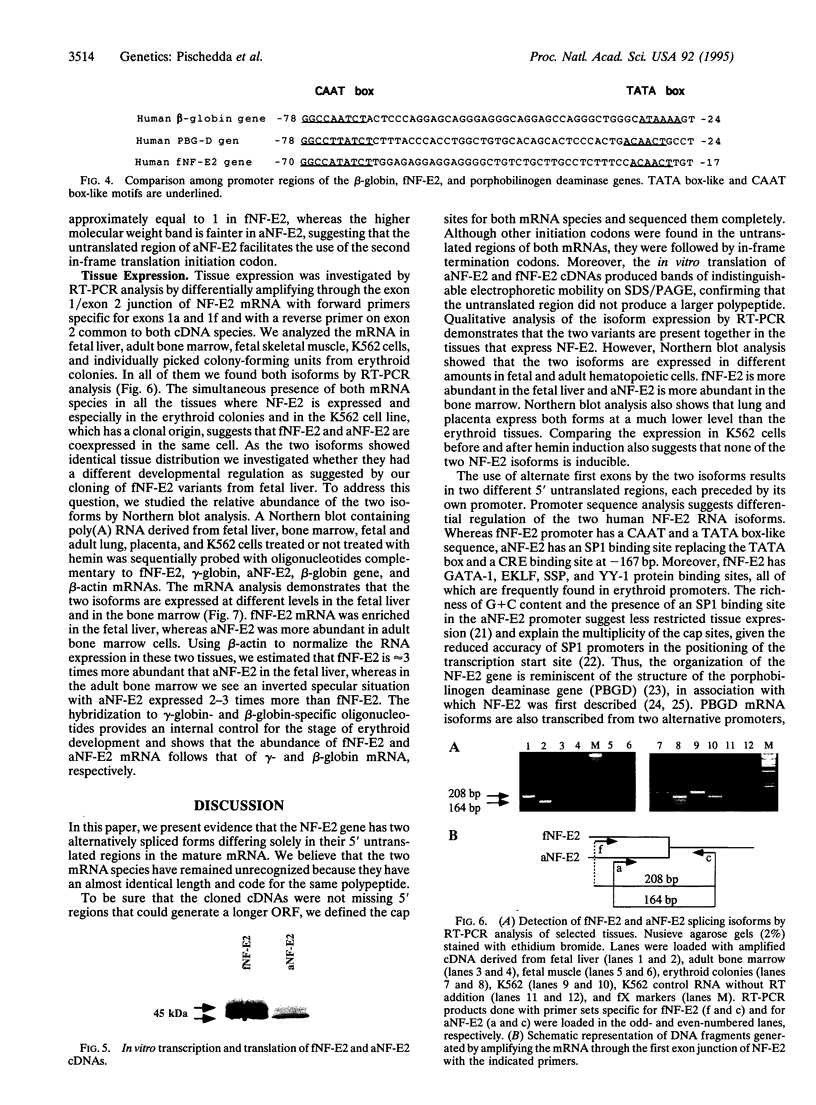
The transcription factor NF-E2 (nuclear factor erythroid 2), interacting via DNA motifs within regulatory regions of several hematopoietic genes, is thought to mediate the enhancer activity of the globin locus control regions. By screening a human fetal liver cDNA library with probes derived from mouse NF-E2, we have isolated a splicing variant of the NF-E2 gene (fNF-E2) that differs in the 5' untranslated region from the previously reported cDNA (aNF-E2). The fNF-E2 isoform is transcribed from an alternative promoter located in the 3' end of the first intron and joined by alternative splicing to the second and third exons, which are shared by both RNA isoforms. Although the two forms produce the same protein, they are expressed in different ratios during development. fNF-E2 is more abundant in the fetal liver and less abundant in the adult bone marrow compared to the previously described form. Their distribution apparently follows the differential expression of fetal and adult hemoglobins.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews N. C., Erdjument-Bromage H., Davidson M. B., Tempst P., Orkin S. H. Erythroid transcription factor NF-E2 is a haematopoietic-specific basic-leucine zipper protein. Nature. 1993 Apr 22;362(6422):722–728. doi: 10.1038/362722a0. [DOI] [PubMed] [Google Scholar]
- Andrews N. C., Kotkow K. J., Ney P. A., Erdjument-Bromage H., Tempst P., Orkin S. H. The ubiquitous subunit of erythroid transcription factor NF-E2 is a small basic-leucine zipper protein related to the v-maf oncogene. Proc Natl Acad Sci U S A. 1993 Dec 15;90(24):11488–11492. doi: 10.1073/pnas.90.24.11488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizkhan J. C., Jensen D. E., Pierce A. J., Wade M. Transcription from TATA-less promoters: dihydrofolate reductase as a model. Crit Rev Eukaryot Gene Expr. 1993;3(4):229–254. [PubMed] [Google Scholar]
- Behringer R. R., Ryan T. M., Palmiter R. D., Brinster R. L., Townes T. M. Human gamma- to beta-globin gene switching in transgenic mice. Genes Dev. 1990 Mar;4(3):380–389. doi: 10.1101/gad.4.3.380. [DOI] [PubMed] [Google Scholar]
- Bucher P., Trifonov E. N. Compilation and analysis of eukaryotic POL II promoter sequences. Nucleic Acids Res. 1986 Dec 22;14(24):10009–10026. doi: 10.1093/nar/14.24.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J. Y., Han X. L., Kan Y. W. Isolation of cDNA encoding the human NF-E2 protein. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):11366–11370. doi: 10.1073/pnas.90.23.11366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Chretien S., Dubart A., Beaupain D., Raich N., Grandchamp B., Rosa J., Goossens M., Romeo P. H. Alternative transcription and splicing of the human porphobilinogen deaminase gene result either in tissue-specific or in housekeeping expression. Proc Natl Acad Sci U S A. 1988 Jan;85(1):6–10. doi: 10.1073/pnas.85.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel J. D. Developmental regulation of human beta-globin gene transcription: a switch of loyalties? Trends Genet. 1993 Sep;9(9):304–309. doi: 10.1016/0168-9525(93)90248-g. [DOI] [PubMed] [Google Scholar]
- Feng W. C., Southwood C. M., Bieker J. J. Analyses of beta-thalassemia mutant DNA interactions with erythroid Krüppel-like factor (EKLF), an erythroid cell-specific transcription factor. J Biol Chem. 1994 Jan 14;269(2):1493–1500. [PubMed] [Google Scholar]
- Ghosh S., Selby M. J., Peterlin B. M. Synergism between Tat and VP16 in trans-activation of HIV-1 LTR. J Mol Biol. 1993 Dec 5;234(3):610–619. doi: 10.1006/jmbi.1993.1615. [DOI] [PubMed] [Google Scholar]
- Gumucio D. L., Shelton D. A., Bailey W. J., Slightom J. L., Goodman M. Phylogenetic footprinting reveals unexpected complexity in trans factor binding upstream from the epsilon-globin gene. Proc Natl Acad Sci U S A. 1993 Jul 1;90(13):6018–6022. doi: 10.1073/pnas.90.13.6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito E., Toki T., Ishihara H., Ohtani H., Gu L., Yokoyama M., Engel J. D., Yamamoto M. Erythroid transcription factor GATA-1 is abundantly transcribed in mouse testis. Nature. 1993 Apr 1;362(6419):466–468. doi: 10.1038/362466a0. [DOI] [PubMed] [Google Scholar]
- Jane S. M., Ney P. A., Vanin E. F., Gumucio D. L., Nienhuis A. W. Identification of a stage selector element in the human gamma-globin gene promoter that fosters preferential interaction with the 5' HS2 enhancer when in competition with the beta-promoter. EMBO J. 1992 Aug;11(8):2961–2969. doi: 10.1002/j.1460-2075.1992.tb05366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu S. J., Rowan S., Bani M. R., Ben-David Y. Retroviral integration within the Fli-2 locus results in inactivation of the erythroid transcription factor NF-E2 in Friend erythroleukemias: evidence that NF-E2 is essential for globin expression. Proc Natl Acad Sci U S A. 1994 Aug 30;91(18):8398–8402. doi: 10.1073/pnas.91.18.8398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna L., Johnsen O., Skartlien A. H., Pedeutour F., Turc-Carel C., Prydz H., Kolstø A. B. Molecular cloning of a putative novel human bZIP transcription factor on chromosome 17q22. Genomics. 1994 Aug;22(3):553–562. doi: 10.1006/geno.1994.1428. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignotte V., Eleouet J. F., Raich N., Romeo P. H. Cis- and trans-acting elements involved in the regulation of the erythroid promoter of the human porphobilinogen deaminase gene. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6548–6552. doi: 10.1073/pnas.86.17.6548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mignotte V., Wall L., deBoer E., Grosveld F., Romeo P. H. Two tissue-specific factors bind the erythroid promoter of the human porphobilinogen deaminase gene. Nucleic Acids Res. 1989 Jan 11;17(1):37–54. doi: 10.1093/nar/17.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller I. J., Bieker J. J. A novel, erythroid cell-specific murine transcription factor that binds to the CACCC element and is related to the Krüppel family of nuclear proteins. Mol Cell Biol. 1993 May;13(5):2776–2786. doi: 10.1128/mcb.13.5.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mujeeb A., Bishop K., Peterlin B. M., Turck C., Parslow T. G., James T. L. NMR structure of a biologically active peptide containing the RNA-binding domain of human immunodeficiency virus type 1 Tat. Proc Natl Acad Sci U S A. 1994 Aug 16;91(17):8248–8252. doi: 10.1073/pnas.91.17.8248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ney P. A., Andrews N. C., Jane S. M., Safer B., Purucker M. E., Weremowicz S., Morton C. C., Goff S. C., Orkin S. H., Nienhuis A. W. Purification of the human NF-E2 complex: cDNA cloning of the hematopoietic cell-specific subunit and evidence for an associated partner. Mol Cell Biol. 1993 Sep;13(9):5604–5612. doi: 10.1128/mcb.13.9.5604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Stamatoyannopoulos J. A., Nienhuis A. W. Therapeutic approaches to hemoglobin switching in treatment of hemoglobinopathies. Annu Rev Med. 1992;43:497–521. doi: 10.1146/annurev.me.43.020192.002433. [DOI] [PubMed] [Google Scholar]
- Tsai S. F., Martin D. I., Zon L. I., D'Andrea A. D., Wong G. G., Orkin S. H. Cloning of cDNA for the major DNA-binding protein of the erythroid lineage through expression in mammalian cells. Nature. 1989 Jun 8;339(6224):446–451. doi: 10.1038/339446a0. [DOI] [PubMed] [Google Scholar]