Abstract
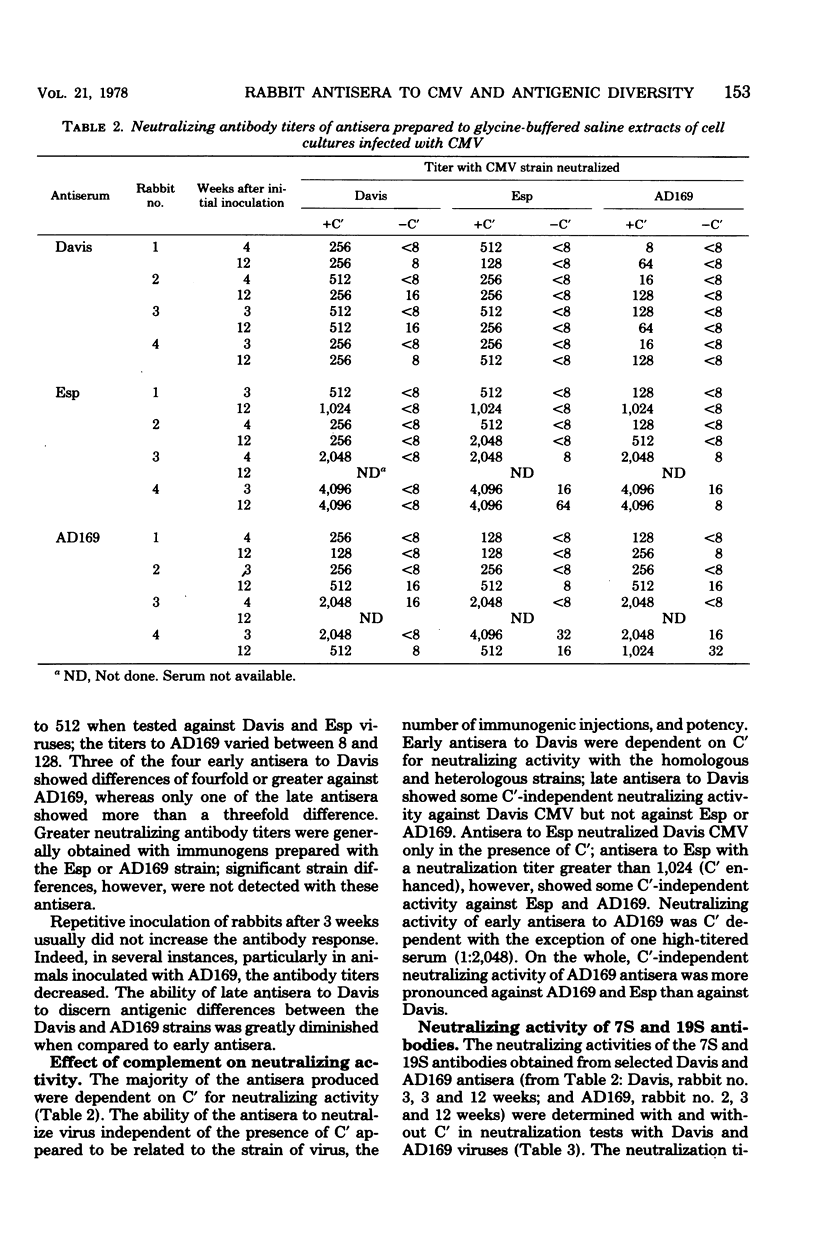
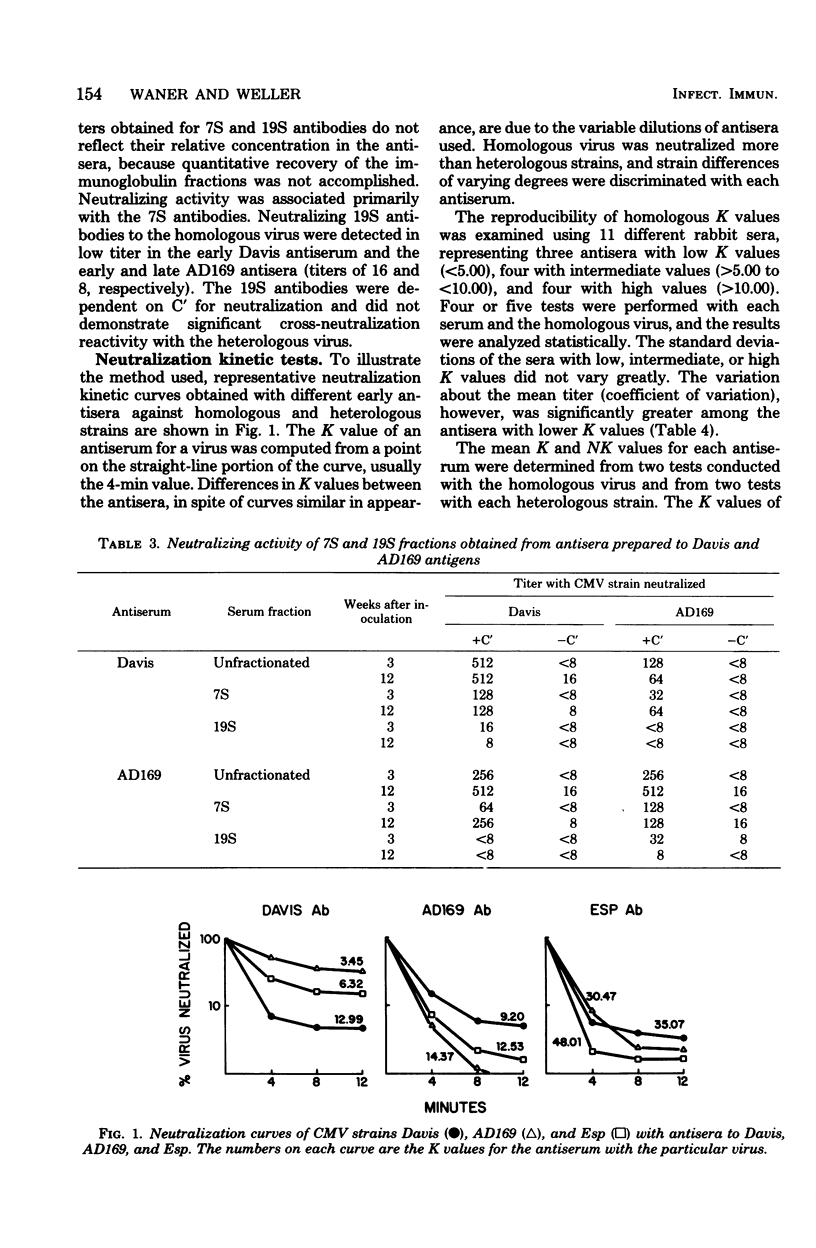
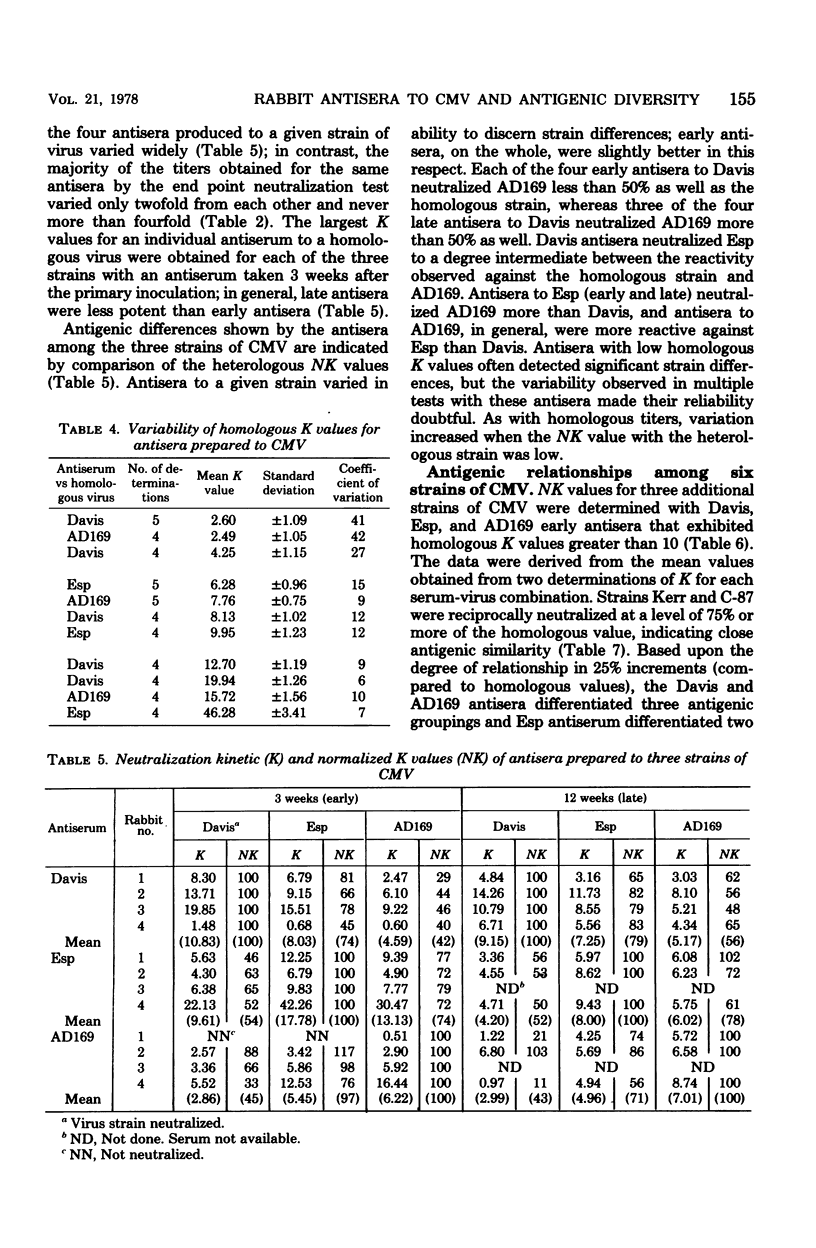
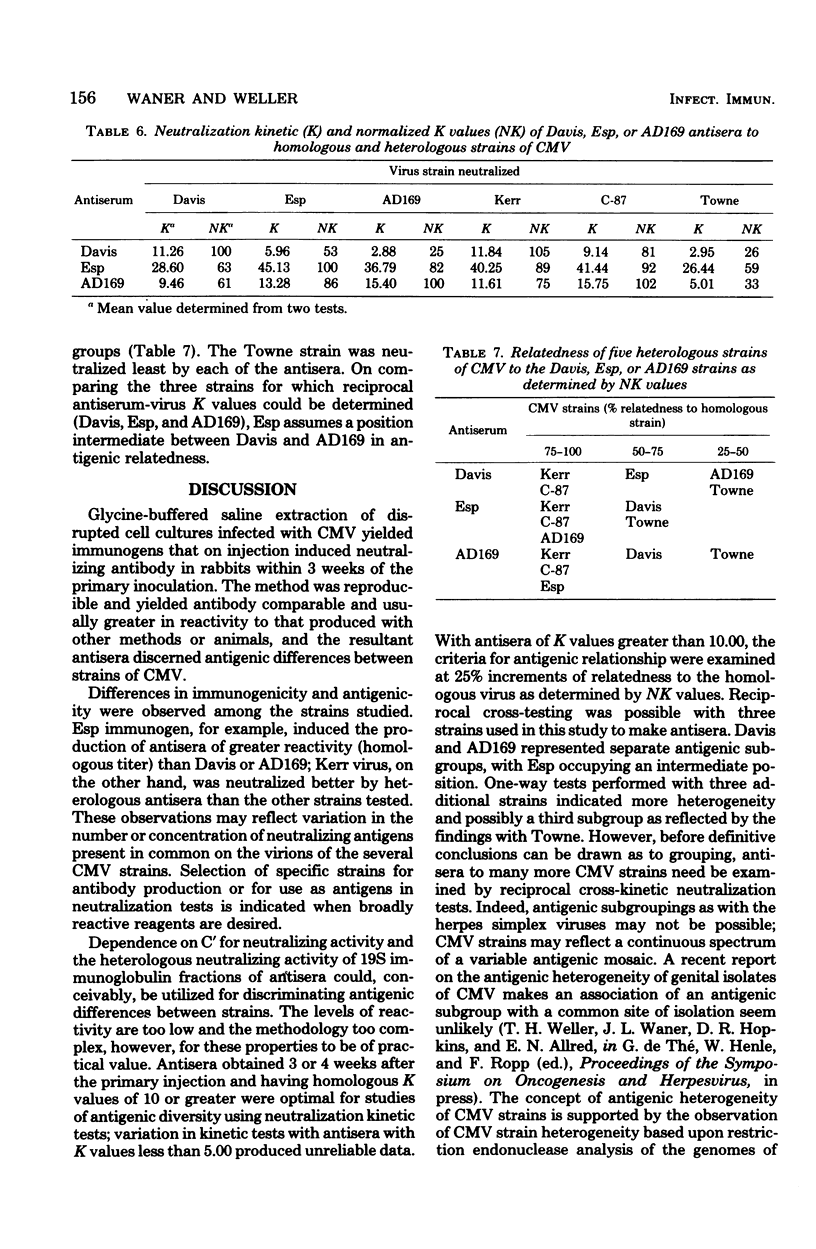
Neutralizing antisera to human cytomegalovirus were produced in rabbits with alkaline-buffered extracts of infected cell cultures. The antibody activity was complement dependent and associated primarily with the 7S immunoglobulin fraction. Antisera with homologous K values greater than 10.00 were shown to be suitable for neutralization kinetic studies and were so used to examine the antigenic relatedness of strains AD169, Davis, Esp, C-87, Kerr, and Towne. Based upon the degree of relationship as determined by normalized K values, antisera to the Davis or AD169 strains discriminated three antigenic groups, and an antiserum to strain Esp discriminated two groups.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen H. K. Serologic differentiation of human cytomegalovirus strains using rabbit hyperimmune sera. Brief report. Arch Gesamte Virusforsch. 1971;33(1):187–191. doi: 10.1007/BF01254177. [DOI] [PubMed] [Google Scholar]
- Andersen H. K. Studies of human cytomegalovirus strain variations by kinetic neutralization tests. Arch Gesamte Virusforsch. 1972;38(4):297–305. doi: 10.1007/BF01262820. [DOI] [PubMed] [Google Scholar]
- BENYESH-MELNICK M., ROSENBERG H. S., WATSON B. VIRUSES IN CELL CULTURES OF KIDNEYS OF CHILDREN WITH CONGENITAL HEART MALFORMATIONS AND OTHER DISEASES. Proc Soc Exp Biol Med. 1964 Nov;117:452–459. doi: 10.3181/00379727-117-29607. [DOI] [PubMed] [Google Scholar]
- CRAIG J. M., MACAULEY J. C., WELLER T. H., WIRTH P. Isolation of intranuclear inclusion producing agents from infants with illnesses resembling cytomegalic inclusion disease. Proc Soc Exp Biol Med. 1957 Jan;94(1):4–12. doi: 10.3181/00379727-94-22841. [DOI] [PubMed] [Google Scholar]
- Chiang W. T., Wentworth B. B., Alexander E. R. The use of an immunofluorescence technique for the determination of antibodies to cytomegalovirus strains in human sera. J Immunol. 1970 Apr;104(4):992–999. [PubMed] [Google Scholar]
- Chiba S., Striker R. L., Jr, Benyesh-Melnick M. Microculture plaque assay for human and simian cytomegaloviruses. Appl Microbiol. 1972 Apr;23(4):780–783. doi: 10.1128/am.23.4.780-783.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreesman G. R., Benyesh-Melnick M. Spectrum of human cytomegalovirus complement-fixing antigens. J Immunol. 1967 Dec;99(6):1106–1114. [PubMed] [Google Scholar]
- Forghani B., Schmidt N. J., Lennette E. H. Antisera to human cytomegalovirus produced in hamsters: reactivity in radioimmunoassay and other antibody assay systems. Infect Immun. 1976 Nov;14(5):1184–1190. doi: 10.1128/iai.14.5.1184-1190.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham B. J., Minamishima Y., Dresman G. R., Haines H. G., Benyesh-Melnick M. Complement-requiring neutralizing antibodies in hyperimmune sera to human cytomegaloviruses. J Immunol. 1971 Dec;107(6):1618–1630. [PubMed] [Google Scholar]
- Haines H. G., Von Essen R., Benyesh-Melnick M., Melnick J. L. Preparation of specific antisera to cytomegaloviruses in goats. Proc Soc Exp Biol Med. 1971 Dec;138(3):846–849. doi: 10.3181/00379727-138-36003. [DOI] [PubMed] [Google Scholar]
- Hampar B., Notkins A. L., Mage M., Keehn M. A. Heterogeneity in the properties of 7 S and 19S rabbit-neutralizing antibodies to herpes simplex virus. J Immunol. 1968 Mar;100(3):586–593. [PubMed] [Google Scholar]
- Huang Y. T., Huang E. S., Pagano J. S. Antisera to human cytomegaloviruses prepared in the guinea pig: specific immunofluorescence and complement fixation tests. J Immunol. 1974 Feb;112(2):528–532. [PubMed] [Google Scholar]
- Kilpatrick B. A., Huang E. S., Pagano J. S. Analysis of cytomegalovirus genomes with restriction endonucleases Hin D III and EcoR-1. J Virol. 1976 Jun;18(3):1095–1105. doi: 10.1128/jvi.18.3.1095-1105.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krech U., Jung M. The development of neutralizing antibodies in guinea pigs following immunization with human cytomegalovirus. Arch Gesamte Virusforsch. 1969;28(2):248–250. doi: 10.1007/BF01249391. [DOI] [PubMed] [Google Scholar]
- Martos L. M., Ablashi D. V., Gilden R. V., Sigüenza R. F., Hampar B. Preparation of immune rabbit sera with neutralizing activity against human cytomegalovirus and varicella-zoster virus. J Gen Virol. 1970;7(2):169–171. doi: 10.1099/0022-1317-7-2-169. [DOI] [PubMed] [Google Scholar]
- McBRIDE W. D. Antigenic analysis of polioviruses by kinetic studies of serum neutralization. Virology. 1959 Jan;7(1):45–58. doi: 10.1016/0042-6822(59)90176-x. [DOI] [PubMed] [Google Scholar]
- Mäntyjärvi R. Preparation of cytomegalovirus immune serum in rabbits with alkaline-extracted virus. Acta Pathol Microbiol Scand. 1968;72(2):345–346. doi: 10.1111/j.1699-0463.1968.tb01348.x. [DOI] [PubMed] [Google Scholar]
- PLUMMER G., BENYESH-MELNICK M. A PLAQUE REDUCTION NEUTRALIZATION TEST FOR HUMAN CYTOMEGALOVIRUS. Proc Soc Exp Biol Med. 1964 Oct;117:145–150. doi: 10.3181/00379727-117-29520. [DOI] [PubMed] [Google Scholar]
- Plotkin S. A., Furukawa T., Zygraich N., Huygelen C. Candidate cytomegalovirus strain for human vaccination. Infect Immun. 1975 Sep;12(3):521–527. doi: 10.1128/iai.12.3.521-527.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROWE W. P., HARTLEY J. W., WATERMAN S., TURNER H. C., HUEBNER R. J. Cytopathogenic agent resembling human salivary gland virus recovered from tissue cultures of human adenoids. Proc Soc Exp Biol Med. 1956 Jun;92(2):418–424. [PubMed] [Google Scholar]
- WELLER T. H., HANSHAW J. B., SCOTT D. E. Serologic differentiation of viruses responsible for cytomegalic inclusion disease. Virology. 1960 Sep;12:130–132. doi: 10.1016/0042-6822(60)90156-2. [DOI] [PubMed] [Google Scholar]
- Waner J. L. Partial characterization of a soluble antigen preparation from cells infected with human cytomegalovirus: properties of antisera prepared to the antigen. J Immunol. 1975 May;114(5):1454–1457. [PubMed] [Google Scholar]


