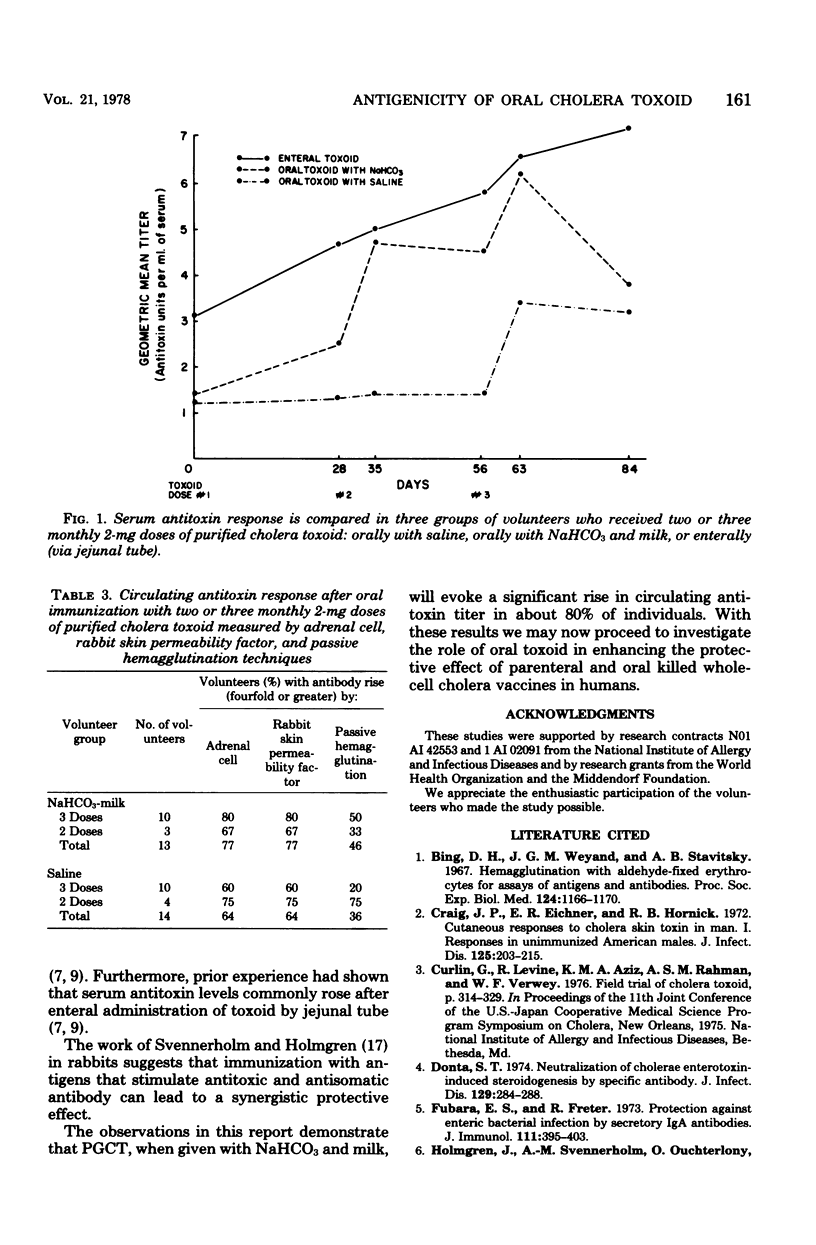
Abstract
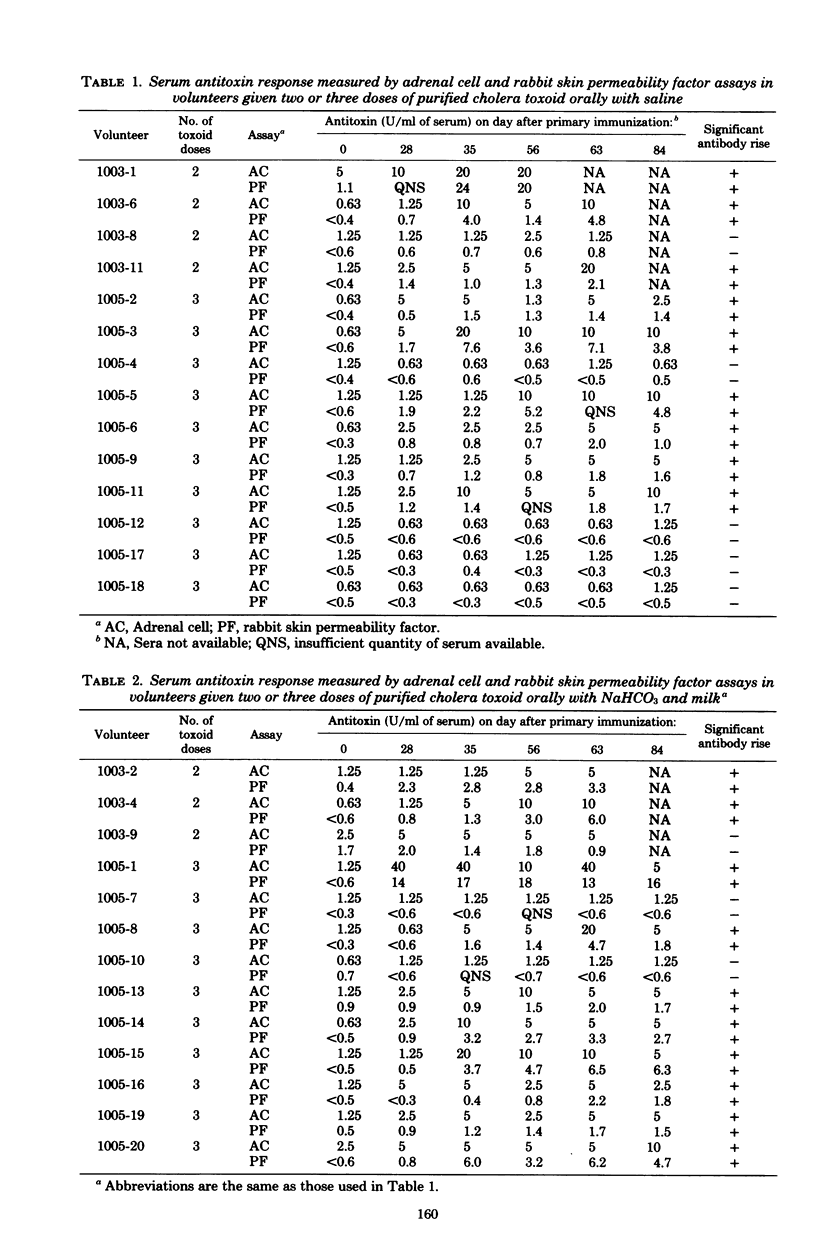
The antigenicity of orally administered glutaraldehyde-treated cholera toxoid was investigated in healthy volunteers. Fourteen volunteers ingested two or three 2-mg doses of toxoid with saline, with the doses spaced at 28-day intervals. Thirteen other volunteers received comparable toxoid doses with NaHCO3 and milk to neutralize gastric acid. Increments in circulating antitoxin levels were used to assay the antigenicity of oral toxoid. Antitoxin was measured by adrenal cell, rabbit skin permeability factor, and passive hemagglutination assays in sera collected on days 0, 28, 35, 56, 63, and 84 after primary immunization. Adrenal cell and rabbit skin assays exhibited identical sensitivity in detecting antitoxin rises in the 27 vaccinees (19/27) and were significantly more sensitive than passive hemagglutination (11/27) (P less than 0.03). Volunteers who ingested toxoid with NaHCO3 and milk had a higher rate of seroconversion (77%) than those who received toxoid with saline (64%); they also had earlier rises in antitoxin titer and consistently higher geometric mean titers on all days tested. These studies demonstrate that purified cholera toxoid is antigenic in humans after oral administration. The possible role of oral toxoid in enhancing the protective effect of killed whole-cell vaccines can now be investigated.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bing D. H., Weyand J. G., Stavitsky A. B. Hemagglutination with aldehyde-fixed erythrocytes for assay of antigens and antibodies. Proc Soc Exp Biol Med. 1967 Apr;124(4):1166–1170. doi: 10.3181/00379727-124-31953. [DOI] [PubMed] [Google Scholar]
- Craig J. P., Eichner E. R., Hornick R. B. Cutaneous responses to cholera skin toxin in man. I. Responses in unimmunized American males. J Infect Dis. 1972 Mar;125(3):203–215. doi: 10.1093/infdis/125.3.203. [DOI] [PubMed] [Google Scholar]
- Donta S. T. Neutralization of cholera enterotoxin-induced steroidogenesis by specific antibody. J Infect Dis. 1974 Mar;129(3):284–288. doi: 10.1093/infdis/129.3.284. [DOI] [PubMed] [Google Scholar]
- Fubara E. S., Freter R. Protection against enteric bacterial infection by secretory IgA antibodies. J Immunol. 1973 Aug;111(2):395–403. [PubMed] [Google Scholar]
- Holmgren J., Svennerholm A. M., Ouchterlony O., Anderson A., Walletström G., Westerberg-Berndtsson U. Antitoxic immunity in experimental cholera: protection, and serum and local antibody responses in rabbits after enteral and parenteral immunization. Infect Immun. 1975 Dec;12(6):1331–1340. doi: 10.1128/iai.12.6.1331-1340.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine M. M., Dupont H. L., Gangarosa E. J., Hornick R. B., Snyder M. J., Libonati J. P., Glaser K., Formal S. B. Shigellosis in custodial institutions. II. Clinical, immunologic and bacteriologic response of institutionalized children to oral attenuated shigella vaccines. Am J Epidemiol. 1972 Jul;96(1):40–49. doi: 10.1093/oxfordjournals.aje.a121431. [DOI] [PubMed] [Google Scholar]
- Pierce N. F., Reynolds H. Y. Immunity to experimental cholera. I. Protective effect of humoral IgG antitoxin demonstrated by passive immunization. J Immunol. 1974 Sep;113(3):1017–1023. [PubMed] [Google Scholar]
- Pierce N. F., Reynolds H. Y. Immunity to experimental cholera. II. Secretory and humoral antitoxin response to local and systemic toxoid administration. J Infect Dis. 1975 Apr;131(4):383–389. doi: 10.1093/infdis/131.4.383. [DOI] [PubMed] [Google Scholar]
- Pierce N. F., Sack R. B., Sircar B. K. Immunity to experimental cholera. III. Enhanced duration of protection after sequential parenteral-oral administration of toxoid to dogs. J Infect Dis. 1977 Jun;135(6):888–896. doi: 10.1093/infdis/135.6.888. [DOI] [PubMed] [Google Scholar]
- Rappaport R. S., Bonde G., McCann T., Rubin B. A., Tint H. Development of a purified cholera toxoid. II. Preparation of a stable, antigenic toxoid by reaction of purified toxin with glutaraldehyde. Infect Immun. 1974 Feb;9(2):304–317. doi: 10.1128/iai.9.2.304-317.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappaport R. S., Pierzchala W. A., Bonde G., McCann T., Rubin B. A. Development of a purified cholera toxoid. III. Refinements in purification of toxin and methods for the determination of residual somatic antigen. Infect Immun. 1976 Sep;14(3):687–693. doi: 10.1128/iai.14.3.687-693.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappaport R. S., Rubin B. A., Tint H. Development of a purified cholera toxoid. I. Purification of toxin. Infect Immun. 1974 Feb;9(2):294–303. doi: 10.1128/iai.9.2.294-303.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svennerholm A. M., Holmgren J. Synergistic protective effect in rabbits of immunization with Vibrio cholerae lipopolysaccharide and toxin/toxoid. Infect Immun. 1976 Mar;13(3):735–740. doi: 10.1128/iai.13.3.735-740.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A. L., Walker W. A. Immunological control mechanism against cholera toxin: interference with toxin binding to intestinal receptors. Infect Immun. 1976 Oct;14(4):1034–1042. doi: 10.1128/iai.14.4.1034-1042.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]


