Abstract
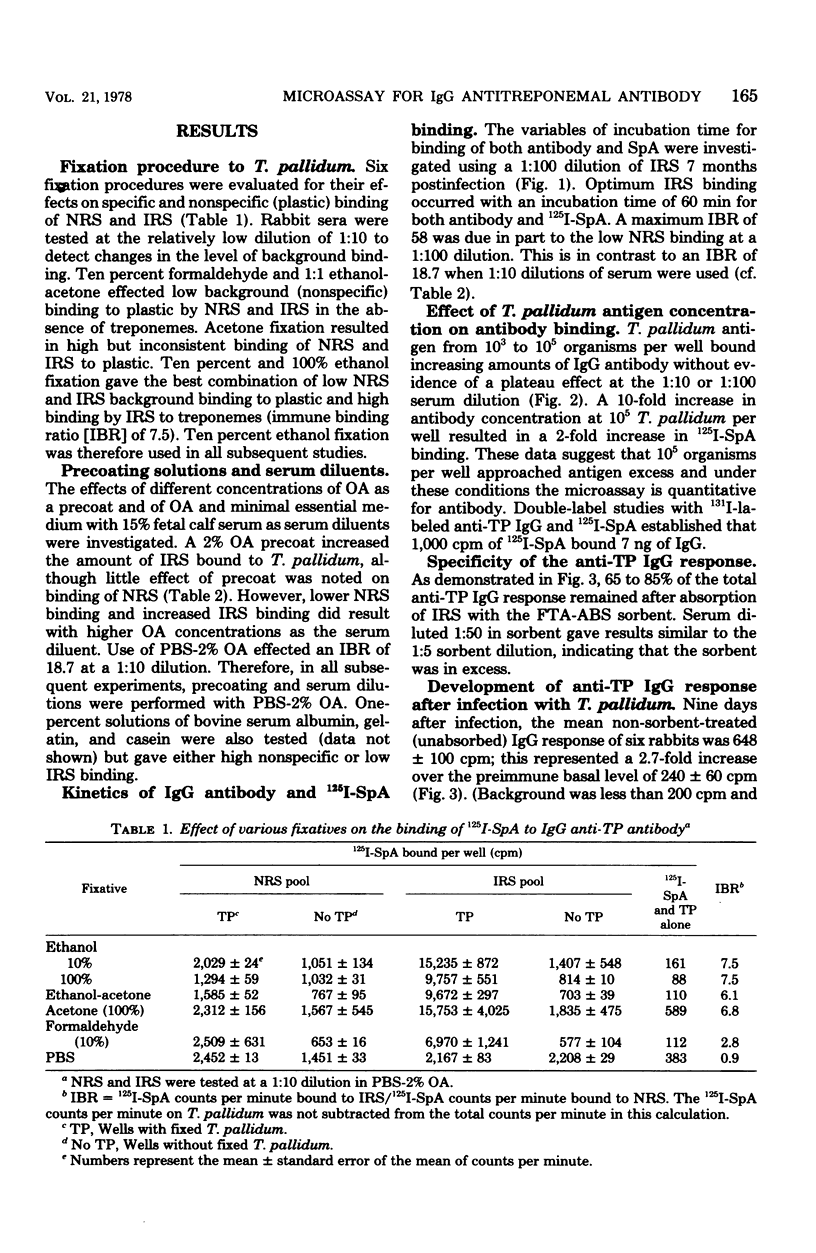
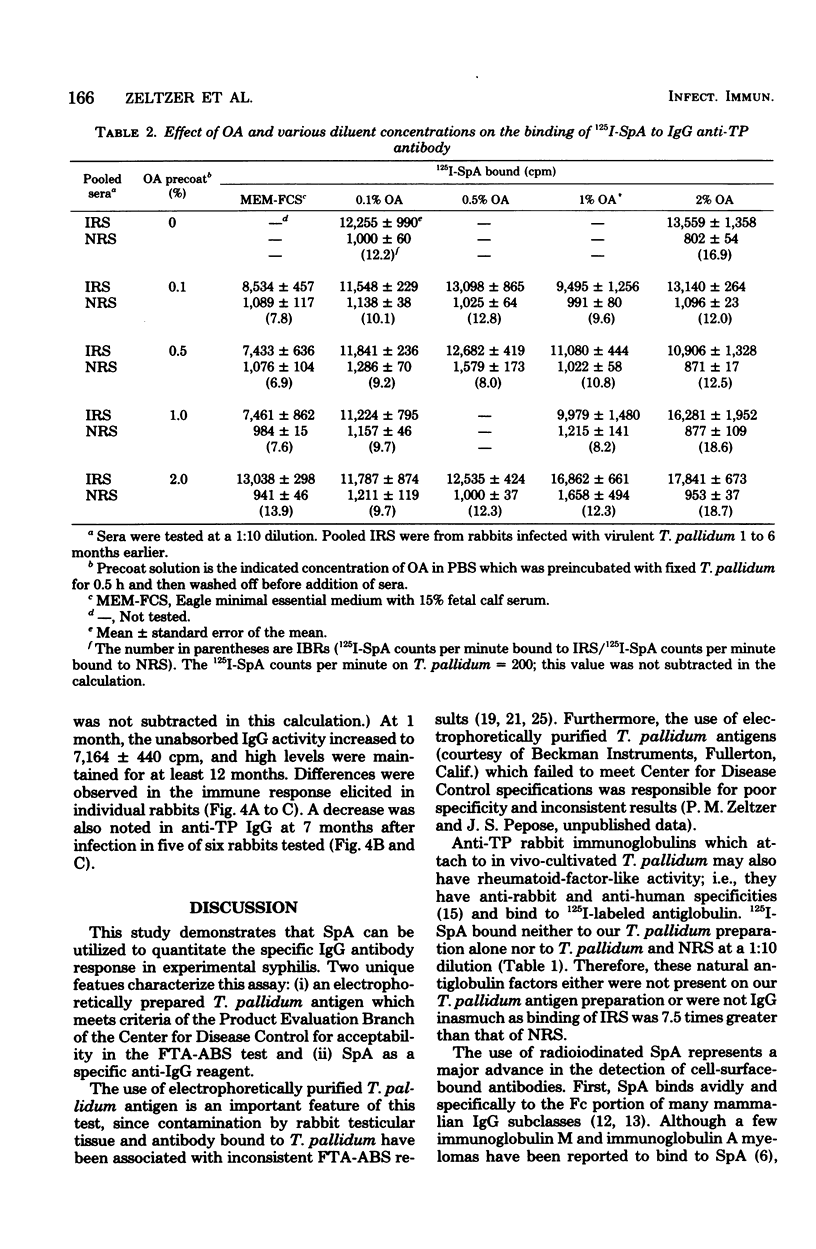
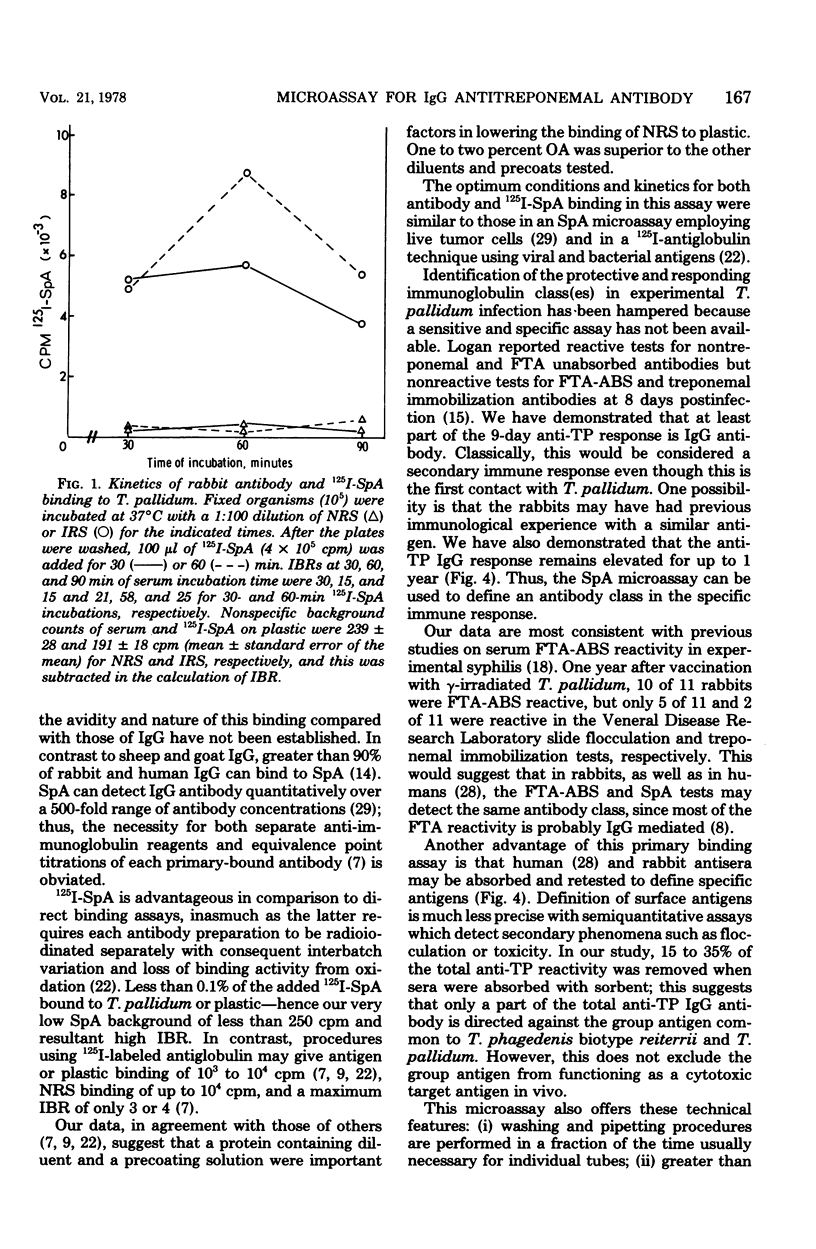
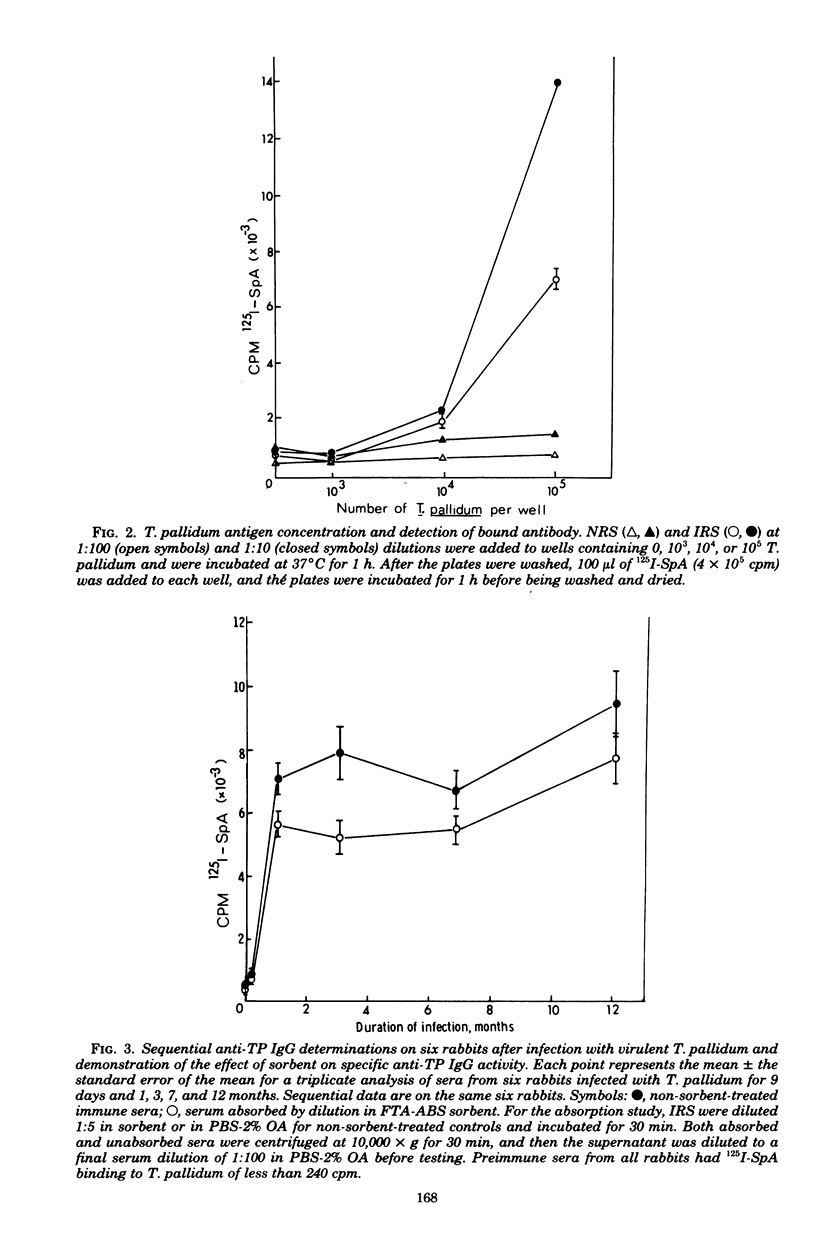
Radioiodinated staphylococcal protein A (SpA) was used to detect the immunoglobulin G (IgG) antibody response to Treponema pallidum in experimental syphilis. This solid-phase assay is based on the principle that SpA binds avidly to the Fc portion of mammalian IgG. The optimal number of organisms for detection of antibody was 10(5) per microwell. Of eight fixatives, 10% ethanol gave an optimum immune binding ratio of infected to normal rabbit serus at a 1:100 serum dilution. Kinetic studies demonstrated maximum binding and the highest immune binding ratio (15:1) with a 60-min incubation each for antibody and (125)I-SpA, respectively. The IgG response in rabbits intratesticularly infected with live T. pallidum and bled at -1, 9, 30, 90, 180, and 480 days was detected first at 9 days, reached a peak at 30 days, and remained elevated for 480 days. Absorption studies with an extract of T. phagedenis biotype reiterii demonstrated that 65 to 85% of the total antitreponemal IgG response was specific for T. pallidum throughout the course of infection. The microassay was quantitative and detected less than 2 ng of antibody.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ankerst J., Christensen P., Kjellén L., Kronvall G. A rountine diagnostic test for IgA and IgM antibodies to rubella virus: absorption of IgG with Staphylococcus aureus. J Infect Dis. 1974 Sep;130(3):268–273. doi: 10.1093/infdis/130.3.268. [DOI] [PubMed] [Google Scholar]
- Bishop N. H., Miller J. N. Humoral immunity in experimental syphilis. I. The demonstration of resistance conferred by passive immunization. J Immunol. 1976 Jul;117(1):191–196. [PubMed] [Google Scholar]
- Bishop N. H., Miller J. N. Humoral immunity in experimental syphilis. II. The relationship of neutralizing factors in immune serum to acquired resistance. J Immunol. 1976 Jul;117(1):197–207. [PubMed] [Google Scholar]
- Christensen K. K., Christensen P., Mårdh P. A., Weström L. Quantitation of serum antibodies to surface antigens of Neisseria gonorrhoeae with radiolabeled protein A of Staphylococcus aureus. J Infect Dis. 1976 Oct;134(4):317–323. doi: 10.1093/infdis/134.4.317. [DOI] [PubMed] [Google Scholar]
- Dorval G., Welsh K. I., Wigzell H. A radioimmunoassay of cellular surface antigens on living cells using iodinated soluble protein A from Staphylococcus aureus. J Immunol Methods. 1975 Jun;7(2-3):237–250. doi: 10.1016/0022-1759(75)90021-6. [DOI] [PubMed] [Google Scholar]
- Harboe M., Fölling I. Recognition of two distinct groups of human IgM and IgA based on different binding to staphylococci. Scand J Immunol. 1974;3(4):471–482. doi: 10.1111/j.1365-3083.1974.tb01280.x. [DOI] [PubMed] [Google Scholar]
- Huang J. C., Berczi I., Froese G., Tsay H. M., Sehon A. H. A microradioimmunoassay for antibodies to tumor-associated antigens. J Natl Cancer Inst. 1975 Oct;55(4):879–886. doi: 10.1093/jnci/55.4.879. [DOI] [PubMed] [Google Scholar]
- Hunter E. F., Maddison S. E., Larsen S. A., Felker M. B., Feeley J. C. Immunoglobulin specificity for the fluorescent treponemal antibody-absorption test conjugate. J Clin Microbiol. 1976 Oct;4(4):338–342. doi: 10.1128/jcm.4.4.338-342.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson H. D., Ziegler D. W. Simplified radioimmunoassay for diagnostic serology. Appl Microbiol. 1972 Nov;24(5):742–749. doi: 10.1128/am.24.5.742-749.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julian A. J., Logan L. C., Norins L. C. Early syphilis: immunoglobulins reactive in immunofluorescence and other serologic tests. J Immunol. 1969 May;102(5):1250–1259. [PubMed] [Google Scholar]
- Julian A. J., Logan L. C., Norins L. C., Scotti A. T. Latent syphilis: immunoglobulins reactive in immunofluorescence and other serological tests. Infect Immun. 1971 Apr;3(4):559–561. doi: 10.1128/iai.3.4.559-561.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronvall G., Seal U. S., Finstad J., Williams R. C., Jr Phylogenetic insight into evolution of mammalian Fc fragment of gamma G globulin using staphylococcal protein A. J Immunol. 1970 Jan;104(1):140–147. [PubMed] [Google Scholar]
- Kronvall G., Williams R. C., Jr Differences in anti-protein A activity among IgG subgroups. J Immunol. 1969 Oct;103(4):828–833. [PubMed] [Google Scholar]
- Lind I., Mansa B. Further investigation of specific and non-specific adsorption of serum globulins to Staphylococcus aureus. Acta Pathol Microbiol Scand. 1968;73(4):637–645. doi: 10.1111/j.1699-0463.1968.tb03221.x. [DOI] [PubMed] [Google Scholar]
- Logan L. C. Rabbit globulin and antiglobulin factors associated with Treponema pallidum growth in rabbits. Br J Vener Dis. 1974 Dec;50(6):421–427. doi: 10.1136/sti.50.6.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER J. N., WHANG S. J., FAZZAN F. P. STUDIES ON IMMUNITY IN EXPERIMENTAL SYPHILIS. I. IMMUNOLOGIC RESPONSE OF RABBITS IMMUNIZED WITH REITER PROTEIN ANTIGEN AND CHALLENGED WITH VIRULENT TREPONEMA PALLIDUM. Br J Vener Dis. 1963 Sep;39:195–198. doi: 10.1136/sti.39.3.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConahey P. J., Dixon F. J. A method of trace iodination of proteins for immunologic studies. Int Arch Allergy Appl Immunol. 1966;29(2):185–189. doi: 10.1159/000229699. [DOI] [PubMed] [Google Scholar]
- Metzger M., Michalska E., Podwińska J., Smogór W. Immunogenic properties of the protein component of Treponema pallidum. Br J Vener Dis. 1969 Dec;45(4):299–304. doi: 10.1136/sti.45.4.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. N. Immunity in experimental syphilis. VI. Successful vaccination of rabbits with Treponema pallidum, Nichols strain, attenuated by -irradiation. J Immunol. 1973 May;110(5):1206–1215. [PubMed] [Google Scholar]
- Miller J. N. Value and limitations of nontreponemal and treponemal tests in the laboratory diagnosis of syphilis. Clin Obstet Gynecol. 1975 Mar;18(1):191–203. doi: 10.1097/00003081-197503000-00017. [DOI] [PubMed] [Google Scholar]
- Roberts M. E., Miller J. N., Binnings G. F. Reduction of nonspecific background staining in the fluorescent treponemal antibody-absorption test. J Bacteriol. 1968 Nov;96(5):1500–1506. doi: 10.1128/jb.96.5.1500-1506.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal J. D., Hayashi K., Notkins A. L. Comparison of direct and indirect solid-phase microradioimmunoassays for the detection of viral antigens and antiviral antibody. Appl Microbiol. 1973 Apr;25(4):567–573. doi: 10.1128/am.25.4.567-573.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjöquist J., Meloun B., Hjelm H. Protein A isolated from Staphylococcus aureus after digestion with lysostaphin. Eur J Biochem. 1972 Sep 25;29(3):572–578. doi: 10.1111/j.1432-1033.1972.tb02023.x. [DOI] [PubMed] [Google Scholar]
- Thomas M. L., Clark J. W., Jr, Cline G. B., Anderson N. G., Russell H. Separation of Treponema pallidum from tissue substances by continuous-flow zonal centrifugation. Appl Microbiol. 1972 Apr;23(4):714–720. doi: 10.1128/am.23.4.714-720.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeltzer P. M., Seeger R. C. Microassay using radioiodinated protein A from Staphylococcus aureus for antibodies bound to cell surface antigens of adherent tumor cells. J Immunol Methods. 1977;17(1-2):163–175. doi: 10.1016/0022-1759(77)90087-4. [DOI] [PubMed] [Google Scholar]



