Abstract
The family of polyhalogenated monoterpenes from Plocamium counts over a hundred known members. Using glyceraldehyde acetonide as a chiral pool precursor, an enantioselective and divergent strategy was developed that provides a blueprint for the synthesis of many of the small yet complex acyclic members of this family. The broad applicability of this approach is demonstrated with the short (eight-step) synthesis of four natural products and three analogues. These syntheses are the first of any members of the acyclic polyhalogenated Plocamium monoterpenes, and permitted the evaluation of their selectivity against a range of tumor cell lines.
Keywords: natural product synthesis, chlorination, stereocontrol, olefination, solid tumors
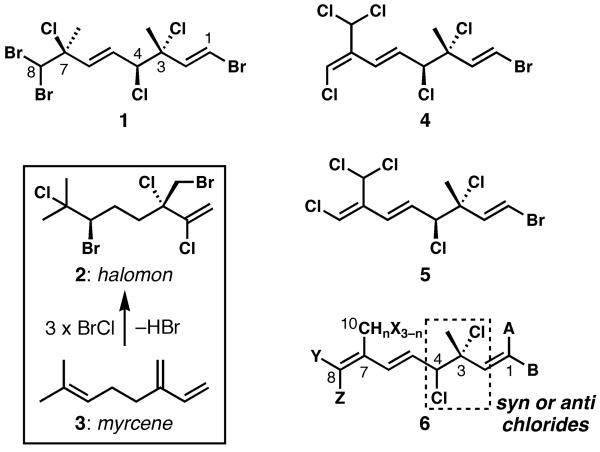
In 1973, Faulkner reported the discovery of (3R,4S,7S)-3,7-dimethyl-1,8,8-tribromo-3,4,7-trichloro-(1E,5E)-octadiene (1, Figure 1) from the digestive glands of the sea hare Aplysia californica as the first in a series of polyhalogenated monoterpenes.[1] Shortly thereafter, it became clear that these types of secondary metabolites were the products of red algae from the genus Plocamium, a major food source for the sea hares.[2] In the time since, over a hundred highly halogenated monoterpenes, both cyclic and acyclic, have been isolated from many different Plocamium species and from various locations.[3]
Figure 1.
Representative acyclic polyhalogenated monoterpenes from Plocamium, and the related natural product halomon with its biogenesis from myrcene. A, B, X, Y = Cl, Br, or H.
The family of acyclic polyhalogenated monoterpenes gained some renown in the 1990s with the discovery that halomon (2) had a unique profile of cytotoxicity when tested against the NCI 60-cell line. It demonstrated particular selectivity for cell lines that are typically resistant to chemotherapy (renal, brain, colon, and non-small-cell lung), with less efficacy toward leukemia and melanoma lines.[4,5] Isolated from the red alga Portieria hornemannii, this compound was the subject of preclinical development at the NCI. Halomon’s biogenesis from myrcene (3) is easily explained by sequential bromochlorination of each alkene followed by a single elimination of HBr (3 → 2). This hypothesis was used as inspiration for Hirama’s exceptional three-step synthesis of racemic halomon.[6] The biogenesis of many of the Plocamium monoterpenes (including 1) is not so easily explained,[3] and a straightforward laboratory conversion of an acyclic monoterpene feedstock to these targets is not likely.
Syntheses of the acyclic polyhalogenated Plocamium monoterpenes have not yet been described,[7] in spite of their reported anticancer and antimalarial activities.[8–10] Previously unpublished work from two of our laboratories (W.H.G. and F.A.V.) established that isomeric compounds 4 and 5[2,11] are selectively cytotoxic to several solid tumor cell lines, as defined by the disk diffusion assay pioneered by F.A.V.[12] Our goal in this work was to generate many more compounds in this series (the natural variability is shown by general structure 6). We targeted a significant array of natural and unnatural analogs for further testing of activity and selectivity, with the eventual goal of performing mechanism of action and pharmacological studies on the most promising candidates. In this communication, we describe a simple strategy for the enantioselective and divergent synthesis of many of these natural products from glyceraldehyde acetonide, with proof-of-principle execution resulting in access to four different natural products and three analogues.
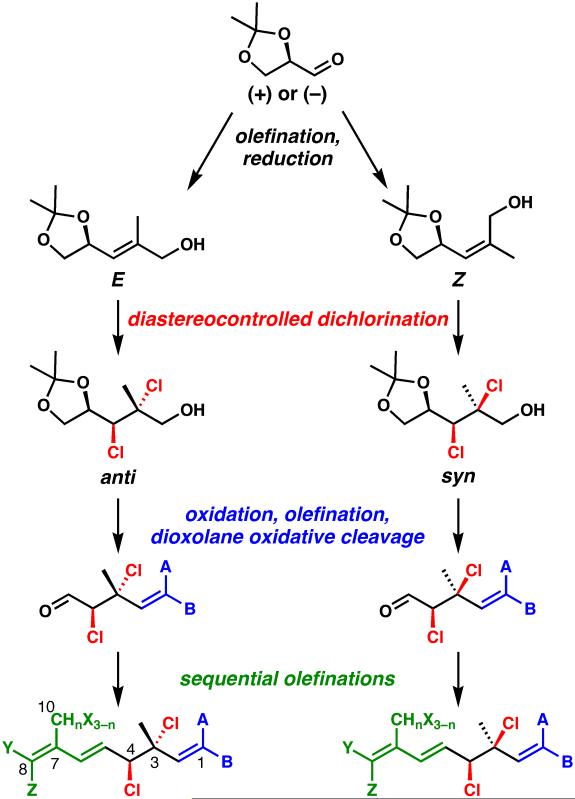
In considering a synthesis of compounds of type 4 and 5, we recognized multiple challenges in spite of the small molecular size of the targets. First, the vicinal secondary and tertiary chloride-bearing centers at C3 and C4, which are present in both the syn and anti diastereomeric forms within this family of natural products,[9] are both allylic and might cause undesired reactivity. Second, the extensive halogenation on either side of the “central” C5–C6 alkene appears to necessitate a convergent approach; however, the method chosen to unite two precursor fragments must be tolerant of the potentially reactive halogen atoms. Third, because we wanted to access tens or hundreds of milligrams of many different natural (and unnatural) polyhalogenated Plocamium monoterpenes for biological evaluation, we placed a premium on the development of a short and scalable, yet divergent sequence that could effectively access dozens of different targets. The culmination of our work to address these challenges is the flexible approach shown in Scheme 1, which permits access to both enantiomeric series, both diastereomeric forms of the C3/C4 vicinal dichloride, and a wide range of halogen substitutions at C1, C8, and C10. It features the non-obvious use of glyceraldehyde acetonide as the source of C4 and C5, as a formal “chiral glyoxal” equivalent that permits the stereocontrolled introduction of the C3 and C4 chlorine-bearing stereogenic centers, and as a linchpin for the entire synthesis.
Scheme 1.
Divergent synthesis plan for the Plocamium monoterpenes. Colored regions are diversifiable. A, B, X, Y = Cl, Br, or H.
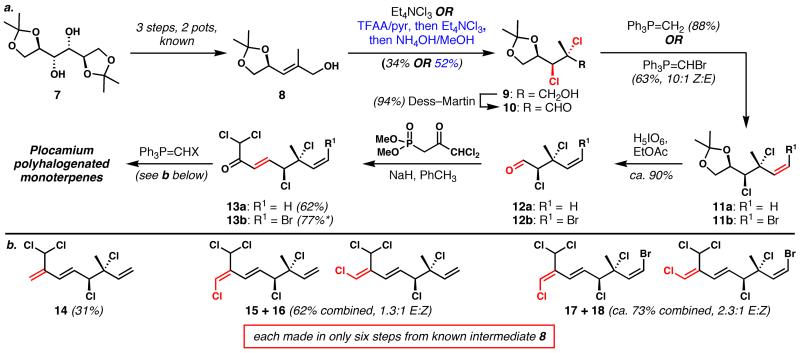
Mannitol diacetonide (7, Scheme 2a) has previously been converted to allylic alcohol 8 via oxidative cleavage to glyceraldehyde acetonide, stereoselective olefination, and ester reduction.[13] Dichlorination of the free allylic alcohol 8, although highly diastereoselective, was plagued with competitive oxidation to an enal[14] that resisted chlorination; this undesired reactivity could not be prevented by tuning of reaction conditions. Therefore, a one-pot sequence of in situ trifluoroacetylation, dichlorination, and deacylation was developed, which afforded higher overall yields and high selectivity (>10:1 d.r.). It is likely that the minimization of A1,3-strain[15] in substrate 8 and its trifluoroacetylated derivative is important, and while the outcome mirrors the studies of Chamberlin and Hehre in iodofunctionalization of allylic alcohols;[16,17] the mechanism underlying stereocontrol is not completely understood. X-ray crystallographic analysis on a mesylated derivative of alcohol 9[18] enabled the unequivocal assignment of absolute and relative configuration for compounds produced via this chemistry.
Scheme 2.
a. General sequence for the synthesis of polyhalogenated monoterpenes. *Olefination product 13b is contaminated by about 10% of eliminated product (conjugated trienone). b. Examples of five completed targets, with yields for the final olefination steps. Geometrical isomers 15/16 and 17/18 were completely separated by HPLC.
Oxidation of alcohol 9 to aldehyde 10 was followed by methylenation or bromomethylenation to afford products 11a or 11b, respectively. Direct oxidative cleavage of the dioxolane produced sensitive aldehydes 12a/12b in high yield. Olefination of these products was plagued by β-elimination under many conditions examined; however, the use of NaH in toluene with the Horner–Wadsworth–Emmons reagent shown provided enones 13a/13b in good yields with high levels of stereocontrol. Finally, methylenation or chloromethylenation provided targets 14–18 in the yields shown (Scheme 2b). The chloromethylenation products were obtained as mixtures at the newly formed alkenes, but they could be separated easily via preparative HPLC.[19] Compounds 14–16 and 18 are known natural products,[2,9] but 17 has not yet been reported.
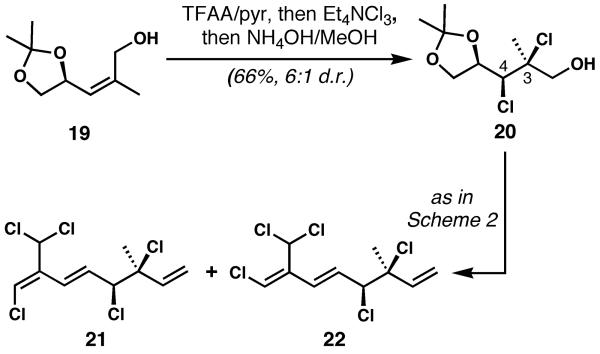
The synthesis as shown in Scheme 2 is highly divergent, and will provide access to many more naturally occurring polyhalogenated natural products from Plocamium as well as unnatural analogs. However, there remains another important aspect of our design: the Z-isomer of allylic alcohol 8 is also readily available (see 19, Scheme 3),[20–22] and we have shown that it also undergoes stereocontrolled dichlorination to procure the syn-C3/C4 dichloride (20). The relative configuration of product 20 was established via X-ray crystallographic analysis of the corresponding aldehyde obtained by oxidation.[23] Dichloroalcohol 20 was converted to 21 and 22 via similar chemistry to that shown in Scheme 2.[23,24] The final products thus produced are in the unnatural enantiomeric series because we used the same enantiomer of glyceraldehyde acetonide as we did for 14–18. Clearly, natural products in both diastereomeric series, as well as in both enantiomeric forms, are available by this route, because glyceraldehyde acetonide is readily available in both mirror image forms. Each of the target molecules (14–18, 21, and 22) was obtained in only six steps from allylic alcohols 8 or 19 (eight steps from inexpensive commercially available mannitol derivative 7). Although some of the steps proceed in only moderate yields and the chemistry is “classical”, these small but complex molecules have never been synthesized before, and the sequence is direct enough to procure plenty of material for biological evaluation.[25]
Scheme 3.
Synthesis of 3,4-syn Plocamium monoterpenes 21 and 22 via diastereocontrolled dichlorination of Z-allylic alcohol 19. See SI for details.
Stereocontrolled access to the C3 and C4 chloride-bearing stereogenic centers of the Plocamium monoterpenes would not be easily accomplished by other existing methods. The two best currently available methods to obtain vicinal dichlorides in enantioenriched form—the asymmetric dichlorination of allylic alcohols put forth by the Nicolaou group[26] and the stereospecific deoxydichlorination of epoxides developed by the Yoshimitsu group[27]—do not appear to tolerate the formation of tertiary chlorides such as C3. Moreover, the ready availability of substrate 8 and the utility of the dioxolane for more than just control of diastereoselectivity in the dichlorination (see below) is particularly attractive.
Although the synthesis of a ten-carbon target via four classical olefination reactions might seem far from ideal, this approach permits maximum divergence both with respect to constitution and configuration (Scheme 1), and suffers from few non-productive operations. The biggest liability, at this stage of development, is the lack of stereocontrol in the final olefination step; this apparent problem is mitigated by the fact that many of these natural products are found in both C7–C8 isomeric forms,[9] and that the isomers are readily separable. Furthermore, in the context of compounds 4 and 5 at least, these isomers are known to equilibrate readily upon exposure to minimally acidic medium.[20] Overall, the strategic use of glyceraldehyde acetonide as a chiral glyoxal equivalent is significant; it not only plays the role of chiral auxiliary to control the absolute configuration of the C3/C4 dichloride, it also serves as a truly effective linchpin for our short synthesis by virtue of the one-step conversion of the dioxolane into an aldehyde. At current count, we have made four naturally occurring polyhalogenated Plocamium monoterpenes, one that is likely to be an as yet undiscovered natural product, and two unnatural enantiomers, thereby showcasing the generality of our approach.
Compounds 14–16 were subjected to the disk diffusion assay that provides an indication of the selectivity of compounds for human solid tumor cell lines compared with the CCRF-CEM human leukemia cell line. These data were compared with the data obtained for halomon (2) and compound 4 using the same assay (Table 1).[10,27] All five compounds exhibited selectivity for solid tumors, with some differences in the selectivity profiles. None showed selectivity for PANC-1, while all were selective for LNCaP, OVCAR-5, and U251N tumor cells with respect to the leukemia cell line. Of the three synthetic compounds evaluated, 15 proved to be the most potent, with an IC50 of 1.3 μg/mL against the HCT-116 colon carcinoma cell line, and it demonstrated similar activity to previously isolated compound 4. This level of activity, coupled with the good selectivity for solid tumors, is sufficient to warrant further studies, including the development of a detailed SAR profile for this class of compounds and the synthesis of more bioavailable analogs. Our versatile synthesis is ideal to fuel these studies.
Table 1.
Selectivity of halomon (2), compound 4, and synthetic compounds 14–16 for different solid tumor cell lines[a] with respect to the CCRF-CEM leukemia cell lines according to the disk diffusion assay,[b] and the activity measured as IC50 against HCT-116 cells. A “✔” indicates selectivity for the indicated cell line.
| compound | H116 | H125 | MCF-7 | LNCaP | OVC-5 | U251N | MDA | PANC-1 | HepG2 |
IC50
(μ/mL) |
|---|---|---|---|---|---|---|---|---|---|---|
| halomon (2) | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | 0.37[c] | ||
| 4 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | 1.3 | |||
| 14 | ✔ | ✔ | ✔ | ✔ | ✔ | 15 | ||||
| 15 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | 1.3 | |
| 16 | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | 3.6 |
H116: HCT-116 human colon carcinoma; H125: human non-small-cell lung carcinoma; MCF-7: human breast adenocarcinoma; LNCaP: androgen-sensitive human prostate adenocarcinoma; OVC-5: OVCAR-5 human ovary carcinoma; U251N: human glioblastoma; MDA: MDA-MB-231 human breast carcinoma; PANC-1: human pancreatic carcinoma; HepG2: human hepatocellular carcinoma.
The disk diffusion soft agar colony formation assay is used to identify compounds that are selective for solid tumor lines. The observation of a substantial differential in the zone of growth inhibition between solid tumor cell lines and leukemia cell lines indicates solid tumor selectivity. See the Supporting Information for raw data.
Insufficient halomon was available to run an IC50 value against HCT-116 cells in our laboratories; this value is taken from reference 4.
Supplementary Material
Acknowledgements
This work was supported by NIH through R01 GM086483 (to C.D.V), F31 CA174176 (to C.V.V.), and R01 CA 100851 (to W.H.G. and F.A.V.), and by the UC Cancer Research Coordinating Committee (to C.D.V.). We thank Dr. John Greaves for support with mass spectrometry and Dr. Joseph Ziller and Jordan Corbey for assistance with X-ray crystallography.
References
- 1.a Faulkner DJ, Stallard MO. Tetrahedron Lett. 1973;14:1171–1174. [Google Scholar]; b Faulkner DJ, Stallard MO, Fayos J, Clardy J. J. Am. Chem. Soc. 1973;95:3413–3414. doi: 10.1021/ja00802a064. [DOI] [PubMed] [Google Scholar]
- 2.Mynderse JS, Faulkner DJ. Tetrahedron. 1975;31:1963–1967. [Google Scholar]
- 3.a Naylor S, Hanke FJ, Manes LV, Crews P. Prog. Chem. Org. Nat. Prod. 1983;44:189–241. For an excellent reviews, see: [Google Scholar]; b Kladi M, Vagias C, Roussis V. Phytochem. Rev. 2004;3:337–366. [Google Scholar]
- 4.Fuller RW, Cardellina JH, II, Kato Y, Brinen LS, Clardy J, Snader KM, Boyd MR. J. Med. Chem. 1992;35:3007–3011. doi: 10.1021/jm00094a012. [DOI] [PubMed] [Google Scholar]
- 5.Andrianasolo EH, France D, Cornell-Kennon S, Gerwick WH. J. Nat. Prod. 2006;69:576–579. doi: 10.1021/np0503956. Halomon’s mechanism of action could be related to its DNA methyl transferase-1 inhibitory activity: [DOI] [PubMed] [Google Scholar]
- 6.Sotokawa T, Noda T, Pi S, Hirama M. Angew. Chem. Int. Ed. 2000;39:3430–3432. doi: 10.1002/1521-3773(20001002)39:19<3430::aid-anie3430>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 7.a Williard PG, de Laszlo SE. J. Org. Chem. 1985;50:3738–3749. Some of the monocyclic polyhalogenated monoterpenoids from Plocamium have been made: [Google Scholar]; b Whitney JM, Parnes JS, Shea KJ. J. Org. Chem. 1997;62:8962–8963. [Google Scholar]
- 8.Afolayan AF, Mann MGA, Lategan CA, Smith PJ, Bolton JJ, Beukes DR. Phytochemistry. 2009;70:597–600. doi: 10.1016/j.phytochem.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 9.Antunes EM, Afolayan AF, Chiwakata MT, Fakee J, Knott MG, Whibley CE, Hendricks DT, Bolton JJ, Beukes DR. Phytochemistry. 2011;72:769–772. doi: 10.1016/j.phytochem.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 10.a Nilewski C, Geisser RW, Carreira EM. Nature. 2009;457:573–576. doi: 10.1038/nature07734. Significant work has been reported toward the chlorosulfolipids, which are only peripherally related: [DOI] [PubMed] [Google Scholar]; b Bedke DK, Shibuya GM, Pereira A, Gerwick WH, Haines TH, Vanderwal CD. J. Am. Chem. Soc. 2009;131:7570–7572. doi: 10.1021/ja902138w. [DOI] [PubMed] [Google Scholar]; c Bedke DK, Shibuya GM, Pereira AR, Gerwick WH, Vanderwal CD. J. Am. Chem. Soc. 2010;132:2542–2543. doi: 10.1021/ja910809c. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Yoshimitsu T, Fujumoto N, Nakatani R, Kojima N, Tanaka T. J. Org. Chem. 2010;75:5425–5437. doi: 10.1021/jo100534d. [DOI] [PubMed] [Google Scholar]; e Yoshimitsu T, Nakatani R, Kobayashi A, Tanaka T. Org. Lett. 2011;13:908–911. doi: 10.1021/ol1029518. [DOI] [PubMed] [Google Scholar]; f Umezawa T, Shibata M, Kaneko K, Okino T, Matsuda F. Org. Lett. 2011;13:904–907. doi: 10.1021/ol102882a. [DOI] [PubMed] [Google Scholar]; g Nilewski C, Deprez NR, Fessard TC, Li DB, Geisser RW, Carreira EM. Angew. Chem. Int. Ed. 2011;50:7940–7943. doi: 10.1002/anie.201102521. [DOI] [PubMed] [Google Scholar]; h Chung W.-j., Carlson JS, Bedke DK, Vanderwal CD. Angew. Chem. Int. Ed. 2013;52:10052–10055. doi: 10.1002/anie.201304565. [DOI] [PMC free article] [PubMed] [Google Scholar]; i Chung W.-j., Vanderwal CD. Acc. Chem. Res. 2014;47:718–728. doi: 10.1021/ar400246w. [DOI] [PMC free article] [PubMed] [Google Scholar]; j Chung W.-j., Carlson JS, Vanderwal CD. J. Org. Chem. 2014;79:2226–2241. doi: 10.1021/jo5000829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. For the purpose of testing, compounds 4 and 5 were reisolated from P. cartilagineum.
- 12.Valeriote FA, Tenney K, Media J, Pietraszkiewicz H, Edelstein M, Johnson TA, Amagata T, Crews P. J. Expl Ther. Oncol. 2012;10:119–134. [PubMed] [Google Scholar]
- 13.Nemoto H, Satoh A, Ando M, Fukumoto K. J. Chem. Soc. Perkin Trans. 1991;1:1309–1314. [Google Scholar]
- 14.Schlama T, Gabriel K, Gouverneur V, Mioskowski C. Angew. Chem. Int. Ed. 1997;36:2342–2344. Mioskowski’s trichloride reagent is known to oxidize alcohols under some circumstances: [Google Scholar]
- 15.Hoffmann RW. Chem. Rev. 1989;89:1841–1860. [Google Scholar]
- 16.a Chamberlin AR, Mulholland RL., Jr. Tetrahedron. 1984;40:2297–2302. [Google Scholar]; b Chamberlin AR, Mulholland RL, Jr., Kahn SD, Hehre WJ. J. Am. Chem. Soc. 1987;109:672–677. [Google Scholar]
- 17.Shibuya GM, Kanady JS, Vanderwal CD. J. Am. Chem. Soc. 2008;130:12514–12518. doi: 10.1021/ja804167v. In our early work toward the chlorosulfolipids, we found that Z-allylic esters underwent diastereocontrolled dichlorination. See: [DOI] [PubMed] [Google Scholar]
- 18. See the Supporting Information for this X-ray crystal structure. When the dichlorination reaction was performed on a multi-gram scale, enough of the minor diastereomer was obtained for X-ray crystallographic analysis of its mesylate. This structure can also be found in the Supporting Information.
- 19.Sabry OM. 2004. pp. 99–133. Facile isomerization of the Z-isomer (5) to the E-isomer (4) under mildly acidic conditions has been reported for very similar systems in studies in the Gerwick laboratory: PhD dissertation Oregon State University.
- 20.Takano S, Kurotaki A, Takahashi M, Ogasawara K. J. Chem. Soc. Perkin Trans. 1987;1:91–97. Compound 19 is known, but has not been made with high stereoselectivity: [Google Scholar]
- 21.Bates RH, Shotwell JB, Roush WR. Org. Lett. 2008;10:4343–4346. doi: 10.1021/ol801852j. The corresponding 3-pentanone ketal, of opposite absolute configuration, has been made stereoselectively: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. We generate compound 19 via a reasonably Z-selective (6:1) Horner–Wadsworth–Emmons olefination, which is much more economical than the corresponding Still–Gennari-modified reaction. See the Supporting Information for details.
- 23. Please see the Supporting Information for details.
- 24. The enantiomer of 21 has not yet been reported, but that of 22 is known (see ref 2). On the basis of the ready equilibration of the “left-hand” trichloropropenyl moieties found in 15–18, 21, and 22, and the fact that in many cases both geometrical isomers are isolated from the same source, it is reasonable to assume that both 17 and ent-21 are natural products that have not yet been described in the literature.
- 25. For example, we have made ca. 40 mg of compound 15 via this route, without any attempt to scale up the synthesis.
- 26.Nicolaou KC, Simmons NL, Ying Y, Heretsch PM, Chen JS. J. Am. Chem. Soc. 2011;133:8134–8137. doi: 10.1021/ja202555m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yoshimitsu T, Fukumoto N, Tanaka T. J. Org. Chem. 2009;74:696–702. doi: 10.1021/jo802093d. [DOI] [PubMed] [Google Scholar]
- 28. A mixture of compounds 4 and 5 and each separate compound were tested and showed similar selectivity to that shown for 4 in Table 1. However, there was only enough material to do the complete set of assays, including IC50 determination, for compound 4.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.