Abstract
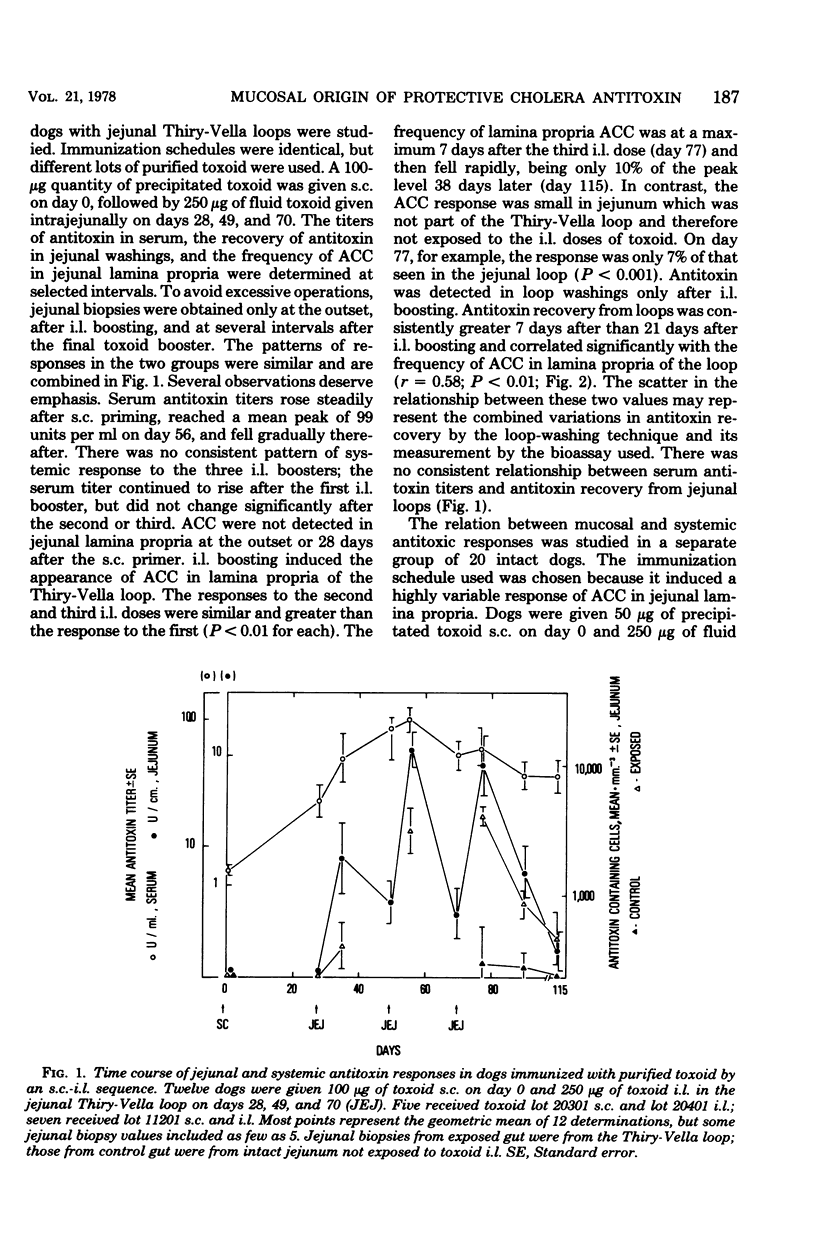
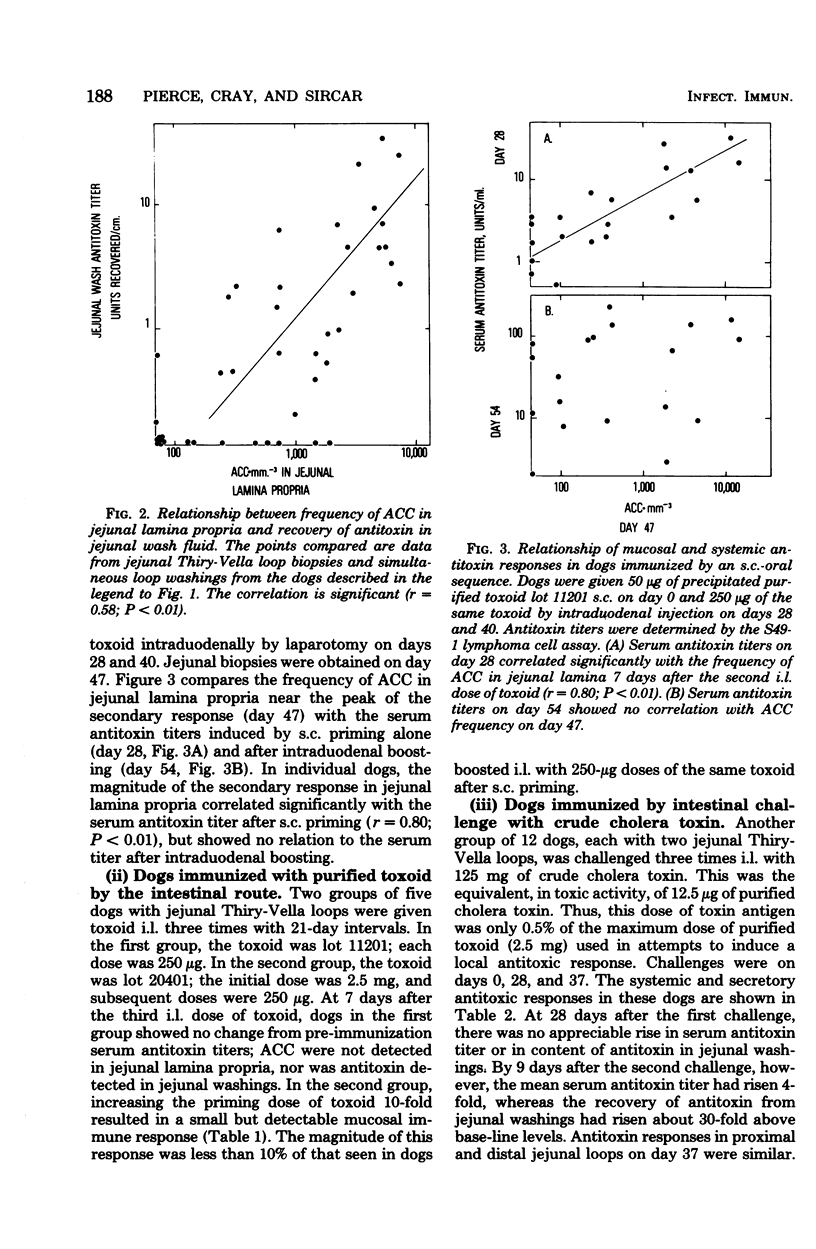
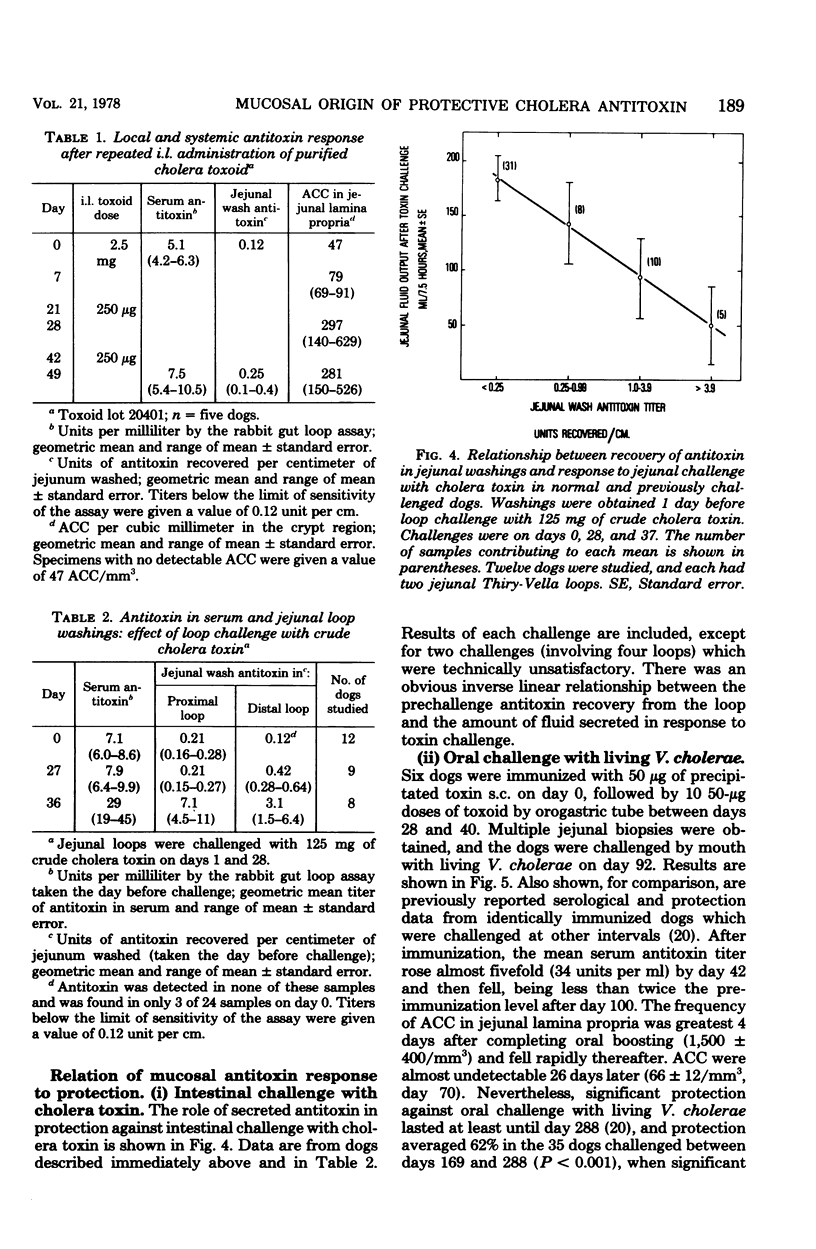
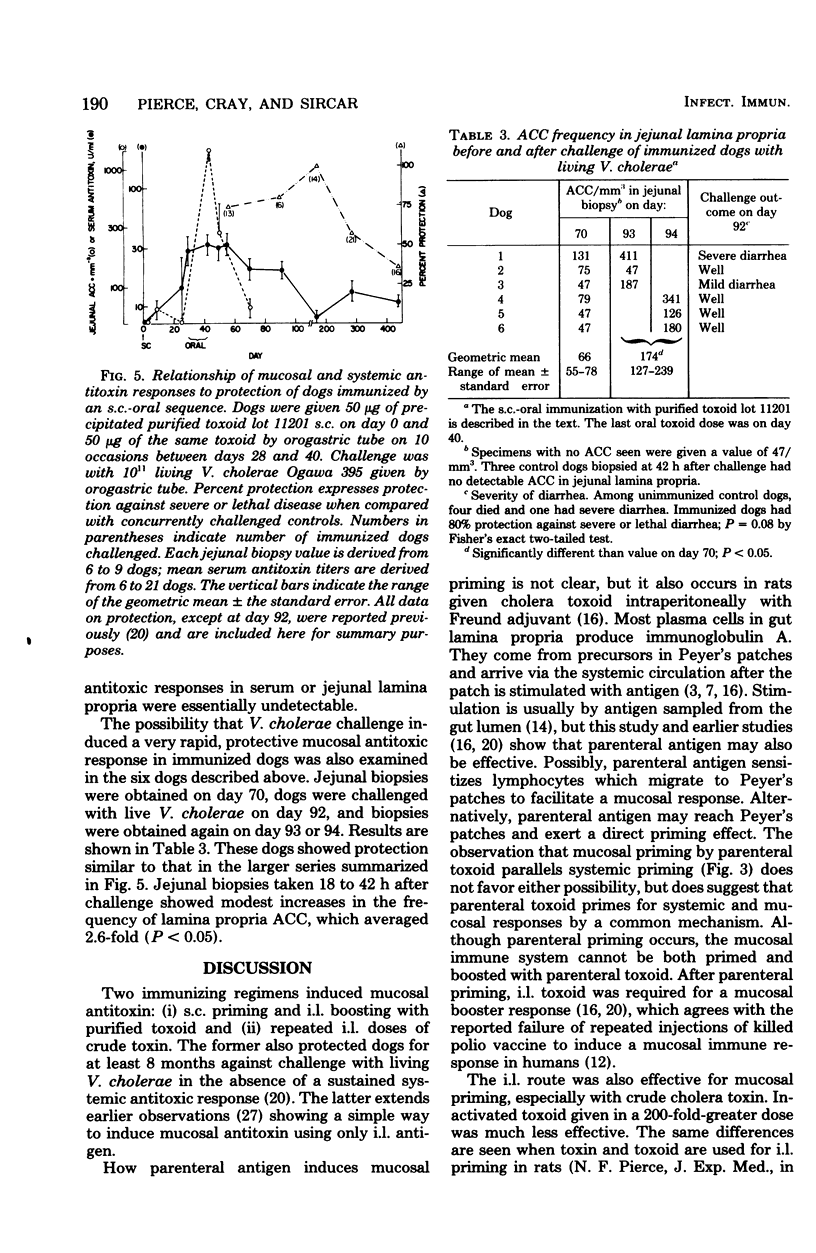
The induction of a jejunal antitoxin response was studied in dogs immunized with cholera toxin or toxoid. Single doses of toxoid given subcutaneously (s.c.) or of toxin given intraluminally (i.l.) were each effective in priming the mucosal immune system, whereas toxoid given i.l. was much less effective. In contrast, toxin and toxoid given i.l. were each effective as booster antigens. The local secondary response was rapid and brief, the peak occurring at about 7 days after i.l. boosting and declining by 90% after 2 more weeks. After s.c. priming and i.l. boosting with toxoid, antitoxin-containing plasma cells appeared predominantly in the portion of jejunum exposed to the i.l. booster. The appearance of antitoxin-containing plasma cells in jejunal lamina propria correlated with the amount of antitoxin recovered in jejunal washings which, in turn, correlated with protection against challenge with cholera toxin. Thus, lamina propria antitoxin-containing plasma cells appeared to be the source of protective antitoxin. However, after sequential s.c.-oral immunization with toxoid, protection against challenge with Vibrio cholerae far outlasted the major systemic and local antitoxin responses and was not obviously explained by either. These studies reveal methods for induction of a mucosal antitoxin response, but leave in question the mechanism of prolonged protection induced by s.c.-oral immunization of dogs.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braun W., Ishizuka M., Winchurch R., Webb D. On the role of cyclic AMP in immune responses. Ann N Y Acad Sci. 1971 Dec 30;185:417–422. doi: 10.1111/j.1749-6632.1971.tb45268.x. [DOI] [PubMed] [Google Scholar]
- Cash R. A., Music S. I., Libonati J. P., Craig J. P., Pierce N. F., Hornick R. B. Response of man to infection with Vibrio cholerae. II. Protection from illness afforded by previous disease and vaccine. J Infect Dis. 1974 Oct;130(4):325–333. doi: 10.1093/infdis/130.4.325. [DOI] [PubMed] [Google Scholar]
- Craig S. W., Cebra J. J. Peyer's patches: an enriched source of precursors for IgA-producing immunocytes in the rabbit. J Exp Med. 1971 Jul 1;134(1):188–200. doi: 10.1084/jem.134.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRETER R., GANGAROSA E. J. ORAL IMMUNIZATION AND PRODUCTION OF COPROANTIBODY IN HUMAN VOLUNTEERS. J Immunol. 1963 Dec;91:724–729. [PubMed] [Google Scholar]
- Fujita K., Finkelstein R. A. Antitoxic immunity in experimental cholera: comparison of immunity induced perorally and parenterally in mice. J Infect Dis. 1972 Jun;125(6):647–655. doi: 10.1093/infdis/125.6.647. [DOI] [PubMed] [Google Scholar]
- GOWANS J. L., KNIGHT E. J. THE ROUTE OF RE-CIRCULATION OF LYMPHOCYTES IN THE RAT. Proc R Soc Lond B Biol Sci. 1964 Jan 14;159:257–282. doi: 10.1098/rspb.1964.0001. [DOI] [PubMed] [Google Scholar]
- Goldblum R. M., Ahlstedt S., Carlsson B., Hanson L. A., Jodal U., Lidin-Janson G., Sohl-Akerlund A. Antibody-forming cells in human colostrum after oral immunisation. Nature. 1975 Oct 30;257(5529):797–798. doi: 10.1038/257797a0. [DOI] [PubMed] [Google Scholar]
- Holmgren J., Andersson A., Wallerstrom G., Ouchterlony O. Experimental studies on cholera immunization. II. Evidence for protective antitoxic immunity mediated by serum antibodies as well as local antibodies. Infect Immun. 1972 May;5(5):662–667. doi: 10.1128/iai.5.5.662-667.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J., Lindholm L., Lönnroth I. Interaction of cholera toxin and toxin derivatives with lymphocytes. I. Binding properties and interference with lectin-induced cellular stimulation. J Exp Med. 1974 Apr 1;139(4):801–819. doi: 10.1084/jem.139.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren J., Svennerholm A. M., Ouchterlony O., Anderson A., Walletström G., Westerberg-Berndtsson U. Antitoxic immunity in experimental cholera: protection, and serum and local antibody responses in rabbits after enteral and parenteral immunization. Infect Immun. 1975 Dec;12(6):1331–1340. doi: 10.1128/iai.12.6.1331-1340.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northrup R. S., Fauci A. S. Adjuvant effect of cholera enterotoxin on the immune response of the mouse to sheep red blood cells. J Infect Dis. 1972 Jun;125(6):672–673. doi: 10.1093/infdis/125.6.672. [DOI] [PubMed] [Google Scholar]
- Ogra P. L., Karzon D. T. Distribution of poliovirus antibody in serum, nasopharynx and alimentary tract following segmental immunization of lower alimentary tract with poliovaccine. J Immunol. 1969 Jun;102(6):1423–1430. [PubMed] [Google Scholar]
- Owen R. L. Sequential uptake of horseradish peroxidase by lymphoid follicle epithelium of Peyer's patches in the normal unobstructed mouse intestine: an ultrastructural study. Gastroenterology. 1977 Mar;72(3):440–451. [PubMed] [Google Scholar]
- Pierce N. F., Gowans J. L. Cellular kinetics of the intestinal immune response to cholera toxoid in rats. J Exp Med. 1975 Dec 1;142(6):1550–1563. doi: 10.1084/jem.142.6.1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce N. F., Kaniecki E. A., Northrup R. S. Protection against experimental cholera by antitoxin. J Infect Dis. 1972 Dec;126(6):606–616. doi: 10.1093/infdis/126.6.606. [DOI] [PubMed] [Google Scholar]
- Pierce N. F., Reynolds H. Y. Immunity to experimental cholera. I. Protective effect of humoral IgG antitoxin demonstrated by passive immunization. J Immunol. 1974 Sep;113(3):1017–1023. [PubMed] [Google Scholar]
- Pierce N. F., Reynolds H. Y. Immunity to experimental cholera. II. Secretory and humoral antitoxin response to local and systemic toxoid administration. J Infect Dis. 1975 Apr;131(4):383–389. doi: 10.1093/infdis/131.4.383. [DOI] [PubMed] [Google Scholar]
- Pierce N. F., Sack R. B., Sircar B. K. Immunity to experimental cholera. III. Enhanced duration of protection after sequential parenteral-oral administration of toxoid to dogs. J Infect Dis. 1977 Jun;135(6):888–896. doi: 10.1093/infdis/135.6.888. [DOI] [PubMed] [Google Scholar]
- Pierce N. F., Wallace C. K. Stimulation of jejunal secretion by a crude Escherichia coli enterotixin. Gastroenterology. 1972 Sep;63(6):439–448. [PubMed] [Google Scholar]
- Rappaport R. S., Bonde G., McCann T., Rubin B. A., Tint H. Development of a purified cholera toxoid. II. Preparation of a stable, antigenic toxoid by reaction of purified toxin with glutaraldehyde. Infect Immun. 1974 Feb;9(2):304–317. doi: 10.1128/iai.9.2.304-317.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappaport R. S., Pierzchala W. A., Bonde G., McCann T., Rubin B. A. Development of a purified cholera toxoid. III. Refinements in purification of toxin and methods for the determination of residual somatic antigen. Infect Immun. 1976 Sep;14(3):687–693. doi: 10.1128/iai.14.3.687-693.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds H. Y., Johnson J. S. Canine immunoglobulins. IV. Copro-immunoglobulins. J Immunol. 1970 Apr;104(4):888–895. [PubMed] [Google Scholar]
- Sack R. B., Carpenter C. C. Experimental canine cholera. I. Development of the model. J Infect Dis. 1969 Feb;119(2):138–149. doi: 10.1093/infdis/119.2.138. [DOI] [PubMed] [Google Scholar]
- Sack R. B., Johnson J., Pierce N. F., Keren D. F., Yardley J. H. Challenge of dogs with live enterotoxigenic Escherichia coli and effects of repeated challenges on fluid secretion in jejunal Thiry-Vella loops. J Infect Dis. 1976 Jul;134(1):15–24. doi: 10.1093/infdis/134.1.15. [DOI] [PubMed] [Google Scholar]
- Svennerholm A. M., Holmgren J. Immunoglobulin and specific-antibody synthesis in vitro by enteral and nonenteral lymphoid tissues after subcutaneous cholera immunization. Infect Immun. 1977 Feb;15(2):360–369. doi: 10.1128/iai.15.2.360-369.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


