Abstract
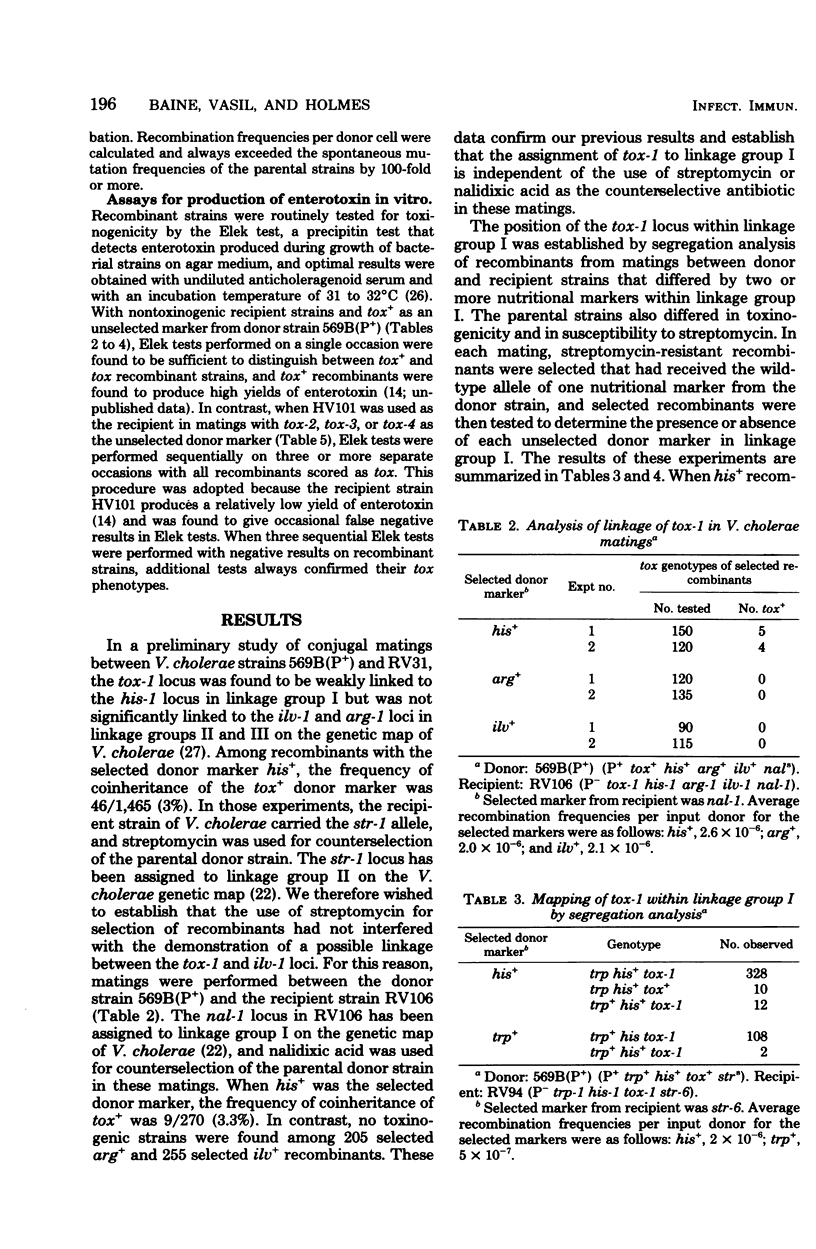
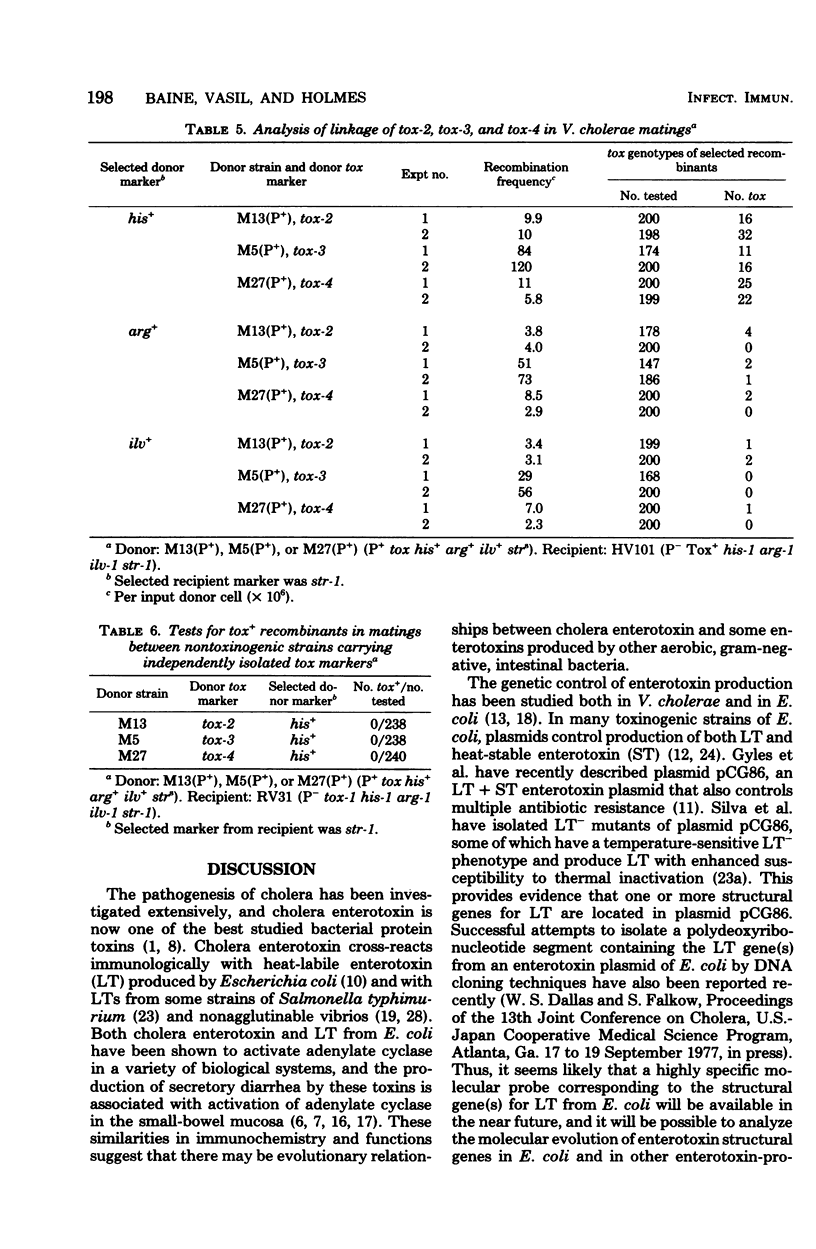
Conjugal mating experiments were performed between donor strains of Vibrio cholerae carrying the vibrio sex plasmid P and recipient strains lacking the P plasmid. Donor and recipient genotypes differed with respect to toxinogenicity (tox), nutritional requirements, and antibiotic susceptibilities. Recombinants carrying selected donor and recipient markers were produced at low frequencies in conjugal matings. Mapping of tox markers was accomplished by scoring for the frequency of coinheritance of the donor tox allele with selected and unselected donor markers. Four independently isolated tox markers were analyzed. Each of these four tox markers was shown to be linked to the his-1 site in linkage group I on the genetic map of V. cholerae. In matings between a recipient strain carrying the tox-1 marker and donor strains carrying either tox-2, tox-3, or tox-4, all selected his+ recombinants remained nontoxinogenic. Matings between multiply marked strains demonstrated that the position of tox-1 with respect to other genetic loci in linkage group I is as follows: met-2--trp-1--asp-1--nal-1--his-1--tox-1. These findings demonstrate that a chromosomal determinant linked to his-1 in linkage group I on the genetic map of V. cholerae is essential for toxinogenesis and suggest that tox-1,tox-2, tox-3, and tox-4 may be alleles of a single tox gene.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Datta A., Parker C. D., Wohlhieter J. A., Baron L. S. Isolation and characterization of the fertility factor P of Vibrio cholerae. J Bacteriol. 1973 Feb;113(2):763–771. doi: 10.1128/jb.113.2.763-771.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Jr, Chen L. C., Curlin G. T., Evans D. G. Stimulation of adenyl cyclase by Escherichia coli enterotoxin. Nat New Biol. 1972 Apr 5;236(66):137–138. doi: 10.1038/newbio236137a0. [DOI] [PubMed] [Google Scholar]
- Field M: Intestinal secretion: effect of cyclic AMP and its role in cholera. N Engl J Med. 1971 May 20;284(20):1137–1144. doi: 10.1056/NEJM197105202842008. [DOI] [PubMed] [Google Scholar]
- Finkelstein R. A., Vasil M. L., Holmes R. K. Studies on toxinogenesis in Vibrio cholerae. I. Isolation of mutants with altered toxinogenicity. J Infect Dis. 1974 Feb;129(2):117–123. doi: 10.1093/infdis/129.2.117. [DOI] [PubMed] [Google Scholar]
- Gyles C. L., Palchaudhuri S., Maas W. K. Naturally occurring plasmid carrying genes for enterotoxin production and drug resistance. Science. 1977 Oct 14;198(4313):198–199. doi: 10.1126/science.333581. [DOI] [PubMed] [Google Scholar]
- Gyles C. L. Relationships among heat-labile enterotoxins of Escherichia coli and Vibrio cholerae. J Infect Dis. 1974 Mar;129(3):277–283. doi: 10.1093/infdis/129.3.277. [DOI] [PubMed] [Google Scholar]
- Gyles C., So M., Falkow S. The enterotoxin plasmids of Escherichia coli. J Infect Dis. 1974 Jul;130(1):40–49. doi: 10.1093/infdis/130.1.40. [DOI] [PubMed] [Google Scholar]
- Holmes R. K., Baine W. B., Vasil M. L. Quantitative measurements of cholera enterotoxin in cultures of toxinogenic wild-type and nontoxinogenic mutant strains of Vibrio cholerae by using a sensitive and specific reversed passive hemagglutination assay for cholera enerotoxin. Infect Immun. 1978 Jan;19(1):101–106. doi: 10.1128/iai.19.1.101-106.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynie S., Sharp G. W. The effect of cholera toxin on intestinal adenyl cyclase. Adv Cyclic Nucleotide Res. 1972;1:163–174. [PubMed] [Google Scholar]
- Kimberg D. V., Field M., Johnson J., Henderson A., Gershon E. Stimulation of intestinal mucosal adenyl cyclase by cholera enterotoxin and prostaglandins. J Clin Invest. 1971 Jun;50(6):1218–1230. doi: 10.1172/JCI106599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oashi M., Shimada T., Fukumi H. In vitro production of enterotoxin and hemorrhagic principle by Vibrio cholerae, NAG. Jpn J Med Sci Biol. 1972 Jun;25(3):179–194. doi: 10.7883/yoken1952.25.179. [DOI] [PubMed] [Google Scholar]
- Parker C., Romig W. R. Self-transfer and genetic recombination mediated by P, the sex factor of Vibrio cholerae. J Bacteriol. 1972 Nov;112(2):707–714. doi: 10.1128/jb.112.2.707-714.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandefur P. D., Peterson J. W. Neutralization of Salmonella toxin-induced elongation of Chinese hamster ovary cells by cholera antitoxin. Infect Immun. 1977 Mar;15(3):988–992. doi: 10.1128/iai.15.3.988-992.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva M. L., Maas W. K., Gyles C. L. Isolation and characterization of enterotoxin-deficient mutants of Escherichia coli. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1384–1388. doi: 10.1073/pnas.75.3.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So M., Crosa J. H., Falkow S. Polynucleotide sequence relationships among Ent plasmids and the relationship between Ent and other plasmids. J Bacteriol. 1975 Jan;121(1):234–238. doi: 10.1128/jb.121.1.234-238.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasil M. L., Holmes R. K., Finkelstein R. A. Conjugal transfer of a chromosomal gene determining production of enterotoxin in vibrio cholerae. Science. 1975 Mar 7;187(4179):849–850. doi: 10.1126/science.1114331. [DOI] [PubMed] [Google Scholar]
- Vasil M. L., Holmes R. K., Finkelstein R. A. Studies on toxinogenesis in Vibrio cholerae. II. An vitro test for enterotoxin production. Infect Immun. 1974 Jan;9(1):195–197. doi: 10.1128/iai.9.1.195-197.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinnaka Y., Carpenter C. C., Jr An enterotoxin produced by noncholera vibrios. Johns Hopkins Med J. 1972 Dec;131(6):403–411. [PubMed] [Google Scholar]


