Abstract
The zebrafish posterior lateral line is formed during early development by the deposition of neuromasts from a migrating primordium. The molecular mechanisms regulating the regional organization and migration of the primordium involve interactions between Fgf and Wnt/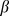 -catenin signaling and the establishment of specific cxcr4b and cxcr7b cytokine receptor expression domains. Itch has been identified as a regulator in several different signaling pathways, including Wnt and Cxcr4 signaling. We identified two homologous itch genes in zebrafish, itcha and itchb, with generalized expression patterns. By reducing itchb expression in particular upon morpholino knockdown, we demonstrated the importance of Itch in regulating lateral line development by perturbing the patterns of cxcr4b and cxcr7b expression. Itch knockdown results in a failure to down-regulate Wnt signaling and overexpression of cxcr4b in the primordium, slowing migration of the posterior lateral line primordium and resulting in abnormal development of the lateral line.
-catenin signaling and the establishment of specific cxcr4b and cxcr7b cytokine receptor expression domains. Itch has been identified as a regulator in several different signaling pathways, including Wnt and Cxcr4 signaling. We identified two homologous itch genes in zebrafish, itcha and itchb, with generalized expression patterns. By reducing itchb expression in particular upon morpholino knockdown, we demonstrated the importance of Itch in regulating lateral line development by perturbing the patterns of cxcr4b and cxcr7b expression. Itch knockdown results in a failure to down-regulate Wnt signaling and overexpression of cxcr4b in the primordium, slowing migration of the posterior lateral line primordium and resulting in abnormal development of the lateral line.
Introduction
The zebrafish lateral line is an insightful system for studies of cellular development as it displays evolutionarily-conserved developmental mechanisms ranging from progenitor migration, neural differentiation and planar cell polarity to sensory transduction [1]. Furthermore, the lateral line is thought to have evolved into sensory structures of the cochlea and inner ear in drier vertebrates, making it an organ of broad interest for developmental neurobiology [2], [3]. The posterior lateral line (pLL) is a mechanosensory organ running along the body and tail of fish and amphibians. It is built during early development through coordinated cell migration, proliferation, epithelial morphogenesis and differentiation of a group of about one hundred cells forming the pLL primordium. The pLL primordium arises from placodal cells that undergo partial epithelial-mesenchymal transition and acquire migratory properties. As the primordium migrates towards the tail along the myoseptum, cells in the trailing zone of the primordium become organized into rosette-like epithelial structures that mature into proneuromasts, which are reiteratively formed and deposited every 3–4 hours. These cells differentiate as the accessory and hair cells of the 6–7 mature neuromasts of the primary pLL. When the primordium reaches the end of the tail, it fragments into a few terminal neuromasts [4]–. Thus, the timing of neuromast deposition and the underlying molecular mechanisms of its regulation are critical for the development of this organ.
The migration of the primordium and the formation of the neuromasts is coordinated by Wnt and Fgf signaling. Through a feedback mechanism, Wnt/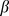 -catenin signaling is restricted to the leading zone of the primordium and Fgf signaling occurs in the trailing zone [7], [8]. These localized activities maintain the polarized activation of two chemokine receptors: cxcr4b is expressed in the leading zone while cxcr7b is restricted to the trailing zone. The differential expression of cxcr4b and cxcr7b is essential for directed collective migration of the primordium cells [1], [9]. However, the mechanisms downstream of these receptors that convey their actions are unclear.
-catenin signaling is restricted to the leading zone of the primordium and Fgf signaling occurs in the trailing zone [7], [8]. These localized activities maintain the polarized activation of two chemokine receptors: cxcr4b is expressed in the leading zone while cxcr7b is restricted to the trailing zone. The differential expression of cxcr4b and cxcr7b is essential for directed collective migration of the primordium cells [1], [9]. However, the mechanisms downstream of these receptors that convey their actions are unclear.
The ubiquitin ligase ITCH has been shown to influence signaling downstream of several important receptors. In particular, ITCH recognizes and down-regulates several SH3-domain proteins, which have been shown to limit epidermal growth factor receptor internalization and signaling [10]. Although no direct link has been established between ITCH and FGF signaling, ITCH targets proteins involved in receptor tyrosine kinase internalization like CBL and SH3GL2 (endophilin) [10]–[12].
ITCH directly interacts with ligand-activated CXCR4 and promotes its ubiquitylation at the plasma membrane [13], [14], which is important for the regulation of CXCR4 trafficking and signaling [14], [15]. In human cell lines, ITCH depletion significantly attenuates CXCR4-induced ERK-1/2 activation and modestly increases CXCR4 surface levels [16].
ITCH also regulates Wnt signaling through its interaction with Disheveled (Dvl) [17]. Dvl is a central mediator of Wnt signaling where it functions as a scaffold protein bridging the receptors and downstream signaling components [18], [19]. In HEK-293 cells, knockdown of ITCH significantly increased Wnt-induced TOPflash activity and the accumulation of free 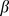 -catenin induced by Wnt3a. Wnt3a-mediated induction of Wnt target genes AXIN2 and NKD1 was also potentiated, suggesting that ITCH negatively regulates the canonical Wnt pathway [17].
-catenin induced by Wnt3a. Wnt3a-mediated induction of Wnt target genes AXIN2 and NKD1 was also potentiated, suggesting that ITCH negatively regulates the canonical Wnt pathway [17].
Given this implication of Itch in the two major signaling pathways, Cxcr4 and Wnt, involved in pLL primordium migration, we investigated the effects of Itch depletion in lateral line formation in zebrafish embryos. Our study presents the first direct demonstration of the implication of the ubiquitin-ligase Itch in the regulation of signal transduction in a living organism.
Results
Itch in Danio rerio
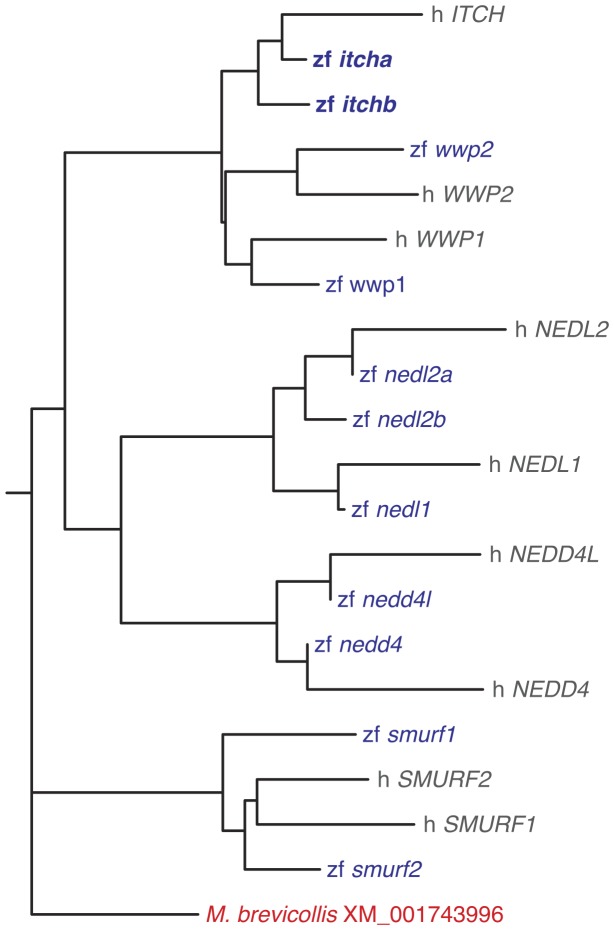
To investigate the impact of itch loss of function in early vertebrate development, we used the well-established model that is the zebrafish [20]. We identified the ITCH orthologues in zebrafish by querying the NCBI database Danio rerio sequences with the amino acid sequence of the human Itch HECT domain (NP_113671.3) using the tBlastn algorithm [21]. This search retrieved 11 complete coding sequences with a maximum nucleotide sequence identity of 43–88%. All the sequences identified encoded predicted proteins with the typical domain architecture of the Nedd4 sub-family of ubiquitin ligases, consisting of a C2 domain, two to four WW domains and the catalytic HECT domain (CWH ligases).
We aligned these cds sequences together with the human sequences for all nine members of the CWH sub-family (Table S1), and constructed a phylogenetic tree using the Phylip package SeqDist algorithm (Fig. 1) [22]. All zebrafish sequences clustered with their human counterparts, giving weight to the hypothesis that they represent orthologues. Two genes grouped with human ITCH. We designate these sequences itcha and itchb, corresponding respectively to NCBI gene IDs 100331274 on chromosome 6 and 100330031 on chromosome 23. Interestingly, the itchb sequence is absent from the ZFIN database but was however confirmed in the latest zebrafish genome sequencing project [23]. Except for itch and nedl2, only one copy of each of the other CWHs was found in the zebrafish genome.
Figure 1. Phylogenetic tree of human and zebrafish members of the CWH family of E3s.
Two zebrafish sequences cluster with the human ITCH gene. The tree was constructed with the neighbor-joining method supported by 1000 bootstraps. Sequences used in the phylogenetic analysis are given in Table S1.
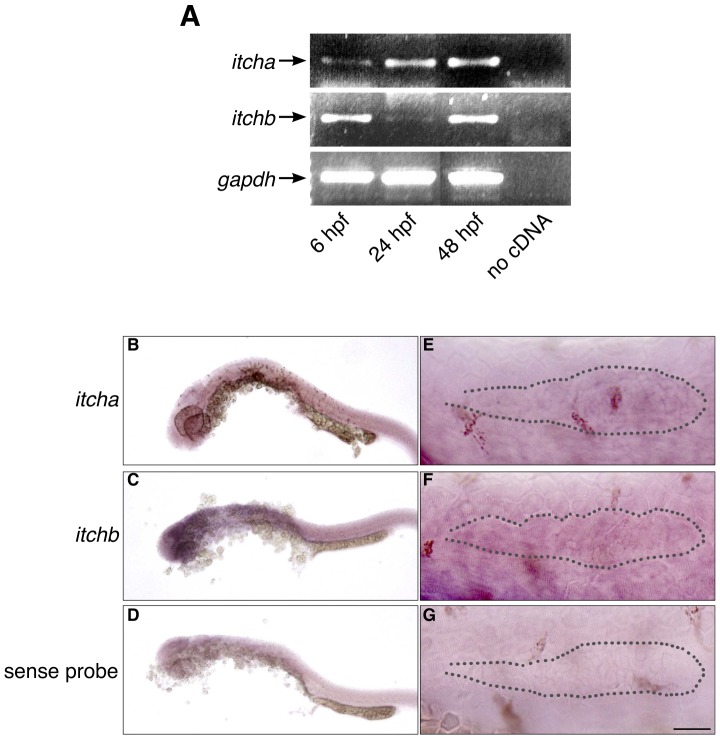
We next examined the expression pattern of itcha and itchb in developing embryos. We preformed reverse-transcription of RNA extracted from embryos at 6, 24, and 48 hours post-fertilization (hpf) followed by PCR amplification with primers designed to specifically amplify itcha or itchb. Both genes were successfully amplified from 6 hpf embryos and their expression was maintained at 48 hpf (Fig. 2 A) and later on (not shown). In situ hybridization experiments were conducted on embryos at 6 and 24 hpf. A generalized pattern of expression was discerned in these experiments, suggesting that both genes are expressed in every tissue (Fig. 2 B–D). This is consistent with reports ITCH gene in mammals where RNA is detected in all analyzed tissues [24]. Faint staining could be observed in the pLL primordium with both itcha and itchb specific probes, distributed throughout the structure, whereas no staining appeared when sense probes were used (Fig. 2 E–G).
Figure 2. Expression of itch genes in zebrafish embryos.
(A) RT-PCR experiment showing early expression of both itcha and itchb genes. RNA was extracted from embryos at approximately 6, 24 and 48 hpf. cDNA was obtained from each stage using 1 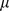 g total RNA. GAPDH was used as an internal control for cDNA amplification. (B–D) Weak, general staining of embryos at 26 hpf revealed with DIG-labelled probes complementary to itcha (B) or itchb (C) or sense sequence (D) as a control. (E–G) The primordium of 26 hpf embryos revealed with DIG-labelled probes complementary to itcha (E) or itchb (F) or sense sequence (G) as a control. Position of the primordium is underlined with a dotted line. Expression of itch genes is general, and itchb is expressed throughout the primordium. Scale bar: (B–D) 250
g total RNA. GAPDH was used as an internal control for cDNA amplification. (B–D) Weak, general staining of embryos at 26 hpf revealed with DIG-labelled probes complementary to itcha (B) or itchb (C) or sense sequence (D) as a control. (E–G) The primordium of 26 hpf embryos revealed with DIG-labelled probes complementary to itcha (E) or itchb (F) or sense sequence (G) as a control. Position of the primordium is underlined with a dotted line. Expression of itch genes is general, and itchb is expressed throughout the primordium. Scale bar: (B–D) 250 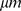 , (E–G) 25
, (E–G) 25 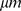 .
.
Knockdown of itcha or itchb affects embryo's growth
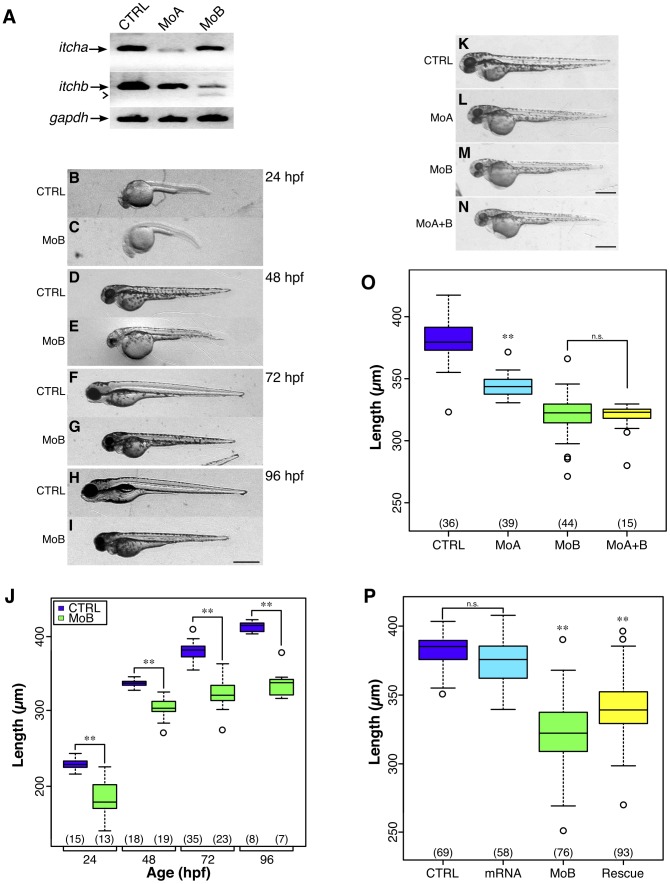
We next examined the impact of loss of expression of itch genes in early development. We designed antisense splice junction morpholino oligonucleotides (MOs) targeting itcha and itchb (Table S2). We first assessed the efficacy of the MOs by performing RT-PCR on RNA extracted from control or injected embryos using oligonucleotide primers flanking the target exons of each MO. There was a marked and specific reduction of itcha mRNA in embryos injected with MOs against itcha but not itchb, and likewise for itchb with the itchb but not the itcha MO, with amplification of the lower, exon-skipped band readily visible in the middle panel (Fig. 3 A). Both MOs induced considerable cell death, visualized by acridine orange staining [25] (Fig. S1). Injection of morpholino has been shown to induce cell death by activating the proapoptotic protein p53, an unspecific side effect efficiently counteracted by downregulation of p53 expression [26]. Therefore, p53 MO [27] was consistently co-injected to reduce the general toxicity effect of MOs.
Figure 3. itch knockdown impairs early zebrafish development.
(A) Splice junction MOs against itcha and itchb efficiently reduced their respective but not opposite mRNAs. RNA was extracted from 48 hpf control embryos or embryos injected with MOs against itcha (MoA) or itchb (MoB). After reverse-transcription, cDNAs from each group were amplified with PCR primers specific for itcha, itchb or GAPDH as a control for 35 cycles. Marked reduction of itcha and itchb was obtained after injection of the appropriate but not the other MO. (B–I) Morphology of embryos injected with MOs targeting itchb (MoB) showed general growth failure, already visible at 24 hpf (B,C) and persisting through to 96 hpf (H,I) as compared to control embryos (CTRL). Scale bar: 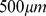 . (J) Total length of control embryos or embryos injected with MOs against itchb (MoB) was measured from the tip of the head to the tip of the tail muscle. Two-factor analysis of variance indicates no significant interaction of injection with age. Pairwise comparison was performed between control and MoB injected embryos at each time point. The number of embryos measured is indicated at the bottom of the graph.
. (J) Total length of control embryos or embryos injected with MOs against itchb (MoB) was measured from the tip of the head to the tip of the tail muscle. Two-factor analysis of variance indicates no significant interaction of injection with age. Pairwise comparison was performed between control and MoB injected embryos at each time point. The number of embryos measured is indicated at the bottom of the graph.  represents statistical significance at
represents statistical significance at 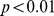 . (K-N) Morphology of control embryos (CTRL), itcha knockdown embryos (MoA), itchb knockdown (MoB) or itcha and itchb knockdown embryos (MoA
. (K-N) Morphology of control embryos (CTRL), itcha knockdown embryos (MoA), itchb knockdown (MoB) or itcha and itchb knockdown embryos (MoA B) at 48 hpf. itcha knockdown embryos were slightly delayed, but were morphologically intact as compared to itchb or itcha plus itchb knockdown embryos. (O) Global growth of injected embryos was assessed by total length measurements at 72 hpf. itcha and itchb knockdown embryos were significantly smaller than control embryos, and itchb knockdown were smaller than itcha as assessed by Kruskal-Wallis one-way ANOVA combined with Dunn's method of group comparison with a significant threshold fixed at
B) at 48 hpf. itcha knockdown embryos were slightly delayed, but were morphologically intact as compared to itchb or itcha plus itchb knockdown embryos. (O) Global growth of injected embryos was assessed by total length measurements at 72 hpf. itcha and itchb knockdown embryos were significantly smaller than control embryos, and itchb knockdown were smaller than itcha as assessed by Kruskal-Wallis one-way ANOVA combined with Dunn's method of group comparison with a significant threshold fixed at 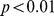 (
( ). itcha plus itchb knockdown embryos were not significantly smaller than itchb knockdown embryos (n.s.). (P) Growth defect of itchb knockdown was partially rescued with injection of in vitro transcribed human ITCH mRNA. Kruskal-Wallis one-way ANOVA combined with Dunn's method of comparison established no-significant differences between control embryos (CTRL) and embryos injected with ITCH mRNA alone (mRNA), whereas the total length was significantly reduced in both itchb MO-injected embryos (MoB) and embryos injected with a combination of itchb MO and ITCH mRNA (Rescue). Total length was significantly larger in the Rescue group than in the MoB group, indicating partial rescue. The number of embryos measured is indicated at the bottom of the graphs. Each graph summarizes at least four different experiments.
). itcha plus itchb knockdown embryos were not significantly smaller than itchb knockdown embryos (n.s.). (P) Growth defect of itchb knockdown was partially rescued with injection of in vitro transcribed human ITCH mRNA. Kruskal-Wallis one-way ANOVA combined with Dunn's method of comparison established no-significant differences between control embryos (CTRL) and embryos injected with ITCH mRNA alone (mRNA), whereas the total length was significantly reduced in both itchb MO-injected embryos (MoB) and embryos injected with a combination of itchb MO and ITCH mRNA (Rescue). Total length was significantly larger in the Rescue group than in the MoB group, indicating partial rescue. The number of embryos measured is indicated at the bottom of the graphs. Each graph summarizes at least four different experiments.  indicates
indicates 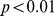 .
.
We then assessed the morphology of injected embryos. Knockdown of either gene caused a general growth delay, the effect of itchb knockdown being generally more severe (Fig. 3 B-I and K-N). itchb knockdown embryos were about two hours late in their development at 24 hpf, judging from the number of somites and pLL primordium migration. They remained smaller throughout the first five days of development, the discrepancy in size tending to increase with time (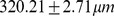 at 72 hpf compared to
at 72 hpf compared to 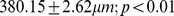 ) (Fig. 3 J,O). Other general features included small head, deformed hindbrain, small eyes, immature fin buds at 72 hpf, pericardium oedema and low survival after 96 hpf. They were able to swim but were generally not responsive to touch and tended to rest on the bottom of the dish when undisturbed (not shown). In comparison, itcha knockdown was less severe, but knockdown embryos were still significantly smaller than control siblings (
) (Fig. 3 J,O). Other general features included small head, deformed hindbrain, small eyes, immature fin buds at 72 hpf, pericardium oedema and low survival after 96 hpf. They were able to swim but were generally not responsive to touch and tended to rest on the bottom of the dish when undisturbed (not shown). In comparison, itcha knockdown was less severe, but knockdown embryos were still significantly smaller than control siblings (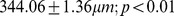 ) (Fig. 3 L–O). The coinjection of both MOs resulted in slightly smaller embryos
) (Fig. 3 L–O). The coinjection of both MOs resulted in slightly smaller embryos 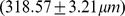 and high mortality (Fig. 3 N,O).
and high mortality (Fig. 3 N,O).
In an attempt to rescue and assess the specificity of the more severe itchb phenotype, we used in vitro-transcribed human ITCH mRNA (that is not targeted by the itchb MOs), injected alone as a control, or in itchb knockdown embryos. Co-injection of ITCH mRNA partially rescued growth defects, with embryos reaching a significantly larger size than upon itchb knockdown 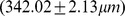 , and was comparable to itcha knockdown (Fig. 3 P).
, and was comparable to itcha knockdown (Fig. 3 P).
itchb is involved in pLL development
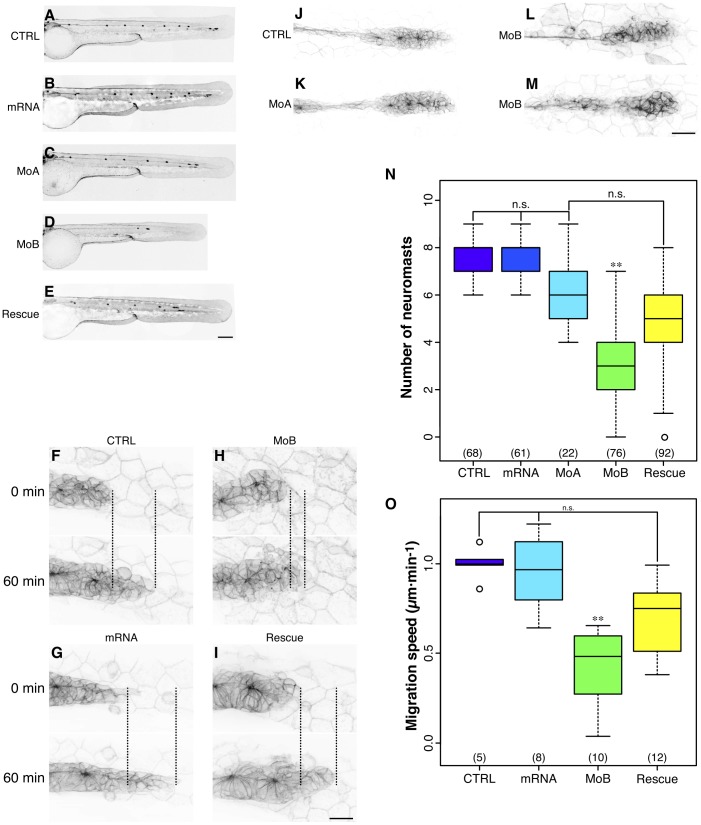
In mammalian cells, Itch has been identified as an important regulator of CXCR4 signaling [14], [16]. This cytokine receptor is well described in Zebrafish for its role in pLL primordium migration [28]. We therefore examined the consequences of itch knockdown on pLL development. We used DiAsp, a vital marker of neuromast hair cells to label the pLL of WT embryos [29]. We also used cldnb:gfp embryos that express GFP in the developing pLL primordium as well as the neuromasts [30]. Both methods yielded identical results and showed that the pLL primordium did not reach the tail tip upon itchb knockdown, usually stalling around the end of the digestive track at 48–52 hpf (Fig. 4 D). In itcha knockdown, the primordium migration was slightly delayed at 48 hpf(Fig. 4 C), but had reached the tail by 52 hpf (not shown). The number of neuromasts formed at 72 hpf was drastically reduced in itchb knockdown, but not in itcha knockdown (Fig. 4 C, E and N).
Figure 4. itchb is involved in pLL primordium migration and lateral line development.
(A–E) Posterior lateral line at 48 hpf cldnb:gfp in embryos injected with vehicle (CTRL), in vitro-transcribed human ITCH mRNA (mRNA), MOs against itcha (MoA), MOs against itchb (MoB) or MOs against itchb and in vitro-transcribed human ITCH mRNA (Rescue). There was a marked reduction in the number of neuromasts in the pLL after itchb knockdown (D), but not in itcha knockdown, although the primordium had not yet reached the end of the tail at this time point (C). The itchb knockdown effect was partially rescued by injection of human ITCH mRNA (E). (F–I) Time-lapse confocal microscopy on 26–30 hpf cldnb:gfp embryos showed that the pLL primordium migration was slowed in itchb knockdown embryos (MoB) compared to vehicle (CTRL), human ITCH mRNA-injected (mRNA) or rescued embryos (Movies S1–S4). (N) The number of neuromasts in the posterior lateral-line was counted after staining with DiAsp in 72 hpf WT embryos injected with vehicle (CTRL), in vitro-transcribed human ITCH mRNA (mRNA), MOs against itcha (MoA), MOs against itchb (MoB) or MOs against itchb and in vitro-transcribed human ITCH mRNA (Rescue). Whereas the number of neuromasts in the pLL was markedly reduced after injection of MOs against itchb, injection of in vitro transcribed ITCH mRNA alone or of MOs against itcha had no effect. The MOs phenotype was rescued by co-injection of ITCH mRNA in itchb knockdown. The number of embryos for each group is indicated at the bottom of the graph.  represents statistical significance at
represents statistical significance at 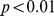 . n.s. = non-significant. (J–M) Morphology of the migrating primordium in 26 hpf embryos (CTRL), itcha knockdown embryos (MoA) and itchb knockdown embryos (MoB). Cells at the tip of the primordium of MoB embryos were slightly disorganized, but the typical rosette formation occurred normally. (O) Migration of the pLL primordium was quantified by measuring the distance between the primordium leading edge in the first frame (
. n.s. = non-significant. (J–M) Morphology of the migrating primordium in 26 hpf embryos (CTRL), itcha knockdown embryos (MoA) and itchb knockdown embryos (MoB). Cells at the tip of the primordium of MoB embryos were slightly disorganized, but the typical rosette formation occurred normally. (O) Migration of the pLL primordium was quantified by measuring the distance between the primordium leading edge in the first frame (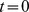 ) compared to the last frame (
) compared to the last frame (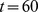 ) as shown in F–I (doted line). The number of embryos counted or imaged is indicated at the bottom of the graph. Each graph summarizes four different experiments.
) as shown in F–I (doted line). The number of embryos counted or imaged is indicated at the bottom of the graph. Each graph summarizes four different experiments.  represents statistical significance at
represents statistical significance at 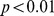 . n.s. = non-significant. EGFP fluorescence is presented as reversed black and white to facilitate visualization in all micrographs. Scale bars: (A–E)
. n.s. = non-significant. EGFP fluorescence is presented as reversed black and white to facilitate visualization in all micrographs. Scale bars: (A–E) 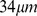 , (F–I)
, (F–I) 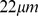 , (L–M)
, (L–M) 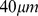 .
.
The primordium maintained its overall morphology, and the characteristic rosette-like organization of the neuromast progenitors was readily identifiable under all conditions, but itchb morphants were often more disorganized and seemed to collapse in the trailing end (Fig. 4 J–M). They also often exhibited less cohesion in the border cells, as can be seen in Movie S3.
To determine if primordium migration was affected by ablation of itchb, we performed time-lapse microscopy experiments in cldnb:gfp embryos. We recorded primordium migration starting just before deposition of the first trunk neuromast in control, morphant or rescued embryos (Fig. 4 F–I and Movies S1–S4). Primordium migration was significantly slower in itchb morphants (Fig. 4 O and Movie S3), as compared to control (Fig. 4 O and Movies S1–S2). Moreover, cells from the primordium leading edge in morphant embryos were disorganized and moved around and back instead of straight toward the tail as in control primordia. This cell behavior suggests that the slowing of pLL primordium migration is not merely the consequence of general growth failure, but due to genuine interference with directional cell migration. The primordium migration defect was rescued by human ITCH mRNA injection (Fig. 4 I and Movie S4). Overall, the migration speed of itchb morphant primordia was reduced by approximately 50% (median speed 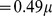 m
m min–1
vs
min–1
vs
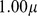 m
m min–1 in controls) and 24% in rescue (median speed
min–1 in controls) and 24% in rescue (median speed 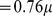 m
m min–1) (Fig. 4 O).
min–1) (Fig. 4 O).
itchb knockdown perturbs signaling events required for pLL primordium migration
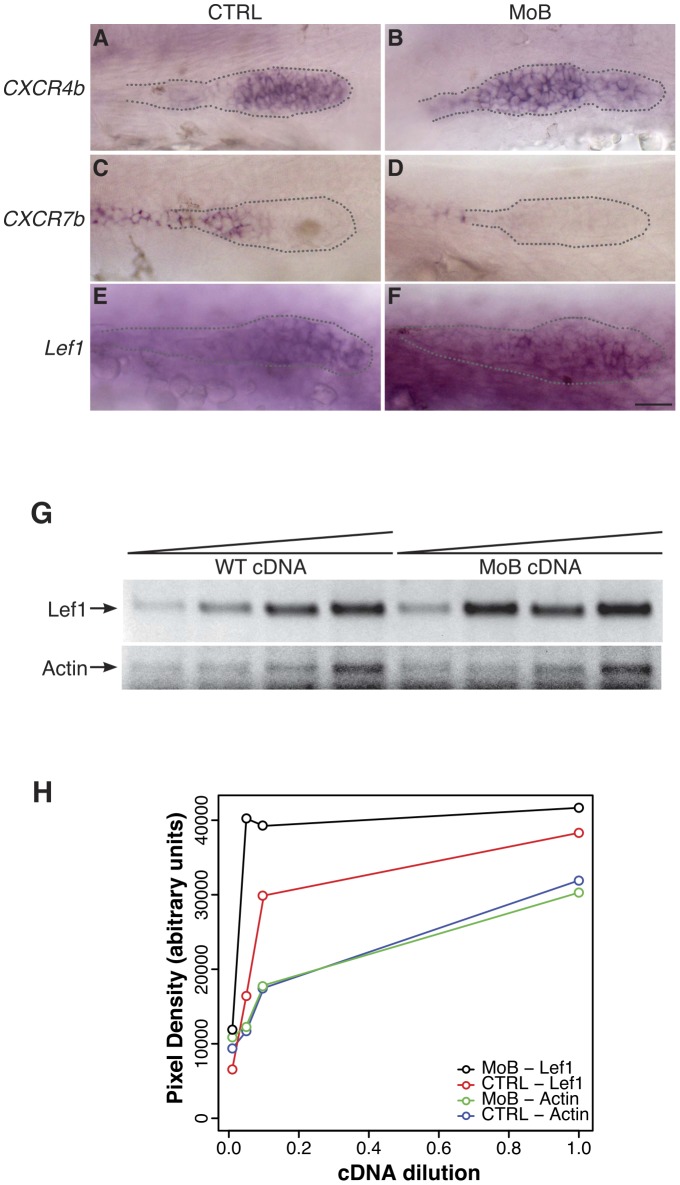
Migration of the primordium along the myoseptum is directed by the cytokine receptor Cxcr4b, expressed in the leading edge of the primordium. Directionality is ensured by expression of another cytokine receptor, Cxcr7b, in the trailing end of the migrating primordium, whose expression is restricted by Cxcr4b and acts as a sink for the ligand Sdf-1, thereby preventing Cxcr4b signaling in the trailing end and allowing directionality of migration [31]. Given the slow migration of the pLL primordium in itchb morphants, we examined cxcr4b and cxcr7b expression in the primordium. We visually assessed in cldnb:gfp embryos that the primordium had reached somite 10 before fixation and in situ hybridization. This occurred at about 28 hpf for control embryos and 30 hpf for itchb morphants. Comparing cxcr4b expression in these stage-matched embryos, while cxcr4b expression was restricted to the leading two-third of the primordium in control embryos (Fig. 5 A), it was clear that cxcr4b was overexpressed in itchb morphants and that its distribution encompassed the entire primordium, extending to the deposited cells behind the migrating primordium (Fig. 5 A and B; representative of 11 embryos in 4 different experiments).
Figure 5. Primordium patterning is altered in itchb knocked-down embryos.
(A–F) RNA in situ hybridization of factors required for primordium patterning in control embryos (vehicle-injected siblings) (CTRL, left panels) and MOs-injected embryos (MoB, right panels) at 30 hpf. cldnb:gfp embryos were used to ensure that the primordium had migrated pass the 10th somite before fixation. (A,B) cxcr4b expression was limited to the leading half of the primordium in control embryos, but extended throughout the primordium after itchb knockdown. (C,D) cxcr7b was limited to the trailing end of control embryos, and almost completely excluded from the primordium in itchb knockdown. (E,F) lef1 was expressed in the leading edge of control embryos. Darker staining indicated higher expression in embryos injected with MOs against itchb (MoB), but leading edge expression was maintained. Scale bar: 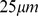 . (G) Semi-quantitative RT-PCR showing enhanced expression of lef1 in embryos injected with MOs against itchb (MoB) as compared to control embryos (WT). Amplification of the actin gene was used as an internal control. The image was inverted to facilitate quantification. (H) Densitometry measurements from the gel presented in G. This is representative of three different experiments.
. (G) Semi-quantitative RT-PCR showing enhanced expression of lef1 in embryos injected with MOs against itchb (MoB) as compared to control embryos (WT). Amplification of the actin gene was used as an internal control. The image was inverted to facilitate quantification. (H) Densitometry measurements from the gel presented in G. This is representative of three different experiments.
Reciprocally, cxcr7b expression, normally present in the trailing end and in the deposited cells in control embryos (Fig. 5 C) was almost completely excluded from the primordium of itchb morphants, and only visible in deposited cells further behind the primordium (Fig. 5 D; representative of 11 embryos from 3 different experiments). This pattern was consistent with the slow primordium migration measured in Figure 4 H and O.
Wnt signaling is increased in itchb morphants
Wnt signaling also plays an important role in pLL primordium migration and is thought to act upstream of cxcr4 [7]. Wei et al. [17] identified Disheveled, an important intermediate in the Wnt signaling pathway as a target of Itch in mammalian cells. To determine if Wnt signaling was modified by itchb knockdown, we examined the expression of the Wnt target gene lef1, whose expression is normally limited to the leading cells of the migrating primordium [7], as seen in control embryos (Fig. 5 E). In itchb morphants lef1 expression appeared to be increased, though it was still restricted to the leading two thirds of the structure (Fig. 5 F).
To get a sense of the general level of lef1 expression in itchb morphants, we performed a semi-quantitative RT-PCR experiment with RNA extracted from stage-matched control (WT) or itchb morphant (MoB) embryos. After reverse transcription, we performed serial dilutions of the cDNA reactions and proceeded to PCR amplification with specific primers for lef1 and actin (Table S3). Amplification of actin was comparable between the two sets of cDNAs (Fig. 5 G), showing comparable extraction and reverse-transcription efficacy. In contrast, lef1 was amplified from much lower amounts of cDNAs from itchb morphants than from control embryos, indicating higher expression of lef1 in this group (Fig. 5 G, H). These data are consistent with general Wnt signaling activation in itchb morphants.
Discussion
itchb is required to maintain primordium migration
This study aimed at exploring how early vertebrate development was affected by knockdown of the ubiquitin ligase itch gene using the vertebrate model organism Danio rerio. We found two genes coding for proteins with similar sequence identity with human ITCH, itcha and itchb. RT-PCR experiments demonstrate that both genes were expressed in zebrafish larvae, and a generalized pattern of expression was observed using in situ hybridization. Duplicated genes are common in zebrafish and in these cases paralogues have been found to present partial genetic redundancy, although they also often diverge in either their function or their expression pattern [32]. Though knockdown of either or both genes affected overall growth, itcha and itchb indeed appeared to differ in their function, the most noticeable distinction being in the effect of the knockdown of itchb but not itcha on perturbation of pLL development.
The process governing pLL primordium migration is well described and involves Wnt, Fgf and cytokine signaling. The principal determinant of pLL primordium migration is the establishment of distinct expression domains of the cytokine receptors cxcr4b and cxcr7b. As the primordium migrates along the myoseptum, cxcr4b is restricted to the leading half of the primordium and inhibits cxcr7b, which consequently is present only in the trailing end [1], [7], [33]. This asymmetric distribution directs primordium migration along the myoseptum in response to Sdf1 secretion and disturbing this equilibrium results in slowed primordium migration speed or stalling of the primordium [1], [7], [33]–[35]. Wnt signaling occurs mainly in the leading region of the pLL primordium and activates Fgf signaling in the medial and trailing region [7]. Fgf signaling organizes the primordium precursor cells in rosettes that will become the neuromasts and restricts Wnt signaling to the leading cells [36], [37].
The expression of cxcr4b is regulated at the transcriptional level by Wnt and oestrogen signaling, as response elements for Lef1 and Esr1 are present in the upstream control region of the cxcr4b gene [34], [35]. lef1 morphants and mutants demonstrate truncated pLL, as the migrating primordium collapses before it reaches the end of the tail caused by decreased cell proliferation and lack of progenitors [4], [35], [38]. Lef1 depletion alone has no effect on cxcr4b or cxcr7b expression, and pLL primordium migration and differentiation appears to be normal. However, increasing Wnt signaling, as occurs in apc mutants, strongly inhibits primordium migration while increasing cxcr4b expression domain and excluding cxcr7b from the primordium [7].
itchb morphants similarly exhibited displacement of the cxcr4b and cxcr7b expression domains, consistent with increased cxcr4b and Wnt signaling. In cultured mammalian cell lines, Itch has been shown to increase both Wnt and Cxcr4 signaling. Itch can regulate the Wnt signaling pathway by recognizing and ubiquitylating phosphorylated Dvl [17]. Dvl is recruited to the activated Wnt receptor complex and activates both the canonical and non-canonical signaling pathways [19]. Upon activation by Wnt, Dvl become hyperphosphorylated, and this phosphorylation is essential to fully activate 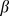 -catenin stabilization [39], [40]. Inactivation of Itch stabilizes phosphorylated Dvl, increasing Wnt signaling [17]. In zebrafish, Dvl degradation has been shown to be implicated in Wnt signaling regulation [41]. On the other hand, Dvl expression has also been shown to be stable in zebrafish embryos during primordium migration [42]. It must be stressed that Itch specifically targets phosphorylated Dvl and promotes its proteasomal degradation [17]. Consequently, Itch depletion could increase Wnt signaling and expression of Wnt signaling pathway target genes, without affecting the overall level of Dvl protein [17].
-catenin stabilization [39], [40]. Inactivation of Itch stabilizes phosphorylated Dvl, increasing Wnt signaling [17]. In zebrafish, Dvl degradation has been shown to be implicated in Wnt signaling regulation [41]. On the other hand, Dvl expression has also been shown to be stable in zebrafish embryos during primordium migration [42]. It must be stressed that Itch specifically targets phosphorylated Dvl and promotes its proteasomal degradation [17]. Consequently, Itch depletion could increase Wnt signaling and expression of Wnt signaling pathway target genes, without affecting the overall level of Dvl protein [17].
CXCR4 is a direct target of ITCH ubiquitin ligase activity in mammalian cells [14], [15]. ITCH is known to regulate CXCR4 internalization and signaling in conjunction with 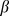 -arestin and STAM [16], [43]. CXCR4 ubiquitylation is required for its rapid ligand-induced lysosomal degradation [15]. ITCH interacts directly with CXCR4 and HRS to direct CXCR4 degradation [14], [44]. Moreover, ITCH is necessary for CXCR4-mediated activation of the ERK/MAPK pathway [16]. It has not been possible to directly measure the effect of Itch depletion on Cxcr4b protein, as antibodies against CXCR4 do not discriminate Cxcr4a and Cxcr4b in Western blot analysis and did not yield a reliable signal in immunofluorescence. Nevertheless, cxcr4b is clearly overexpressed in the primordium at the mRNA level, which could be a direct effect of its increased signaling. It is known that Sdf1/Cxcr4 signaling exerts a positive feedback on cxcr4b expression in the primordium [1], [34]. Therefore, increased cxcr4b expression is consistent with Cxcr4b protein stabilization. Cxcr4b also exerts transcriptional control over cxcr7b expression, effectively excluding Cxcr7b form the Cxcr4b expression domain [1]. This is consistent with the reduced cxcr7b signal in itchb morphants.
-arestin and STAM [16], [43]. CXCR4 ubiquitylation is required for its rapid ligand-induced lysosomal degradation [15]. ITCH interacts directly with CXCR4 and HRS to direct CXCR4 degradation [14], [44]. Moreover, ITCH is necessary for CXCR4-mediated activation of the ERK/MAPK pathway [16]. It has not been possible to directly measure the effect of Itch depletion on Cxcr4b protein, as antibodies against CXCR4 do not discriminate Cxcr4a and Cxcr4b in Western blot analysis and did not yield a reliable signal in immunofluorescence. Nevertheless, cxcr4b is clearly overexpressed in the primordium at the mRNA level, which could be a direct effect of its increased signaling. It is known that Sdf1/Cxcr4 signaling exerts a positive feedback on cxcr4b expression in the primordium [1], [34]. Therefore, increased cxcr4b expression is consistent with Cxcr4b protein stabilization. Cxcr4b also exerts transcriptional control over cxcr7b expression, effectively excluding Cxcr7b form the Cxcr4b expression domain [1]. This is consistent with the reduced cxcr7b signal in itchb morphants.
Defects in pLL primordium migration in itchb morphants can thus be attributed to increased Wnt and Cxcr4b signaling in vivo. Since the phenotypic manifestation of perturbed Cxcr4b signaling and Wnt overactivation are confounded, it is not possible to discriminate which pathway is most affected by itchb depletion, though most likely itchb affects both.
Neuromast deposition is altered in zebrafish depleted of itchb
Although neuromast deposition is clearly a consequence of and influenced by pLL primordium migration, manipulations specifically altering neuromast deposition have no effect on pLL primordium migration [45]. The processes are thus independently regulated by overlapping signals. Examining the pLL of itchb deficient embryos, it was clear that both the number of neuromasts and the rate of primordium migration were reduced by half. Reduced neuromast deposition is observed when the proliferation rate is decreased, for example by treating the embryos with DNA replication inhibitors such as hydroxyurea [45]. Increased cell death also leads to fewer neuromast deposition, as seen in bap28 homozygous mutants and tcf7 ATG morphants. Importantly, the reduction in proneuromast number in tcf7 MO injected embryos is non-specific and not due to loss of tcf7 function, and was completely rescued by the coinjection of p53 MOs [45]. Both itcha and itchb MOs indeed induced significant cell death when injected alone, but this effect was reversed by addition of p53 MO. Moreover, only itchb morphants exhibited defects in neuromast deposition, and this effect was rescued by injection of human Itch mRNA. p53-mediated cell-death therefore is unlikely to be responsible for the neuromast deposition defects. Nevertheless, reduction of Itch has been related to increased cell death or reduced proliferation in a number of cases. First, p63 and p73, two isoforms of p53, are direct targets of Itch, and their stabilization through Itch depletion is known to activate apoptotic pathways [46]–[48]. Although it has not been reported specifically in the primordium, p63-mediated cell death occurs in zebrafish embryos and larvae [49]. In mammalian cells, ITCH depletion increased LATS1, a serine/threonine kinase in the Hippo pathway, enhancing FAS-induced apoptosis and reducing proliferation, survival, and migration [50]. This is consistent with results showing that in HEK-293T cells, ITCH depletion decreased cell survival and enhanced TRAIL-induced cell-death [51]. Increased cell death is thus a plausible explanation for loss of neuromasts in itchb morphants, but its assessment is difficult due to the recognized caveat in the use of MOs to examine apoptotic pathways [26].
It must be noted that Itch depletion has also been shown to induce cell proliferation through a number of signaling pathways. In hematopoietic stem cells, Itch deficiency increases proliferation by stabilizing Notch1 signaling [52]. In the zebrafish pLL, Notch signaling determines differentiation of pLL ganglion neurons and, later on, of hair cells [53], [54]. Although it was shown that preventing hair cell differentiation leads to fewer neuromasts in the pLL [55], the effect of promoting Notch signaling on this system is unknown. Moreover, since DiAsp was successfully accumulated by those neuromasts that were formed, we conclude that itchb morphant hair cells were functional. In the context of Hedgehog signaling, Itch depletion promoted tumorigenicity, preventing the formation of a degradation complex between Itch, Numb and Gli1. This had the effect of stabilizing Gli1 and promoting medulloblastoma growth [56]. There is thus a multitude of pathways through which Itch could influence both cell-death and cell proliferation in the pLL primordium that could result in the formation of fewer neuromasts. A transcriptomic or proteomic approach could yield knowledge about how Itchb affects neuromast formation.
In the zebrafish pLL primordium, proliferation is regulated mainly by Wnt and Fgf signaling, and seems to be largely independent of cell differentiation [45]. Increasing Wnt signaling alone leads to increased proliferation, but the process is dependent on Fgf signaling [45], [57]. In short, Fgf signaling promotes proliferation whereas Wnt signaling limits the proliferation zone to the trailing end of the primordium, where rosette formation occurs, while there is little proliferation in the leading zone that instead directs migration [45]. The domain of cxcr7b expression seems to delimit the zone where the depositing cells reside, although it is not clear how Cxcr7b activity mediates this process [45]. cxcr7b expression is limited by Wnt signaling and lef1 expression that occurs in the leading zone of the primordium [7]. lef1 is a target gene of Wnt signaling and its expression increases in apc mutants [7]. We show here that Itch depletion in zebrafish resulted in increased Wnt signaling, as was shown by higher lef1 expression, consistent with the identification of Dvl as a target of Itch ubiquitylation in mammalian cells [17].
Increased Wnt signaling in zebrafish is associated with slower migration of the primordium, but is not sufficient to induce deposition of fewer neuromasts. Apart from increased apoptosis, the reduction in neuromast number in itchb morphants could occur through altered Fgf signaling [45], [58]. No direct impact of Itch on Fgf signaling has been reported so far in the literature. Fgfr1 is a direct target of the closely related Nedd4-1 ubiquitin ligase and the inhibition of this interaction leads to an important increase in Fgf signaling that alters anterior development in zebrafish [59]. Ubiquitylation is important to regulate Fgfr internalization and signaling, both directly and indirectly through the regulation of Sprouty 2 [59], [60]. Tyrosine kinase receptor internalization is influenced by ubiquitylation of the endocytic machinery, and targets of Itch have been implicated in this process [10]–[12], [61]–[65]. However, should Itch be directly involved in Fgf regulation, one would expect that itchb depletion would lead to increased Fgf signaling. Increasing Fgf signaling does not lead to reduced proliferation, but instead leads to the formation of supplementary rosettes in the migrating primordium [37]. It does not seem likely then that the Fgf pathway is perturbed independently of Wnt and Cxcr4b in itchb morphants.
The HECT-domain ubiquitin ligase Itch is an important negative regulator of signaling. It has been mainly linked to immunological responses, but is widely expressed and likely involved in many developmental and regulatory signaling events.
In mice, Itch deficiency results in spontaneous development of late onset and progressively lethal systemic autoimmune-like disease, attributable to biased differentiation of CD4 cells into TH2 cells and chronic activation [66]. The inflammatory response is also attributable to expansion of the B1b lymphocytes leading to IgM elevation and IgE production [67]. These immunological defects are mainly attributable to accumulation of the transcription factor JunB, in the absence of Itch [66], [67].
cells into TH2 cells and chronic activation [66]. The inflammatory response is also attributable to expansion of the B1b lymphocytes leading to IgM elevation and IgE production [67]. These immunological defects are mainly attributable to accumulation of the transcription factor JunB, in the absence of Itch [66], [67].
In human, in addition to multisystem autoimmune diseases akin to the Itchy mice phenotype, patients with ITCH mutations displayed morphologic and developmental abnormalities [68]. Together, the results described above confirm that Itch constitutes an important signaling hub in the cell, maintaining the balance in several important signaling pathways. Different vertebrate models, including the zebrafish introduced here, are likely to unveil the molecular defects underlying these Itch-related pathologies.
Materials and Methods
Ethics Statement and Transgenic Animals
A colony of wild-type Longfin zebrafish (Danio rerio) was bred and maintained according to standard procedures in our animal facility [69]. The transgenic line Tg(cldnb:lynEGFP) expressing membrane-tethered EGFP (enhanced GFP) under the claudinb promoter was used as it labels the migrating lateral line primordial, the neuromast organs as well as the chain of interneuromast cells deposited during migration [30]. All experiments were performed in compliance with the guidelines of the Canadian Council for Animal Care and approved by the Comité de déontologie de l'expérimentation sur les animaux (CDEA) of the University of Montreal. Embryos were anesthetized in 0.02% tricain (MS-222, Sigma) in Embryo medium prior to all experiments.
Antisense Morpholino Oligonucleotides and RNA Injections
To knockdown the zebrafish itcha and itchb genes, we designed splice-junction blocking MOs specific to the donor and acceptor splice-sites of itcha and itchb exons 12 and 13 (Gene Tools, Philomath, OR). MOs and mRNAs were diluted in nuclease-free water with 0.2% FastGreen vital dye to judge of injection volume. To avoid toxicity effects, p53 MO was coinjected [27]. All MOs are listed in the file (Table S2).
Human ITCH mRNA was transcribed from the I.M.A.G.E. Consortium (LLNL) cDNA Clone 4838366 [70] encoding human ITCH linearized with BamHI using the mMESSAGE Machine T7 kit (Ambion, Austin, TX).
Reverse Transcription-PCR
Total RNA was extracted from pools of approximately 50 embryos, treated as stated, using TRIzol Reagent (Invitrogen, Carlsbad, CA). cDNA was synthesized from 1 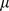 g total RNA using the SuperScript VILO cDNA Synthesis Kit (Invitrogen, Carlsbad, CA). All amplifications were carried on using Phusion High Fidelity DNA polymerase (New England Biolab, Ipswich, MA).
g total RNA using the SuperScript VILO cDNA Synthesis Kit (Invitrogen, Carlsbad, CA). All amplifications were carried on using Phusion High Fidelity DNA polymerase (New England Biolab, Ipswich, MA).
Semi-quantitative PCR reactions were setup using serial dilutions of cDNAs followed by 30 cycles of amplification. All primers are listed in the file (Table S3).
Lateral Line Staining
The lateral line of control or injected embryos was labeled using the vital dye 4-(4-diethylaminostyryl)-N-methylpyridinium iodine (4-di-2-ASP, Invitrogen) diluted to 0.5 mM in embryo medium according to an established procedure [71]. Embryos were dechorionated manually and staged at 72 hpf then incubated in the solution for 30 minutes at 28.5°C. They were then washed three times for 10 minutes in fresh embryo medium and anesthetized in tricain before imaging on an epifluorescence dissection microscope (Olympus) equipped with a Flea2 CCD Camera (IEEE 1394, Point Grey Research Inc. Richmond, BC, Canada). This protocol allows for visualization of full neuromasts that were counted for each fish.
Acridine Orange Staining
Zebrafish were incubated in 1 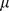 g/ml acridine orange for 30 min then repeatedly washed in embryo medium. Larvae were anesthetized in tricain and mounted in low melting point agarose before being visualized under a 10X water immersion lens mounted on a Quorum Technologies spinning disk confocal microscope as bellow.
g/ml acridine orange for 30 min then repeatedly washed in embryo medium. Larvae were anesthetized in tricain and mounted in low melting point agarose before being visualized under a 10X water immersion lens mounted on a Quorum Technologies spinning disk confocal microscope as bellow.
Confocal Microscopy and Time-Lapse Recordings
Embryos were anesthetized in 0.02% tricain (MS-222) in embryo medium and embedded in 1% low melting point agarose. Imaging was performed on a Quorum Technologies spinning-disk confocal microscope (Quorum WaveX Technology Inc Guelph, On, Canada) mounted on an upright Olympus BX61W1 fluorescence microscope with water-immersion lenses. The setup was fitted with a Hamamatsu ORCA-ER camera and image acquisition was done with the Volocity software (Perkin-Elmer) and analyzed with the ImageJ software (NIH). Stacks were acquired at 1 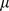 m thickness and assembled in ImageJ. When necessary, adjacent frames were aligned and stitched together using Photoshop CS 5.1 (Adobe Systems Incorporated) Auto-Blend Layers function. For time-lapse microscopy, images were captured every three minutes for 60 minutes. To quantify the primordium migration, the distance between the tip of the primordium at
m thickness and assembled in ImageJ. When necessary, adjacent frames were aligned and stitched together using Photoshop CS 5.1 (Adobe Systems Incorporated) Auto-Blend Layers function. For time-lapse microscopy, images were captured every three minutes for 60 minutes. To quantify the primordium migration, the distance between the tip of the primordium at 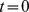 and the tip of the primordium at
and the tip of the primordium at 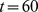 minutes was measured in ImageJ after superposition of the images.
minutes was measured in ImageJ after superposition of the images.
Whole-Mount In Situ Hybridization
In situ hybridization was performed using sense and antisense probes designed against the zebrafish orthologs of itch (Table S4) to view endogenous localization of itch mRNA. Embryos of 6 hpf and 24 hpf were processed for in situ hybridization as previously described [72]. To measure the impact of itch down-regulation on signaling in the migrating primordium, probes against cxcr4b, cxcr7b and lef1 (Table S4), were synthesized and used in an identical procedure on MO and control injected cldnb:gfp embryos age-staged at 26 hpf according to primordium migration.
Supporting Information
Cell death in the pLL primordium of
itcha
and
itchb
morphants. (A–C), acridine orange staining in the primordium region of control cldnb:gfp embryos (CTRL), itcha knockdown (MoA), and itchb knockdown (MoB). p53 MO was omitted in this experiment. In these conditions, cell death occurred in the primordium cells, predominantly in the trailing end of the migrating primordium in both itcha (B) and itchb (C) morphants. Acridine orange staining is visible as brighter dots representing the nucleus of apoptotic cells over the dimmer EGFP signal in the cell membrane of the transgenic primordium. Scale bar: 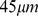 .
.
(TIF)
Accession number of sequences used for alignment.
(PDF)
Sequence of morpholino oligonucleotides.
(PDF)
Sequence of the PCR primers used in this study.
(PDF)
cDNA regions used as probes in in situ hybridization experiments.
(PDF)
CTRL. Example of a pLL primordium migration at 28 hpf in a control embryo. Stacks were acquired at 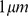 thickness and assembled in ImageJ. Movie length: 60 min.
thickness and assembled in ImageJ. Movie length: 60 min.
(AVI)
mRNA. Example of a pLL primordium migration at 28 hpf in a human ITCH-injected embryo (a second control). Stacks were acquired at 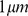 thickness and assembled in ImageJ. Movie length: 60 min.
thickness and assembled in ImageJ. Movie length: 60 min.
(AVI)
MoB. Example of a pLL primordium failing to migrate at 30 hpf in a itchb-morpholino-injected embryo. Stacks were acquired at 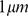 thickness and assembled in ImageJ. Movie length: 60 min.
thickness and assembled in ImageJ. Movie length: 60 min.
(AVI)
Rescue. Example of a pLL primordium migration at 28 hpf in a itchb-morpholino-injected embryo after rescue with human ITCH mRNA. Stacks were acquired at 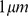 thickness and assembled in ImageJ. Movie length: 60 min.
thickness and assembled in ImageJ. Movie length: 60 min.
(AVI)
Acknowledgments
We are grateful to G. Laliberté and M. Drits for help with animal care.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
Funding provided by AA Natural Sciences and Engineering Research Council of Canada (www.nserc-crsng.ca) grant No. 288238, PD Natural Sciences and Engineering Research Council of Canada (www.nserc-crsng.ca) grant, PD Canada Research Chair in Neuroscience (http://www.chairs-chaires.gc.ca) and PD Fonds de recherche en santé du Québec (http://www.frsq.gouv.qc.ca/fr/index.shtml). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Dambly-Chaudière C, Cubedo N, Ghysen A (2007) Control of cell migration in the development of the posterior lateral line: antagonistic interactions between the chemokine receptors cxcr4 and cxcr7/rdc1. BMC Dev Biol 7: 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Dambly-Chaudière C, Sapède D, Soubiran F, Decorde K, Gompel N, et al. (2003) The lateral line of zebrafish: a model system for the analysis of morphogenesis and neural development in vertebrates. Biol Cell 95: 579–87. [DOI] [PubMed] [Google Scholar]
- 3. Chitnis AB, Nogare DD, Matsuda M (2012) Building the posterior lateral line system in zebrafish. Dev Neurobiol 72: 234–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Valdivia LE, Young RM, Hawkins TA, Stickney HL, Cavodeassi F, et al. (2011) Lef1-dependent wnt/beta-catenin signalling drives the proliferative engine that maintains tissue homeostasis during lateral line development. Development 138: 3931–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ghysen A, Dambly-Chaudière C (2004) Development of the zebrafish lateral line. Curr Opin Neurobiol 14: 67–73. [DOI] [PubMed] [Google Scholar]
- 6. Metcalfe WK, Kimmel CB, Schabtach E (1985) Anatomy of the posterior lateral line system in young larvae of the zebrafish. J Comp Neurol 233: 377–89. [DOI] [PubMed] [Google Scholar]
- 7. Aman A, Piotrowski T (2008) Wnt/beta-catenin and fgf signaling control collective cell migration by restricting chemokine receptor expression. Dev Cell 15: 749–61. [DOI] [PubMed] [Google Scholar]
- 8. Aman A, Piotrowski T (2011) Cell-cell signaling interactions coordinate multiple cell behaviors that drive morphogenesis of the lateral line. Cell Adh Migr 5: 499–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Valentin G, Haas P, Gilmour D (2007) The chemokine sdf1a coordinates tissue migration through the spatially restricted activation of cxcr7 and cxcr4b. Curr Biol 17: 1026–31. [DOI] [PubMed] [Google Scholar]
- 10. Azakir BA, Angers A (2009) Reciprocal regulation of the ubiquitin ligase itch and the epidermal growth factor receptor signaling. Cell Signal 21: 1326–36. [DOI] [PubMed] [Google Scholar]
- 11. Soubeyran P, Kowanetz K, Szymkiewicz I, Langdon WY, Dikic I (2002) Cbl-cin85-endophilin complex mediates ligand-induced downregulation of egf receptors. Nature 416: 183–7. [DOI] [PubMed] [Google Scholar]
- 12. Petrelli A, Gilestro GF, Lanzardo S, Comoglio PM, Migone N, et al. (2002) The endophilin-cin85-cbl complex mediates ligand-dependent downregulation of c-met. Nature 416: 187–90. [DOI] [PubMed] [Google Scholar]
- 13. Bhandari D, Robia SL, Marchese A (2009) The e3 ubiquitin ligase atrophin interacting protein 4 binds directly to the chemokine receptor cxcr4 via a novel ww domain-mediated interaction. Mol Biol Cell 20: 1324–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Marchese A, Raiborg C, Santini F, Keen JH, Stenmark H, et al. (2003) The e3 ubiquitin ligase aip4 mediates ubiquitination and sorting of the g protein-coupled receptor cxcr4. Dev Cell 5: 709–22. [DOI] [PubMed] [Google Scholar]
- 15. Marchese A, Benovic JL (2001) Agonist-promoted ubiquitination of the g protein-coupled receptor cxcr4 mediates lysosomal sorting. J Biol Chem 276: 45509–12. [DOI] [PubMed] [Google Scholar]
- 16. Malik R, Soh UJK, Trejo J, Marchese A (2012) Novel roles for the e3 ubiquitin ligase atrophin-interacting protein 4 and signal transduction adaptor molecule 1 in g protein-coupled receptor signaling. J Biol Chem 287: 9013–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wei W, Li M, Wang J, Nie F, Li L (2012) The e3 ubiquitin ligase itch negatively regulates canonical wnt signaling by targeting dishevelled protein. Mol Cell Biol 32: 3903–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Clevers H (2006) Wnt/beta-catenin signaling in development and disease. Cell 127: 469–80. [DOI] [PubMed] [Google Scholar]
- 19. Gao C, Chen YG (2010) Dishevelled: The hub of wnt signaling. Cell Signal 22: 717–27. [DOI] [PubMed] [Google Scholar]
- 20. Kabashi E, Champagne N, Brustein E, Drapeau P (2010) In the swim of things: recent insights to neurogenetic disorders from zebrafish. Trends Genet 26: 373–81. [DOI] [PubMed] [Google Scholar]
- 21. Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, et al. (1997) Gapped blast and psi-blast: a new generation of protein database search programs. Nucleic Acids Res 25: 3389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Felsenstein J (1989) Phylip - phylogeny inference package (version 3.2). Cladistics 5: 164–166. [Google Scholar]
- 23. Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, et al. (2013) The zebrafish reference genome sequence and its relationship to the human genome. Nature 496: 498–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Uhlen M, Oksvold P, Fagerberg L, Lundberg E, Jonasson K, et al. (2010) Towards a knowledge-based human protein atlas. Nat Biotechnol 28: 1248–1250. [DOI] [PubMed] [Google Scholar]
- 25. Sprague J, Bayraktaroglu L, Clements D, Conlin T, Fashena D, et al. (2006) The zebrafish information network: the zebrafish model organism database. Nucleic Acids Res 34: D581–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gerety SS, Wilkinson DG (2011) Morpholino artifacts provide pitfalls and reveal a novel role for pro-apoptotic genes in hindbrain boundary development. Dev Biol 350: 279–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Langheinrich U, Hennen E, Stott G, Vacun G (2002) Zebrafish as a model organism for the identification and characterization of drugs and genes affecting p53 signaling. Curr Biol 12: 2023–8. [DOI] [PubMed] [Google Scholar]
- 28. Breau MA, Wilson D, Wilkinson DG, Xu Q (2012) Chemokine and fgf signalling act as opposing guidance cues in formation of the lateral line primordium. Development 139: 2246–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Collazo A, Fraser SE, Mabee PM (1994) A dual embryonic origin for vertebrate mechanoreceptors. Science 264: 426–30. [DOI] [PubMed] [Google Scholar]
- 30. Haas P, Gilmour D (2006) Chemokine signaling mediates self-organizing tissue migration in the zebrafish lateral line. Dev Cell 10: 673–80. [DOI] [PubMed] [Google Scholar]
- 31. Ghysen A, Dambly-Chaudière C (2007) The lateral line microcosmos. Genes Dev 21: 2118–30. [DOI] [PubMed] [Google Scholar]
- 32. Prince VE, Pickett FB (2002) Splitting pairs: the diverging fates of duplicated genes. Nat Rev Genet 3: 827–37. [DOI] [PubMed] [Google Scholar]
- 33. Li Q, Shirabe K, Kuwada JY (2004) Chemokine signaling regulates sensory cell migration in zebrafish. Dev Biol 269: 123–36. [DOI] [PubMed] [Google Scholar]
- 34. Gamba L, Cubedo N, Ghysen A, Lutfalla G, Dambly-Chaudière C (2010) Estrogen receptor esr1 controls cell migration by repressing chemokine receptor cxcr4 in the zebrafish posterior lateral line system. Proc Natl Acad Sci U S A 107: 6358–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gamba L, Cubedo N, Lutfalla G, Ghysen A, Dambly-Chaudiere C (2010) Lef1 controls patterning and proliferation in the posterior lateral line system of zebrafish. Dev Dyn 239: 3163–71. [DOI] [PubMed] [Google Scholar]
- 36. Nechiporuk A, Raible DW (2008) Fgf-dependent mechanosensory organ patterning in zebrafish. Science 320: 1774–7. [DOI] [PubMed] [Google Scholar]
- 37. Lecaudey V, Cakan-Akdogan G, Norton WHJ, Gilmour D (2008) Dynamic fgf signaling couples morphogenesis and migration in the zebrafish lateral line primordium. Development 135: 2695–705. [DOI] [PubMed] [Google Scholar]
- 38. McGraw HF, Drerup CM, Culbertson MD, Linbo T, Raible DW, et al. (2011) Lef1 is required for progenitor cell identity in the zebrafish lateral line primordium. Development 138: 3921–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bryja V, Schulte G, Rawal N, Grahn A, Arenas E (2007) Wnt-5a induces dishevelled phosphorylation and dopaminergic differentiation via a ck1-dependent mechanism. J Cell Sci 120: 586–95. [DOI] [PubMed] [Google Scholar]
- 40. González-Sancho JM, Brennan KR, Castelo-Soccio LA, Brown AMC (2004) Wnt proteins induce dishevelled phosphorylation via an lrp5/6- independent mechanism, irrespective of their ability to stabilize beta-catenin. Mol Cell Biol 24: 4757–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Schneider I, Schneider PN, Derry SW, Lin S, Barton LJ, et al. (2010) Zebrafish nkd1 promotes dvl degradation and is required for left-right patterning. Dev Biol 348: 22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lum WM, Robertson JK, Van Raay TJ (2011) Dishevelled2 is a stable protein during early zebrafish development. Zebrafish 8: 65–71. [DOI] [PubMed] [Google Scholar]
- 43. Marchese A (2009) Ubiquitination of chemokine receptors. Methods Enzymol 460: 413–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bhandari D, Trejo J, Benovic JL, Marchese A (2007) Arrestin-2 interacts with the ubiquitin-protein isopeptide ligase atrophin-interacting protein 4 and mediates endosomal sorting of the chemokine receptor cxcr4. J Biol Chem 282: 36971–9. [DOI] [PubMed] [Google Scholar]
- 45. Aman A, Nguyen M, Piotrowski T (2011) Wnt/β-catenin dependent cell proliferation underlies segmented lateral line morphogenesis. Dev Biol 349: 470–82. [DOI] [PubMed] [Google Scholar]
- 46. Rossi M, Aqeilan RI, Neale M, Candi E, Salomoni P, et al. (2006) The e3 ubiquitin ligase itch controls the protein stability of p63. Proc Natl Acad Sci U S A 103: 12753–12758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rossi M, De Simone M, Pollice A, Santoro R, La Mantia G, et al. (2006) Itch/aip4 associates with and promotes p63 protein degradation. Cell Cycle 5: 1816–22. [DOI] [PubMed] [Google Scholar]
- 48. Hansen TM, Rossi M, Roperch JP, Ansell K, Simpson K, et al. (2007) Itch inhibition regulates chemosensitivity in vitro. Biochem Biophys Res Commun 361: 33–6. [DOI] [PubMed] [Google Scholar]
- 49. Pyati UJ, Gjini E, Carbonneau S, Lee JS, Guo F, et al. (2011) p63 mediates an apoptotic response to pharmacological and disease-related er stress in the developing epidermis. Dev Cell 21: 492–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Salah Z, Melino G, Aqeilan RI (2011) Negative regulation of the hippo pathway by e3 ubiquitin ligase itch is sufficient to promote tumorigenicity. Cancer Res 71: 2010–20. [DOI] [PubMed] [Google Scholar]
- 51. Azakir BA, Desrochers G, Angers A (2010) The ubiquitin ligase itch mediates the antiapoptotic activity of epidermal growth factor by promoting the ubiquitylation and degradation of the truncated c-terminal portion of bid. FEBS Journal 277: 1319–1330. [DOI] [PubMed] [Google Scholar]
- 52. Rathinam C, Matesic LE, Flavell RA (2011) The e3 ligase itch is a negative regulator of the homeostasis and function of hematopoietic stem cells. Nat Immunol 12: 399–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Mizoguchi T, Togawa S, Kawakami K, Itoh M (2011) Neuron and sensory epithelial cell fate is sequentially determined by notch signaling in zebrafish lateral line development. J Neurosci 31: 15522–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Matsuda M, Chitnis AB (2010) Atoh1a expression must be restricted by notch signaling for effective morphogenesis of the posterior lateral line primordium in zebrafish. Development 137: 3477–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Schüler S, Hauptmann J, Perner B, Kessels MM, Englert C, et al. (2013) Ciliated sensory hair cell formation and function require the f-bar protein syndapin i and the wh2 domain-based actin nucleator cobl. J Cell Sci 126: 196–208. [DOI] [PubMed] [Google Scholar]
- 56. Di Marcotullio L, Greco A, Mazzà D, Canettieri G, Pietrosanti L, et al. (2011) Numb activates the e3 ligase itch to control gli1 function through a novel degradation signal. Oncogene 30: 65–76. [DOI] [PubMed] [Google Scholar]
- 57. Head JR, Gacioch L, Pennisi M, Meyers JR (2013) Activation of canonical wnt/β-catenin signaling stimulates proliferation in neuromasts in the zebrafish posterior lateral line. Developmental Dynamics 242: 832–846. [DOI] [PubMed] [Google Scholar]
- 58. Matsuda M, Nogare DD, Somers K, Martin K, Wang C, et al. (2013) Lef1 regulates dusp6 to influence neuromast formation and spacing in the zebrafish posterior lateral line primordium. Development 140: 2387–2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Persaud A, Alberts P, Hayes M, Guettler S, Clarke I, et al. (2011) Nedd4-1 binds and ubiquitylates activated FGFR1 to control its endocytosis and function. The EMBO Journal 30: 3259–3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Edwin F, Anderson K, Patel TB (2010) Hect domain-containing e3 ubiquitin ligase nedd4 interacts with and ubiquitinates sprouty2. J Biol Chem 285: 255–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Omerovic J, Santangelo L, Puggioni EMR, Marrocco J, Dall'Armi C, et al. (2007) The e3 ligase aip4/itch ubiquitinates and targets erbb-4 for degradation. FASEB J 21: 2849–62. [DOI] [PubMed] [Google Scholar]
- 62. Akimov V, Rigbolt KTG, Nielsen MM, Blagoev B (2011) Characterization of ubiquitination dependent dynamics in growth factor receptor signaling by quantitative proteomics. Mol Biosyst 7: 3223–33. [DOI] [PubMed] [Google Scholar]
- 63. Sorkin A, Von Zastrow M (2002) Signal transduction and endocytosis: close encounters of many kinds. Nat Rev Mol Cell Biol 3: 600–14. [DOI] [PubMed] [Google Scholar]
- 64. Urbé S, McCullough J, Row P, Prior IA, Welchman R, et al. (2006) Control of growth factor receptor dynamics by reversible ubiquitination. Biochem Soc Trans 34: 754–6. [DOI] [PubMed] [Google Scholar]
- 65. Angers A, Ramjaun AR, McPherson PS (2004) The hect domain ligase itch ubiquitinates endophilin and localizes to the trans-golgi network and endosomal system. J Biol Chem 279: 11471–9. [DOI] [PubMed] [Google Scholar]
- 66. Fang D, Elly C, Gao B, Fang N, Altman Y, et al. (2002) Dysregulation of t lymphocyte function in itchy mice: a role for itch in th2 differentiation. Nat Immunol 3: 281–7. [DOI] [PubMed] [Google Scholar]
- 67. Parravicini V, Field AC, Tomlinson PD, Basson MA, Zamoyska R (2008) Itch-/- alphabeta and gammadelta t cells independently contribute to autoimmunity in itchy mice. Blood 111: 4273–7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lohr NJ, Molleston JP, Strauss KA, Torres-Martinez W, Sherman EA, et al. (2010) Human itch e3 ubiquitin ligase deficiency causes syndromic multisystem autoimmune disease. Am J Hum Genet 86: 447–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Westerfield M (1995) The zebrafish book: a guide for the laboratory use of zebrafish (Danio rerio). Institute of Neuroscience. University of Oregon.
- 70. Lennon G, Auffray C, Polymeropoulos M, Soares MB (1996) The i.m.a.g.e. consortium: an integrated molecular analysis of genomes and their expression. Genomics 33: 151–2. [DOI] [PubMed] [Google Scholar]
- 71. Alexandre D, Ghysen A (1999) Somatotopy of the lateral line projection in larval zebrafish. Proc Natl Acad Sci U S A 96: 7558–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Thisse C, Thisse B (2008) High-resolution in situ hybridization to whole-mount zebrafish embryos. Nat Protoc 3: 59–69. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Cell death in the pLL primordium of
itcha
and
itchb
morphants. (A–C), acridine orange staining in the primordium region of control cldnb:gfp embryos (CTRL), itcha knockdown (MoA), and itchb knockdown (MoB). p53 MO was omitted in this experiment. In these conditions, cell death occurred in the primordium cells, predominantly in the trailing end of the migrating primordium in both itcha (B) and itchb (C) morphants. Acridine orange staining is visible as brighter dots representing the nucleus of apoptotic cells over the dimmer EGFP signal in the cell membrane of the transgenic primordium. Scale bar: 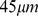 .
.
(TIF)
Accession number of sequences used for alignment.
(PDF)
Sequence of morpholino oligonucleotides.
(PDF)
Sequence of the PCR primers used in this study.
(PDF)
cDNA regions used as probes in in situ hybridization experiments.
(PDF)
CTRL. Example of a pLL primordium migration at 28 hpf in a control embryo. Stacks were acquired at 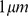 thickness and assembled in ImageJ. Movie length: 60 min.
thickness and assembled in ImageJ. Movie length: 60 min.
(AVI)
mRNA. Example of a pLL primordium migration at 28 hpf in a human ITCH-injected embryo (a second control). Stacks were acquired at 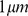 thickness and assembled in ImageJ. Movie length: 60 min.
thickness and assembled in ImageJ. Movie length: 60 min.
(AVI)
MoB. Example of a pLL primordium failing to migrate at 30 hpf in a itchb-morpholino-injected embryo. Stacks were acquired at 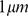 thickness and assembled in ImageJ. Movie length: 60 min.
thickness and assembled in ImageJ. Movie length: 60 min.
(AVI)
Rescue. Example of a pLL primordium migration at 28 hpf in a itchb-morpholino-injected embryo after rescue with human ITCH mRNA. Stacks were acquired at 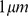 thickness and assembled in ImageJ. Movie length: 60 min.
thickness and assembled in ImageJ. Movie length: 60 min.
(AVI)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.