Abstract
Background
During robot assisted laparoscopic radical prostatectomy (RALRP), a CO2 pneumoperitoneum (CO2PP) is applied and the patient is placed in a head-down position. Intracranial pressure (ICP) is expected to acutely increase under these conditions. A non-invasive method, the optic nerve sheath diameter (ONSD) measurement, may warn us that the mechanism of protective cerebrospinal fluid (CSF) shifts becomes exhausted.
Methods
After obtaining IRB approval and written informed consent, ONSD was measured by ocular ultrasound in 20 ASA I–II patients at various stages of the RALRP procedure: baseline awake, after induction, after applying the CO2PP, during head-down position, after resuming the supine position, in the postoperative anaesthesia care unit, and on day one postoperatively. Cerebral perfusion pressure (CPP) was calculated as the mean arterial (MAP) minus central venous pressure (CVP).
Results
The ONSD did not change during head-down position, although the CVP increased from 4.2(2.5) mm Hg to 27.6(3.8) mm Hg. The CPP was decreased 70 min after assuming the head-down position until 15 min after resuming the supine position, but remained above 60 mm Hg at all times.
Conclusion
Even though ICP has been documented to increase during CO2PP and head-down positioning, we did not find any changes in ONSD during head-down position. These results indicate that intracranial blood volume does not increase up to a point that CSF migration as a compensation mechanism becomes exhausted, suggesting any increases in ICP are likely to be small.
Introduction
During robot assisted laparoscopic radical prostatectomy (RALRP), adequate surgical exposure requires the application of a CO2 pneumoperitoneum (CO2PP) and steep head-down position (up to 45°). The effects on the cardiopulmonary system are mild and well tolerated [1], [2], but those on intracerebral physiology remain poorly documented. According to the waterfall model, cerebral perfusion pressure (CPP) equals mean arterial pressure (MAP) minus central venous pressure (CVP) or intracranial pressure (ICP), whichever is higher [3]. Using only CVP and MAP measurements, CPP was found to remain within acceptable limits. In addition, although the sensitivity of near-infrared spectroscopy (NIRS) to detect cerebral ischemia is debatable, increases or slight decreases in cerebral oximetry readings during head-down position are reassuring [1], [4], [5].
The effect of ICP on CPP cannot easily be determined intraoperatively during RALRP, but in animal studies ICP has been shown to increase by up to 10 mm Hg above baseline with CO2PP and head-down position [6]–[11]. Transcranial Doppler measurement during RALRP revealed an increase in calculated zero flow pressure of equal magnitude as the measured increase in cerebral venous pressure, indicating that ICP does not exceed CVP [12]. More importantly, this increase in zero flow pressure did not further expand over the course of the operation, indicating no increase in cerebrovascular resistance and consequently no haemodynamically relevant increase in cerebral extracellular water content [1], [12].
When intracranial pressure varies within physiological limits, the consequences of the increase in ICP are restricted through compensatory mechanisms such as intracerebral blood and cerebrospinal fluid (CSF) shifts. It is only after exhausting these mechanisms that ICP would start to increase exponentially. Nevertheless, while permanent neurologic sequellae after robotic prostatectomy are rare in the absence of pre-existing intracranial pathology and effective CPP seems to be maintained within autoregulatory limits, severe complications have been reported [13], [14], which shows that it is possible at times these compensatory mechanisms are exhausted or near exhausted.
A new non-invasive method, the optic nerve sheath diameter (ONSD) measurement, may provide information about whether the mechanism of protective CSF fluid shifts to attenuate ICP increases becomes or is threatened to become exhausted in the individual patient undergoing RALRP.
Ocular sonography is safely used for ophthalmic evaluation since more than twenty years [15], and has a fast learning curve: novice sonologists need only 25 scans to obtain adequate results [16], with limited variability in measurement of ONSD, as median intra-observer and inter-observer variations were shown to be respectively less than 0.2 and 0.3 mm [17], [18], [19].
The CSF in the intracranial subarachnoidal space is connected to the CSF in the dural sheath around the optic nerve (ONS). Because CSF is incompressible, the ICP is directly transmitted to the fluid in the optic nerve sheath. Due to the elastic subarachnoidal trabecular anatomy, the optic nerve sheet is most distensible 3 mm behind the globe, thus making it the best point for ONSD interpretation.
The arachnoid surrounding the optic nerves approx. 3 mm proximal to the fovea has a highly ramified meshwork of delicate trabeculae [20]. The distensibilty of these trabeculae allows this chamber to inflate in case of raised ICP with an equilibration time of only a few minutes. In the ICU and emergency medicine literature, the ONSD was shown to correlate well with acute ICP changes [21]–[26]. The ONSD was reported to increase at CSF pressures between 15 and 30 mm Hg [21]. Inversely, a constant ONSD indicates that CSF pressure does not appreciably exceed these values. Reported cut-off values indicative of intracranial hypertension, defined as intracranial pressure above 20 mm Hg, vary from 5.0 mm to 5.9 mm, with a specificity of 86% and sensitivity of 79% [23]. Several recent clinical studies [17], [19], [22], [25] have compared sonographic ONSD with invasive gold standard methods for measuring ICP. Simultaneous measurements of ONSD and invasive ICP show a good relationship between both variables (r = 0.71 and r = 0.68) [19], [22], with similar cut-off values in acute neurocritical care patients. The best cutoff value was 5.7 or 5.8 mm for predicting elevated ICP (≥ or >20 mm Hg). The probability of having high ICP was very low (less than 5%) when ONSD was less than 5.8 mm. When comparing sonographic ONSD and ICP measured with a ventricular drain, the best cut-off value for detecting ICP>15 mm Hg (or 20 cm H2O) was 5 mm with a sensitivity and specificity of respectively 88% and 93% [22]. Moreover, changes in ONSD are strongly related to ICP variations (r = 0.73) [19], indicating that most probably, trends are even more indicative of relative changes in ICP than absolute values.
In patients undergoing RALRP, a constant ONSD would indicate that CSF migration as a compensation mechanism does not become exhausted, providing indirect evidence that ICP does not increase sufficiently to compromise cerebral perfusion. Because the ONSD was reported to increase at CSF pressures between 15 and 30 mm Hg [21], a constant ONSD also indicates that CSF pressure does not appreciably exceed these values. Because most patients awake uneventfully, and because our former observations showed that CPP values and cerebral oxygenation [1] remain within physiological ranges, we hypothesize that the ONSD does not change significantly during RALPR. In this study, the absence of significant changes in ONSD suggests that still an assuring safety margin in capacity for intracranial volume shift exists.
Methods
After obtaining IRB approval and written informed consent, 20 ASA I–II patients undergoing RALRP were enrolled. Sample size calculation for our study was designed to detect an increase of 0.4 mm in ONSD between subsequent patient positioning. Measurements in 26 people with no disease and in 28 people with elevated intracranial pressure demonstrated a mean(SD) ONSD of 4.6(0.3) mm in normal adults versus 6.4(0.7) mm in the presence of increased ICP [27]. For an estimated SD of 0.5 mm between subsequent steady-state ONSD measurements, a power of 95% and an α-error of 5%, at least 19 patients should be included [28]. Therefore, we included 20 patients in total.
Patients were premedicated with 0.5 mg oral alprazolam one hour prior to surgery. After preoxygenation, anaesthesia was induced with sufentanil (0.1 µg/kg) and propofol (2–3 mg/kg). Intubation of the trachea was facilitated with rocuronium (0.6 mg/kg) and anaesthesia was maintained with sevoflurane at an end-expired concentration of 0.7–1 MAC in O2/air (FIO2 = 0.40) using a Zeus anaesthesia machine (Dräger, Lübeck, Germany). Ventilation using tidal volumes of 6–8 ml/kg with a maximal airway pressure of 25 cm H2O was adjusted to maintain the end-expired CO2 partial pressure (PECO2) between 30 and 40 mmHg; positive end-expiratory pressure was not applied. The head was positioned in neutral position on a pillow designed to give additional support to the head and both shoulders to prevent the patient from sliding when placed in the head-down position. Head-down position was achieved by tilting the table to an angle range of 40° to 45°, adjusted to surgical exposure and laparoscopic accessibility.
Arterial blood pressure and CVP were measured in the left radial artery and right internal jugular vein, respectively. Both pressure transducers were positioned at the level of the external ear canal. Cerebral perfusion pressure (CPP), calculated as MAP minus CVP, was maintained above 60 mm Hg either by adjusting the sevoflurane concentration or by administering phenylephrine (100 µg boluses IV). Crystalloids (Hartmann, Braun) were administered as maintenance fluid, calculated for weight and additional third space losses. The insufflation pressure during the procedure was limited to 15 mm Hg. After anastomosis of the urethra, 20 mg furosemide was administered (per surgical protocol). Postoperatively, patients remained sedated with propofol and ventilated in the PACU by local protocol, to hasten turn-over, to diminish risk of airway difficulties (as sometimes seen after prolonged head-down position with pneumoperitoneum) and to have an extra measurement in supine position with a patient who is still sedated. One hour after arrival in the PACU, propofol administration was discontinued, the patients were allowed to awaken, and the trachea was extubated.
Ocular ultrasound measurements (GE Healthware Vivid.q) were made with a 13 MHz linear probe as previously described [21]. Eyelids were taped closed, and a thick layer of water-containing ultrasound gel was applied. The ONSD was measured at the entry zone of the optic nerve in the globe, 3 mm behind the papilla, perpendicular on the axis of the optic nerve. Transducer depth was set at 4 cm. ONSD measurements were made at 14 well defined moments during the procedure: before induction of anaesthesia (T0), 10 min after intubation, 10 min after insufflation, after 10–40–70–100–130–160–190 min of full Trendelenburg, 15–60 min after resuming supine position, awake in the PACU and one day postoperatively. All data were obtained by one of two observers (PV, KS). During each phase, the ONSD was measured once in the sagittal and once in the horizontal plane of each eye. The average of these 4 values was calculated. At those same moments, MAP, CVP and PECO2 were recorded. To increase reproducibility, each image was reviewed by the second investigator, blinded for patient name and time point measured. Values are presented as mean (SD). Changes in MAP, CVP, CPP and PECO2 values and differences of ONSD between measurements were analyzed with ANOVA for repeated measurements, followed by the Holm-Sidak test (SigmaPlot (Systat Software Inc, Vista Centre, Hounslow, London, UK)). Statistical significance level was set at 5%.
Results
Reliable measurements could be performed in all patients. Data of the 20 patients were normally distributed. The age of the patients was 63(50–82) yr with a weight of 83(13) kg, a height of 177(6) cm and a body mass index of 26.6(3.7) kg m−2. Total fluid administration during the procedure was 1558(337) ml, total blood loss was 457(265) ml and total time in Trendelenburg position was 213(58) minutes. There were 9 patients with arterial hypertension and 1 with diabetes.
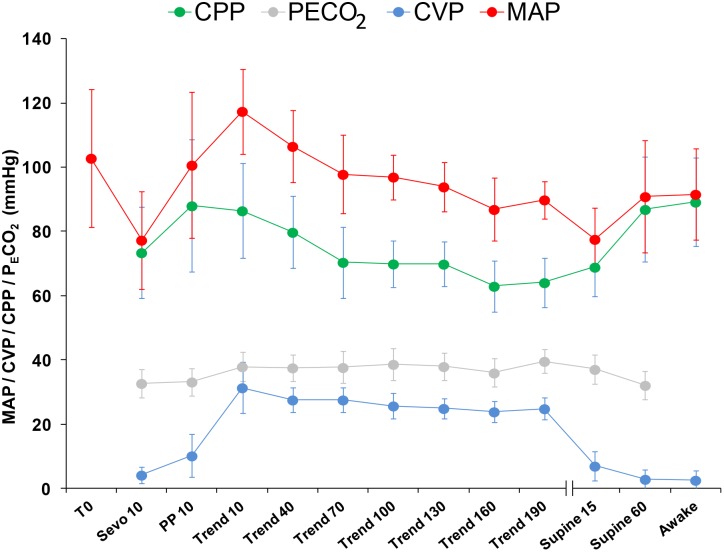
Figure 1 and table 1 show the evolution of MAP, CVP, CPP and PECO2 over the course of the procedure. CPP increased from 73(14) mm Hg after intubation to 86(15) mm Hg in full Trendelenburg and decreased again to a lowest value of 63(8) mm Hg at 160 min of Trendelenburg. It normalised to 87(16) mm Hg at 60 min after resuming the supine position. CPP remained above 60 mm Hg at all times. The CVP increases from 4.2(2.5) mm Hg after intubation to a maximum of 27.6(3.8) mm Hg after 70 min of Trendelenburg. PECO2 increased from 32.8(4.4) mm Hg after intubation (TSevo10) to a maximum of 39.6(3.7) mm Hg after 190 min of Trendelenburg and CO2PP, and returned to baseline values 60 min after resuming the supine position. CPP, CVP and PECO2 values changed significantly (p<0.05) between baseline and full Trendelenburg.
Figure 1. Evolution of physiological variables over the course of the procedure.
Evolution of the mean (SD) values of the Cerebral Perfusion Pressure (CPP), End-tidal CO2 Pressure (PECO2), Central Venous Pressure (CVP) and Mean Arterial Pressure (MAP) over the course of the procedure at different time points at 14 well defined moments during the procedure: before induction of anaesthesia (T0), 10 minutes after intubation, 10 minutes after insufflation, after 10–40–70–100–130–160–190 minutes of full Trendelenburg, 15–60 minutes after resuming supine position and awake at the PACU.
Table 1. Evolution of ONSD (mm), CPP (mm Hg) and PECO2 (mm Hg) over the course of the procedure at 14 defined moments during the procedure: before induction of anaesthesia (T0), 10 minutes after intubation, 10 minutes after insufflation, after 10–40–70–100–130–160–190 minutes of full Trendelenburg, 15–60 minutes after resuming supine position, awake at the PACU (PA) and one day postoperatively (PO).
| ONSD | CPP | PECO2 | ||||
| Mean | SD | Mean | SD | Mean | SD | |
| T0 | 4.9 | 0.2 | N/A | N/A | N/A | N/A |
| Sevo 10 | 4.9 | 0.3 | 73* | 14 | 33* | 4 |
| PP 10 | 5.0 | 0.2 | 88* | 21 | 33* | 4 |
| Trend 10 | 5.0* | 0.3 | 87* | 15 | 38 | 5 |
| Trend 40 | 5.0* | 0.3 | 80 | 11 | 38 | 4 |
| Trend 70 | 5.0* | 0.3 | 70** | 11 | 38 | 5 |
| Trend 100 | 5.0* | 0.2 | 70** | 7 | 39 | 5 |
| Trend 130 | 5.0 | 0.2 | 70** | 7 | 38 | 4 |
| Trend 160 | 5.0 | 0.2 | 63** | 8 | 36 | 4 |
| Trend 190 | 5.1 | 0.2 | 64** | 8 | 40 | 4 |
| Supine 15 | 5.0 | 0.2 | 69** | 9 | 37 | 5 |
| Supine 60 | 4.9 | 0.2 | 87* | 16 | 32* | 4 |
| Awake PA | 4.8** | 0.2 | 89* | 14 | 0 | 0 |
| Awake PO | 4.8** | 0.2 | N/A | N/A | 0 | 0 |
Values are shown in mean (SD).
ONSD: ** differs from * (except POD1 from T100).
CPP: ** differs from *.
PECO2: * lower than other values.
The ONSD remained constant during the entire observation period (Figure 2 and table 1). All original data is freely available for reanalysis on request.
Figure 2. Evolution of ONSD over the course of the procedure.
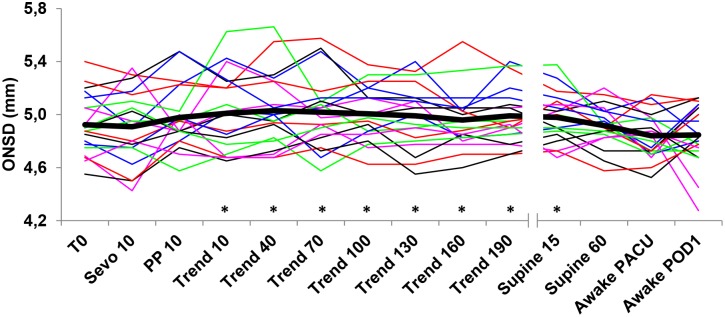
Evolution of the ONSD in individual patients (thin lines) and mean value (thick line) over the course of the procedure. *indicates significant differences with post-operative values.
Discussion
The ONSD does not change when patients undergoing RALRP are placed in a steep head-down position while a CO2PP is being applied. Previous studies in animals, as in humans suggest that applying a CO2PP and assuming the head-down position acutely increases ICP up to 10 mm Hg above baseline [9], [29]–[31]. However, since MAP and CVP increase to the same extent, and CVP is apparently higher than ICP, no net change in CPP should occur. CPP calculated as MAP – CVP has been documented to remain within the limits of cerebral blood flow autoregulation [3].
The hydrostatic pressure difference caused by the head down position causes both CVP and ICP to increase by the same amount relative to the pressure in the right atrium, but ICP could arguably still increase more than CVP. Institution of CO2PP and head-down position induces an increase in arterial blood volume and/or venous blood volume [3], [21]. The Monro-Kellie doctrine states that within the fixed and incompressible intracranial volume, its constituents (blood, CSF and brain tissue) impose a volume equilibrium where increases in volume of one compartment must be compensated by decreases in volume of the others [34]. As long as the increase in intracranial blood volume can be compensated by an equal decrease in CSF (by shifting the CSF out of the rigid container that the skull is), the ICP will not increase dramatically. Animal research has demonstrated that the normal mammal brain has a remarkable capacity to translocate CSF fluid to the vascular compartment [35]. The human brain can also translocate CSF very rapidly: to maintain ICP at 20 mm Hg, 2 mL min−1 of fluid has to be infused intrathecally [34]. Thus, an increase in intracranial blood volume can quickly be compensated by an efflux of CSF. Once the intracranial veins have reached their maximal dilatation, which was likely the case given the venous pressure of up to 27.6(3.8) mm Hg, no further increase in intracranial blood volume is to be expected. The Monro-Kellie doctrine therefore prompts us to examine whether in our patients sufficient CSF could be translocated to cushion this intracranial volume shift to prevent unacceptable increases in ICP and/or if these reserves are being exhausted (indicating cerebral tissue might be at risk of ischemia). Briefly, we expected that the combination of position and pneumoperitoneum would consistently raise ICP, and wanted to study if enough compensation mechanisms exist to keep this increase within acceptable limits. Our study reveals that if CSF translocation would be required to attenuate the increase in ICP in our patient population, this mechanism does not become exhausted up to a point where the ONSD increases. However, a significantly increased ICP due to positioning may be a rare event itself, given the relative rarity of complications consistent with an elevated ICP. Therefore, absence of ONSD increase in our results does not necessarily guarantee an equal safety zone in all patients.
In the patient undergoing RALRP in the presence of intracranial pathology with intracranial hypertension, or with a disturbed blood-brain barrier, head-down with a CO2PP does carry a risk: two patients undergoing radical cystectomy in head-down position during 7 and 10 hours respectively, were reported to have generalised cerebral edema, radiologically diagnosed after neurological deterioration in the PACU [36]. One of these patients had preexisting intracranial pathology. Consequently, an important limitation of our study is that in our patient population, strict exclusion of patients with a high a-priori risk for intracranial hypertension was respected, and therefore our reassuring conclusions should not be extrapolated to patients with pre-existing intracranial pathology.
While ONSD is not a perfect surrogate for ICP change, our current findings indicate that compensatory mechanisms including CSF translocation from the cranial vault to the spinal CSF compartment or the blood compartment are sufficient to attenuate ICP increases. Also pathology studies in animals have shown no evidence of tissue edema [32], [33]. Remarkably, another study with similar methodology and patient population to ours, did report a small but significant increase of ONSD of 12% during RALRP [37]. It has been suggested that some of the postoperative agitation seen in some of the patients might be related to the presence of some mild cerebral edema, but no evidence exist to either substantiate or refute this claim. MRI studies might be useful to help address this question.
Our findings support the existing claims that this steep patient positioning can be performed safely in a large patient population, and its risks should be balanced to the surgical advantages for optimal patient treatment. Importantly however, in mentioned previous studies, as in our current study, procedure duration was only moderate while duration of head -down position probably is a critical factor in generation of intracranial hypertension and possible cerebral edema, as already suggested by other authors [36]. More research is needed to determine the safety and effects on ICP of steep Trendelenburg positioning in longer lasting procedures, such as cystectomy. In addition, patients with intracranial pathologies were not included in our study, and our results should as such be restricted to patients without these conditions.
To summarise, RALRP requires a combination of a CO2PP and head-down positioning, which is known to increase intracranial pressure. The ONSD, previously validated as a means to help diagnose intracranial hypertension in the presence of head trauma, did not change in this setting. Our results in this study population with an apparent lack of intracranial pathology indicate that adequate compensation mechanisms may attenuate intracranial effects induced by the CO2PP and the head-down position, possibly by translocation of CSF towards the spinal canal and the vascular compartment. Additional research is needed to clarify the effects of prolonged head-down position in intracranial pressure in procedures of longer duration [38].
Ethics statement
Our research protocol was approved by the “Ethisch Committee OLV Ziekenhuis, Aalst, Belgium” on January 5th 2012. After obtaining written informed consent, 20 ASA I–II patients undergoing RALRP were enrolled.
No animals were involved in this study.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
The authors received no specific funding for this work. This study was solely supported by departmental and institutional funding: Department of Anaesthesiology and Intensive care medicine, OLV Clinic, Aalst, Belgium. Moorselbaan 164, 9300 Aalst, Belgium. No individuals other than the named authors played any role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No current funding sources for this study were used other than institutional fundings.
References
- 1. Kalmar AF, Foubert L, Hendrickx JF, Mottrie A, Absalom A, et al. (2010) Influence of steep Trendelenburg position and CO(2) pneumoperitoneum on cardiovascular, cerebrovascular, and respiratory homeostasis during robotic prostatectomy. Br J Anaesth. 104: 433–9. [DOI] [PubMed] [Google Scholar]
- 2. Haas S, Haese A, Goetz AE, Kubitz JC (2011) Haemodynamics and cardiac function during robotic-assisted laparoscopic prostatectomy in steep Trendelenburg position. Int J Med Robot. 7: 408–13. [DOI] [PubMed] [Google Scholar]
- 3. Munis JR, Lozada LJ (2000) Giraffes, siphons, and starling resistors. Cerebral perfusion pressure revisited. J Neurosurg Anesthesiol 12: 290–6. [DOI] [PubMed] [Google Scholar]
- 4. Closhen D, Treiber AH, Berres M, Sebastiani A, Werner C, et al. (2014) Robotic assisted prostatic surgery in the Trendelenburg position does not impair cerebral oxygenation measured using two different monitors: A clinical observational study. Eur J Anaesthesiol. 31: 104–9. [DOI] [PubMed] [Google Scholar]
- 5. Jeong H, Lee SH, Jang EA, Chung SS, Lee J, et al. (2012) Haemodynamics and cerebral oxygenation during arthroscopic shoulder surgery in beach chair position under general anaesthesia. Acta Anaesthesiol Scand. 56: 872–9. [DOI] [PubMed] [Google Scholar]
- 6. Kotani J, Momota Y, Sugioka S, Umemura A, Ueda Y (1992) Effect of head-down tilt on intracranial pressure and sagittal sinus pressure during general anesthesia in cats. Anesth Prog. 39: 209–11. [PMC free article] [PubMed] [Google Scholar]
- 7. Tatebayashi K, Asai Y, Maeda T, Shiraishi Y, Miyoshi M, et al. (2003) Effects of head-down tilt on the intracranial pressure in conscious rabbits. Brain Res. 977: 55–61. [DOI] [PubMed] [Google Scholar]
- 8. Halverson AL, Barrett WL, Iglesias AR, Lee WT, Garber SM, et al. (1999) Decreased cerebrospinal fluid absorption during abdominal insufflation. Surg Endosc. 13: 797–800. [DOI] [PubMed] [Google Scholar]
- 9. Halverson A, Buchanan R, Jacobs L, Shayani V, Hunt T, et al. (1998) Evaluation of mechanism of increased intracranial pressure with insufflation. Surg Endosc. 12: 266–9. [DOI] [PubMed] [Google Scholar]
- 10. Brosnan RJ, Steffey EP, LeCouteur RA, Imai A, Farver TB, et al. (2002) Effects of body position on intracranial and cerebral perfusion pressures in isoflurane-anesthetized horses. J Appl Physiol. 92: 2542–6. [DOI] [PubMed] [Google Scholar]
- 11. Doi M, Kawai Y (1998) Mechanisms of increased intracranial pressure in rabbits exposed to head-down tilt. Jpn J Physiol. 48: 63–9. [DOI] [PubMed] [Google Scholar]
- 12. Kalmar AF, Dewaele F, Foubert L, Hendrickx JF, Heeremans EH, et al. (2012) Cerebral haemodynamic physiology during steep Trendelenburg position and CO2 pneumoperitoneum. Br J Anaesth. 108: 478–84. [DOI] [PubMed] [Google Scholar]
- 13. Michael J, Danic MJ, Chow M, Alexander G, Bhandari A, et al. (2007) Anesthesia considerations for robotic-assisted laparoscopic prostatectomy: a review of 1,500 cases. J Robotic Surg 1: 119–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Ficarra V, Novara G, Artibani W, Cestari A, Galfano A, et al. (2009) Retropubic, laparoscopic, and robot-assisted radical prostatectomy: a systematic review and cumulative analysis of comparative studies. Eur Urol. 55: 1037–63. [DOI] [PubMed] [Google Scholar]
- 15. Munk PL, Vellet AD, Levin M, Lin DT, Collyer RT (1991) Sonography of the eye, Am J Roentgenol. 157: 1079–86. [DOI] [PubMed] [Google Scholar]
- 16. Tayal VS, Neulander M, Norton HJ, Foster T, Saunders T, et al. (2007) Emergency department sonographic measurement of optic nerve sheath diameter to detect findings of increased intracranial pressure in adult head injury patients. Ann Emerg Med. 49: 508–14. [DOI] [PubMed] [Google Scholar]
- 17. Soldatos T, Karakitsos D, Chatzimichail K, Papathanasiou M, Gouliamos A, et al. (2008) Optic nerve sonography in the diagnostic evaluation of adult brain injury. Crit Care. 12: R67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ballantyne SA, O’Neill G, Hamilton R, Hollman AS (2002) Observer variation in the sonographic measurement of optic nerve sheath diameter in normal adults. Eur J Ultrasound 15: 145–9. [DOI] [PubMed] [Google Scholar]
- 19. Geeraerts T, Merceron S, Benhamou D, Vigue B, Duranteau J (2008) Non-invasive assessment of intracranial pressure using ocular sonography in neurocritical care patients. Intensive Care Med. 34: 2062–67. [DOI] [PubMed] [Google Scholar]
- 20. Killer HE, Laeng HR, Flammer J, Groscurth P (2003) Architecture of arachnoid trabeculae, pillars, and septa in the subarachnoid space of the human optic nerve: anatomy and clinical considerations. Br J Ophthalmol. 87: 777–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hansen HC, Helmke K (1997) Validation of the optic nerve sheath response to changing cerebrospinal fluid pressure: ultrasound findings during intrathecal infusion tests. J Neurosurg. 87: 34–40. [DOI] [PubMed] [Google Scholar]
- 22. Kimberly HH, Shah S, Marill K, Noble V (2008) Correlation of optic nerve sheath diameter with direct measurement of intracranial pressure. Acad Emerg Med. 15: 201–4. [DOI] [PubMed] [Google Scholar]
- 23. Moretti R, Pizzi B (2011) Ultrasonography of the optic nerve in neurocritically ill patients. Acta Anaesthesiol Scand. 55: 644–52. [DOI] [PubMed] [Google Scholar]
- 24. Newman WD, Hollman AS, Dutton GN, Carachi R (2002) Measurement of optic nerve sheath diameter by ultrasound: a means of detecting acute raised intracranial pressure in hydrocephalus. Br J Ophthalmol. 86: 1109–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Geeraerts T, Launay Y, Martin L, Pottecher J, Vigué B, et al. (2007) Ultrasonography of the optic nerve sheath may be useful for detecting raised intracranial pressure after severe brain injury. Intensive Care Med 33: 1704–1711. [DOI] [PubMed] [Google Scholar]
- 26. Geeraerts T, Duranteau J, Benhamou D (2008) Ocular sonography in patients with raised intracranial pressure: the papilloedema revisited. Crit Care. 12: 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Girisgin AS, Kalkan E, Kocak S, Cander B, Gul M, et al. (2007) The role of optic nerve ultrasonography in the diagnosis of elevated intracranial pressure. Emerg Med J 24: 251–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Faul F, Erdfelder E, Lang AG, Buchner A (2007) G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 39: 175–91. [DOI] [PubMed] [Google Scholar]
- 29. Mavrocordatos P, Bissonnette B, Ravussin P (2000) Effects of neck position and head elevation on intracranial pressure in anaesthetized neurosurgical patients: preliminary results. J Neurosurg Anesthesiol. 12: 10–4. [DOI] [PubMed] [Google Scholar]
- 30. Josephs LG, Este-McDonald JR, Birkett DH, Hirsch EF (1994) Diagnostic laparoscopy increases intracranial pressure. J Trauma. 36: 815–8. [DOI] [PubMed] [Google Scholar]
- 31. Rosenthal RJ, Hiatt JR, Phillips EH, Hewitt W, Demetriou AA, et al. (1997) Intracranial pressure: Effects of pneumoperitoneum in a large-animal model. Surg Endosc 11: 376–80. [DOI] [PubMed] [Google Scholar]
- 32. Shimoyama R, Kawai Y (2000) Histological examination on edema formation in the rabbit brain exposed to head-down tilt. J Gravit Physiol. 7: 83–4. [PubMed] [Google Scholar]
- 33. Shimoyama R, Miyata H, Ohama E, Kawai Y (2000) Does edema formation occur in the rabbit brain exposed to head-down tilt? Jpn J Physiol. 50: 141–7. [DOI] [PubMed] [Google Scholar]
- 34. Eklund A, Smielewski P, Chambers I, Alperin N, Malm J, et al. (2007) Assessment of cerebrospinal fluid outflow resistance. Med Biol Eng Comput. 45: 719–35. [DOI] [PubMed] [Google Scholar]
- 35. Kalmar AF, De Ley G, Van Den Broecke C, Van Aken J, Struys MM, et al. (2009) Influence of an increased intracranial pressure on cerebral and systemic haemodynamics during endoscopic neurosurgery: an animal model. Br J Anaesth. 102: 361–8. [DOI] [PubMed] [Google Scholar]
- 36. Pandey R, Garg R, Darlong V, Punj J, Chandralekha, et al (2010) Unpredicted neurological complications after robotic laparoscopic radical cystectomy and ileal conduit formation in steep trendelenburg position: two case reports. Acta Anaesthesiol Belg. 61: 163–6. [PubMed] [Google Scholar]
- 37. Kim MS, Bai SJ, Lee JR, Choi YD, Kim YJ, et al. (2014) Increase in intracranial pressure during carbon dioxide pneumoperitoneum with steep trendelenburg positioning proven by ultrasonographic measurement of optic nerve sheath diameter. J Endourol. 28: 801–6. [DOI] [PubMed] [Google Scholar]
- 38. Rollins M, Flood P (2012) Imaging intracranial pressure: An introduction to ultrasonography of the optic nerve sheath. Anesthesiology 116: 983–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.