Abstract
L-Glutamate is a major oxidative fuel for the small intestine. However, few studies have demonstrated the effect of L-glutamate on the intestinal architecture and signaling of amino acids in the small intestine. The aim of this study was to investigate the effects of dietary L-glutamate supplementation on the intestinal architecture and expressions of jejunal mucosa amino acid receptors and transporters in weaning piglets. A total of 120 weaning piglets aged 35±1 days with an average body weight at 8.91±0.45 kg were randomly allocated to two treatments with six replicates of ten piglets each, fed with diets containing 1.21% alanine, or 2% L-glutamate. L-Glutamate supplementation increased the activity of glutamate oxaloacetate transaminase (GOT) in the jejunal mucosa. Also, the mRNA expression level of jejunal mucosa glutamine synthetase (GS) was increased by L-glutamate supplementation. The height of villi in duodenal and jejunal segments, and the relative mRNA expression of occludin and zonula occludens protein-1 (ZO-1) in jejunal mucosa were increased by dietary L-glutamate supplementation. L-Glutamate supplementation increased plasma concentrations of glutamate, arginine, histidine, isoleucine, leucine, methionine, phenylalanine and threonine. L-Glutamate supplementation also increased the relative mRNA expression of the jejunal mucosa Ca2+-sensing receptor (CaR), metabotropic glutamate receptor 1 (mGluR1) and metabotropic glutamate receptor 4 (mGluR4), and neutral amino acid transporter B0-like (SLC1A5) in the jejunal mucosa. These findings suggest that dietary addition of 2% L-glutamate improves the intestinal integrity and influences the expression of amino acid receptors and transporters in the jejunum of weaning, which is beneficial for the improvement of jejunal nutrients for digestion and absorption.
Introduction
L-Glutamate is one of the most common amino acid in animal and plant proteins as well as in milk [1]–[3]. L-Glutamate is also a functional amino acid in cell metabolism and signaling [4]. Emerging evidence shows that L-glutamate is a critical oxidative substrate for the intestinal mucosa [5], [6] and a precursor of important molecules, such as glutathione and the polyglutamated folate cofactors [7], [8]. These facts suggest that dietary glutamate may be a key factor for the maintenance of mucosal health. Tracer studies in piglets and rats have confirmed that dietary glutamate is catabolized almost completely in the small intestinal to yield ATP and CO2 [9]. The intestinal epithelial cells have high ATP production and utilization, which is in rapid renewal and responsible for the nutrient absorption process [6], [10]. Therefore, dietary L-glutamate supplementation may improve intestinal health in weaning piglets.
In addition, besides its nutritional role, L-glutamate is an important excitatory neurotransmitter in the body. Some studies have demonstrated that multiple glutamate receptors and transporters have been found in the gastrointestinal tract [11]–[13]. Importantly, emerging evidence also showed that oral administration of monosodium glutamate increased the expression of glutamate receptors and transporters in the gastrointestinal tract of young piglets [14]. In other studies, luminal L-glutamate was shown to enhance duodenal mucosal defense mechanisms via multiple G protein-coupled receptors (GPCRs), including the taste receptor type 1, member 1 and the taste receptor type 1, member 3 (T1R1/T1R3) heterodimer, and the metabotropic glutamate receptors (mGluR) [15], [16]. Thus, glutamate is a functional amino acid which beneficially enhances nutrient sensing and transport in the gastrointestinal tract. Generally, the gut is the primary organ that is involved in digestion, absorption and metabolism of dietary nutrients. The important process for absorption of free amino acid is mainly mediated by specific transporter in the jejunum [17], [18]. The different amino acid transporters have been identified as the major intestinal transporters for neutral, basic, and acidic amino acid, such as solute carrier family 7, member 7 (SLC7A7), neutral amino acid transporter B0-like (SLC1A5), solute carrier family 7, member 9 (SLC7A9), solute carrier family 6, member 19 (SLC6A19) and solute carrier family 1, member 1 (SLC1A1) [17]. Previous reports have indicated that the importance of amino acid in the small intestine is to maintain homeostasis of overall protein nutrition in body [19]. However, the effects of L-glutamate supplementation on the absorption of amino acids in the small intestine of weaning piglets are still unknown. We hypothesized that dietary supplementation with L-glutamate may modulate the mRNA expression of amino acid receptors and transporters, which are associated with the absorption in the small intestine of weaning piglets.
Based on the foregoing, the purpose of the present study was to evaluate the effects of L-glutamate supplementation on the intestinal architecture, expressions of amino acid receptors, transporters and intestinal tight proteins in the jejunum of weaning piglets.
Materials and Methods
Animals and experimental design
All the procedures including animal care and experiment treatments in the present study were approved by the Institutional Animal Care and Use Committee of Nanjing Agricultural University. A total of 120 crossbred males (castrated, at 10 days of the age) piglets aged 35±1 days with an average body weight of 8.91±0.45 kg were randomly allocated to two treatment groups. Each treatment group had six pens of ten piglets. The diets were formulated with 1.21% alanine or 2% L-glutamate on the basis of maize-soybean- based diets at the expense of the same amount of maize. The dosage of L-glutamate supplementation and alanine as isonitrogenous control were according to the previous studies [20]–[22]. Alanine and L-glutamate were obtained from Tiancheng Pharmaceutical Co. (Tianjin, China). Diets were formulated to meet or exceed requirements suggested by the National Research Council (NRC 2012) [23] ( Table 1 ). The basal diets contained 19.96% crude protein and 2.62% glutamate, which were analyzed as previously described [1]. During the entire experimental period, piglets were allowed ad libitum access to feed and water. The room temperature was maintained at 25–27°C. All the piglets were fed three times per day at 06.30, 11.00, and 18.00 hours.
Table 1. Ingredients and nutrient content of the basal diets of weaning piglets.
| Ingredients | (%) | Nutrient content (%) | |
| Maize | 49.50 | Crude protein ‡ | 19.96 |
| Wheat flour | 11.06 | Crude fat ‡ | 17.57 |
| Soybean meal | 19.30 | Crude fiber | 2.00 |
| Expanded soybean | 9.50 | Ash ‡ | 6.44 |
| Whey powder | 5.00 | Net energy(MJ/kg) | 10.13 |
| Fish meal | 2.50 | Lys | 1.24 |
| DL-Methionine | 0.11 | Met + Cys | 0.69 |
| Lysine-HCl | 0.37 | Thr | 0.74 |
| Threonine | 0.11 | Trp | 0.21 |
| Dicalcium phosphate | 0.62 | Arg | 1.15 |
| Limestone | 0.63 | His | 0.43 |
| Salt | 0.30 | Ile | 0.71 |
| Vitamin and mineral premix† | 1.00 | Leu | 1.41 |
| Phe | 0.81 | ||
| Val | 0.78 | ||
| Ca | 0.70 | ||
| Available phosphorus | 0.33 | ||
Premix per kg diet provided: iron 150 mg; copper 171.5 mg; zinc 109.5 mg; manganese 32 mg; selenium 0.45 mg; iodine 0.40 mg; choline, 500 mg; retinyl acetate, 11000 IU; cholecalciferol, 2000 IU; DL-α-tocopheryl acetate, 30 IU; menadione sodium bisulphite, 4.4 mg; thiamin mononitrate, 1.5 mg; riboflavin, 6 mg; pyridoxine hydrochloride, 3 mg; cyanocobalamin, 3.2 mg; D-pantothenic acid, 15 mg; nicotinic acid, 33 mg; D-biotin, 0.20 mg; folic acid, 1.65 mg.
Nutrient content of the diets were the value of measurement.
Collection of samples
At 63 days of the age, 2 h after the last meal, six piglets were randomly selected from each treatment (one pig from each pen) based on average body weight for sampling. Blood samples (10 mL) were obtained from the jugular vein into heparinized tubes, followed immediately by centrifugation at 3000 g for 10 min at 4°C. The supernatant fluid (plasma) was collected and immediately stored at −20°C for amino acids analysis. Immediately after blood sampling, piglets were anaesthetized with an intravenous injection of sodium pentobarbital (50 mg/kg BW) and bled by exsanguinations. The entire small intestine was then immediately collected and flushed with pre-cooled saline, to remove their contents. Intestinal mucosa was scraped from the underlying musculature with a glass microscope slide and was immediately frozen in liquid nitrogen and stored at −80°C until required for further analysis. Segments of duodenum, jejunum, and ileum (∼3 cm in length for each segment) were fixed in 4% paraformaldehyde for subsequent morphometric analysis.
Determination of glutamate oxaloacetate transaminase (GOT) and glutamate pyruvate transaminase (GPT) activities in jejunal mucosa
The activities of GOT and GPT in the jejunal mucosa were determined using commercially available kits (Nanjing Jiancheng Bioengineering Institute, Nanjing China) according to the manufacturer's instruction. Each treatment had six independent piglet samples. The assays were performed in triplicate for each sample, and the mean values of each samples was calculated for statistical.
Determination of plasma amino acids
Amino acids (AA) were determined following acid hydrolysis using a Hitachi L-8900 amino acid analyzer (Hitachi, Tokyo, Japan). Plasma AA contents were determined as previously described [24]. Briefly, 1 mL of plasma and 2.5 mL of 7.5% trichloroacetic acid were mixed thoroughly and centrifuged at 12,000×g at 4°C for 15 min. The supernatant was filtered through a 0.45 µm membrane and then analyzed for AA using an ion-exchange AA analyzer (Hitachi).
Examination of small-intestinal architecture
Paraformaldehyde-fixed duodenum, jejunum and ileum samples (3 cm) were embedded in paraffin and cut approximately 5 µm thick using a microtome and then stained with hematoxylin and eosin. In each section, villus height (determined as the distance from the villus tip to the crypt mouth) and their associated crypt depth (measured from the crypt mouth to the base) were measured using a light microscope (Nikon, Japan) with a computer-assisted morphometric system. The means of measurements was calculated to yield three values per piglet. These procedures were also processed by an observer unaware of the dietary treatments.
RNA extraction and cDNA synthesis
Jejunal mucosa sample was pulverized in liquid nitrogen. Total RNA was extracted from tissue samples using the TRIzol reagent (Invitrogen Company, Carlsbad, CA, USA) and treated with DNase I (Invitrogen Company) according to the manufacturer's guidelines. RNA was reverse transcribed to complementary DNA (cDNA) using a PrimeScript 1st Strand cDNA Synthesis Kit (Takara, Ostu, Japan) according to the manufacturer's protocol. Primers were designed with software (Oligo 7.0; Molecular Biology Insights, Cascade, CO) according to the gene sequence of pig to produce an amplification product ( Table 2 ).
Table 2. Primer pairs used in the RT-PCR.
| Genes | Accession no. | Primers | Sequences (5′→3′) |
| GLUD1 | NM_001244501.1 | Forward | ACCCACAGCAGAGTTCCAAG |
| Reverse | TCAGGTCCAGTCCCAGGTTA | ||
| GS | AY216477.1 | Forward | AGTGTGTTAGTGGGGAGGGA |
| Reverse | GCCATCCATTTACGCCGAAC | ||
| Occludin | NM_001163647.2 | Forward | GCTTTGGTGGCTATGGAAGT |
| Reverse | CCAGGAAGAATCCCTTTGCT | ||
| ZO-1 | XM_005659811.1 | Forward | GAGTTTGATAGTGGCGTT |
| Reverse | GTGGGAGGATGCTGTTGT | ||
| Claudin-1 | NM_001244539.1 | Forward | GCCCTACTTTGCTGCTCCT |
| Reverse | TTCTGGTTGTTCCCACACG | ||
| CaR | NM_001278748 | Forward | TGCCCAGATGACTTCTGGTCC |
| Reverse | GCACGAGATGCAGAGCACGAAGC | ||
| T1R1 | XM_003356140 | Forward | TCCCTGGGCTTCATACTGG |
| Reverse | TTCTCTGGCAAGTCCTTACCC | ||
| T1R3 | NM_001113288 | Forward | AGGAAATCAACAACGGATCG |
| Reverse | CTGCGTGTAGTCGCAGTAGG | ||
| mGluR1 | XM_005659163.1 | Forward | GAAGTGATGGATGGGCAGAC |
| Reverse | AACTCAGGGAACCAGGGATT | ||
| mGluR4 | XM_005665894.1 | Forward | CAAGACCAACCGCATCTACC |
| Reverse | GTCCACCACAAACCACACG | ||
| SLC1A1 | NM_001164649 | Forward | CAAACTGGGCCTTTAACTGG |
| Reverse | TGTTGCTGAACTGGAGGAGA | ||
| SLC6A19 | XM_003359855 | Forward | CACAACAACTGCGAGAAGGA |
| Reverse | CCGTTGATAAGCGTCAGGAT | ||
| SLC1A5 | XM_003355984 | Forward | GCCAGCAAGATTGTGGAGAT |
| Reverse | GAGCTGGATGAGGTTCCAAA | ||
| SLC7A9 | NM_001110171 | Forward | GAACCCAAGACCACAAATC |
| Reverse | ACCCAGTGTCGCAAGAAT | ||
| SLC7A7 | NM_001110421 | Forward | AGGAGAACCCACAGATTAGC |
| Reverse | GCGGAGGAGGAGAAGAA | ||
| β-actin | XM_003357928 | Forward | ATGCTTCTAGACGGACTGCG |
| Reverse | GTTTCAGGAGGCTGGCATGA |
All these primer sequences were designed based on the accession numbers described above.
GLUD1 glutamate dehydrogenase 1, GS glutamine Synthetase, ZO-1 zonula occludens protein-1, CaR Ca2+-sensing receptor, T1R1 taste receptor type 1 member 1, T1R3 taste receptor type 1 member 3, mGluR1 metabotropic glutamate receptor 1, mGluR4 metabotropic glutamate receptor 4, SLC1A1 solute carrier family 1 (neuronal/epithelial high affinity glutamate transporter, system Xag), member 1, SLC6A19 solute carrier family 6 (neutral amino acid transporter), member 19, SLC1A5 neutral amino acid transporter B0-like, SLC7A9 solute carrier family 7 (glycoprotein-associated amino acid transporter light chain, bo,+ system), member 9, SLC7A7 solute carrier family 7 (amino acid transporter light chain, y+L system), member 7.
Quantification of mRNA levels
The yield and quality of the RNAs were checked spectrophotometrically using OD260 and OD280 measurements (ND-100, NanoDrop Technologies, Rockland, DE). All samples had an OD260/OD280 ratios between 1.8 and 2.0. The integrity of the RNA preparations was verified by visualization of the 18 S and 28 S ribosomal bands stained with ethidium bromide after electrophoresis on 2% agarose gels (E-gel; Invitrogen Inc., Carlsbad, CA). Real time RT-PCR (SYBR Premix Ex Taq, catalogue no. RR420A) was performed with a total volume of 20 µL system containing 10 µL SYBR Premix Ex Taq (2×), 0.4 µL ROX Reference Dye (50×), 2.0 µL cDNA and 0.4 µL each of forward and reverse primers according to the instructions of manufacturer. PCR program consisted of one cycle at 95°C for 30 s, 40 cycles at 95°C for 5 s and 60°C for 34 s. A melting curve was conducted to verify the specificity of PCR amplified product. The mRNA abundance values for each sample were normalized using β-actin as an internal control according to the 2−ΔΔCT method [25].
Statistical Analysis
Data are expressed as means with SEM. Statistical analysis of all data was performed by independent-sample T-test with SPSS software (16.0, SPSS Inc., Chicago, USA). The individual piglet was considered the experimental unit for intestinal architecture, plasma amino acids and gene expression. All statements of significance were based on a probability of less than 0.05.
Results
GOT and GPT activities in jejunal mucosa
The activities of GOT and GPT in the jejunal mucosa of weaning piglets are shown in Table 3 . Compared with the control group, dietary L-glutamate supplementation increased (P<0.05) the activity of GOT in the jejunal mucosa. The activity of GPT in the jejunal mucosa did not differ between the groups.
Table 3. Effects of dietary L-glutamate supplementation on jejunal mucosa GOT and GPT in weaning piglets (n = 6).
| Items | Dietary treatments | SEM | P value | |
| Ala | Glu | |||
| GOT (U/g of protein) | 46.08 | 55.73 | 2.21 | 0.02 |
| GPT (U/g of protein) | 11.76 | 12.46 | 0.91 | 0.72 |
GOT, glutamate oxaloacetate transaminase, GPT glutamate pyruvate transaminase.
Ala alanine, Glu L-glutamate. SEM standard error of the means.
Ala was used as the control of isonitrogen balance.
Relative mRNA expression of jejunal mucosa glutamine synthetase (GS) and glutamine synthetase (GLUD1)
The abundances of GS and GLUD1 mRNAs in the jejunal mucosa of weaning piglets are shown in Fig. 1 . Dietary L-glutamate supplementation increased the mRNA expression of jejunal mucosa GS (P<0.05), but did not affect the mRNA expression of jejunal mucosa GLUD1.
Figure 1. Relative mRNA expression of jejunal moucosa glutamine synthetase (GS) and glutamate dehydrogenase 1(GLUD1).
Ala, diet supplemented with 12.1 g alanine/kg, Glu, diet supplemented with 20 g glutamate/kg. mRNA expression levels were normalized using β-actin as an internal control. Values are expressed as mean±SEM. a, bMean values within different letters were significantly different (P<0.05).
Concentrations of amino acids in plasma
The concentrations of plasma amino acids in weaning piglets are shown in Table 4 . Compared with the control group, dietary L-glutamate supplementation increased (P<0.05) the concentrations of arginine, histidine, isoleucine, leucine, methionine, phenylalanine, threonine valine, alanine, glutamate, glyine, proline, serine tyrosine and ornithine in the plasma.
Table 4. Effects of dietary L-glutamate supplementation on intestinal morphology in weaning piglets (n = 6).
| Items | Dietary treatments | SEM | P value | |
| Ala | Glu | |||
| Duodenum | ||||
| Villus height (µm) | 367.94 | 390.87 | 3.89 | 0.002 |
| Crypt depth (µm) | 224.69 | 240.95 | 3.93 | 0.037 |
| Villus height: Crypt depth | 1.65 | 1.63 | 0.02 | 0.762 |
| Jejunum | ||||
| Villus height (µm) | 355.10 | 376.23 | 4.45 | 0.015 |
| Crypt depth (µm) | 160.05 | 171.01 | 3.26 | 0.093 |
| Villus height: Crypt depth | 2.24 | 2.21 | 0.03 | 0.698 |
| Ileum | ||||
| Villus height (µm) | 309.04 | 304.67 | 3.19 | 0.502 |
| Crypt depth (µm) | 181.01 | 183.03 | 4.88 | 0.839 |
| Villus height: Crypt depth | 1.75 | 1.70 | 0.04 | 0.595 |
Ala alanine, Glu L-glutamate, SEM, standard error of the mean.
Ala was used as the control of isonitrogen balance.
Small intestinal architecture
Table 5 shows the small intestinal architecture of weaning piglets. Compared with the control, dietary L-glutamate addition significantly increased (P<0.05) duodenal villus height and crypt depth. Moreover, the jejunal villus height was also significantly increased (P<0.05) compared with the control. Feeding L-glutamate to weaning piglets did not significantly affected villus height or crypt depth in the ileum. Interestingly, compared with the control, there were no significant differences in the ratios of villus height or crypt depth in the entire small intestine.
Table 5. Effect of dietary L-glutamate supplementation on the concentrations plasma amino acid in weaning piglets (n = 6).
| Amino acid | Dietary treatments | SEM | P value | |
| Ala | Glu | |||
| Arg | 238.43 | 312.99 | 12.99 | <0.001 |
| His | 164.03 | 244.67 | 16.41 | 0.006 |
| Ile | 131.55 | 180.57 | 9.52 | 0.003 |
| Leu | 138.29 | 201.03 | 11.24 | 0.001 |
| Lys | 132.45 | 167.97 | 7.94 | 0.016 |
| Met | 167.20 | 243.63 | 15.68 | 0.006 |
| Phe | 102.24 | 134.34 | 7.20 | 0.017 |
| Thr | 143.40 | 189.77 | 14.05 | 0.100 |
| Val | 129.48 | 173.49 | 8.22 | 0.002 |
| Ala | 197.93 | 234.96 | 12.03 | 0.130 |
| Asp | 22.77 | 22.68 | 1.03 | 0.967 |
| Cys | 374.23 | 382.74 | 14.30 | 0.782 |
| Glu | 110.00 | 164.58 | 10.39 | 0.002 |
| Gly | 192.55 | 236.21 | 11.05 | 0.041 |
| Pro | 219.88 | 234.24 | 8.24 | 0.410 |
| Ser | 175.56 | 232.54 | 11.41 | 0.005 |
| Tyr | 145.28 | 214.48 | 12.53 | 0.001 |
| Orn | 131.83 | 172.07 | 6.73 | <0.001 |
| Cit | 160.08 | 186.13 | 4.76 | 0.001 |
Ala alanine, Glu L-glutamate, SEM standard error of the mean.
The unit of amino acid concentratin was nmoL/Ml.
Ala was used as the control of isonitrogen balance.
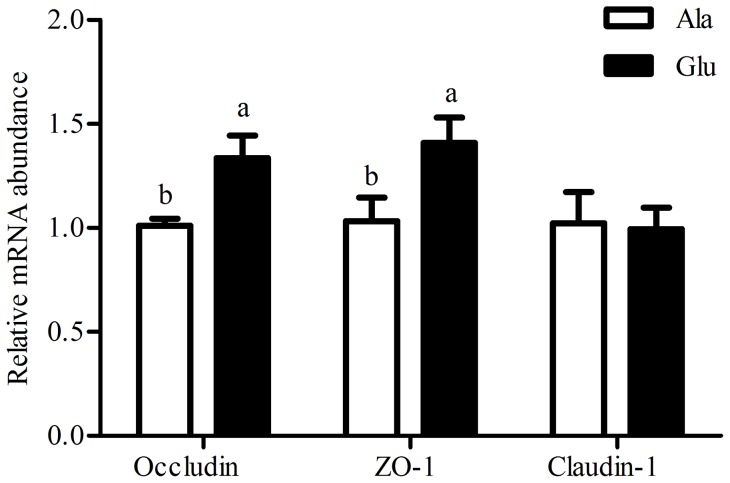
Relative mRNA expression of jejunal mucosa occludin, zonula occludens protein-1 (ZO-1) and claudin-1
The abundance of occludin, ZO-1 and claudin-1 in the jejunal mucosa are shown in Fig. 2 . Compared with the control, dietary L-glutamate supplementation significantly increased (P<0.05) mRNA expression of occludin and ZO-1 in the jejunal mucosa. However, L-glutamate supplementation in the diet of weaning piglets did not affect claudin-1 expression in the jejunum mucosa.
Figure 2. Relative mRNA expression of jejunal mucosa occludin, zonula occludens protein-1 (ZO-1), and claudin-1.
Ala, diet supplemented with 12.1 g alanine/kg, Glu, diet supplemented with 20 g glutamate/kg. mRNA expression levels were normalised using β-actin as an internal control. Values are expressed as mean ± SEM. a, bMean values within different letters were significantly different (P<0.05).
Relative mRNA expression of jejunal mucosa amino acid receptors and transporters
The abundance of amino acids (CaR, T1R1, T1R3, mGluR1 and mGluR4) and transporters (SLC1A5, SLC7A9 SLC6A19, SLC7A7 and SLC1A1) mRNAs in the jejunal mucosa of weaning piglets are shown in Table 6 . Compared with the control, dietary L-glutamate supplementation significantly increased (P<0.05) mRNA expression of CaR, mGluR1, mGluR4 and SLC1A5 in the jejunum mucosa. No significant differences in the mRNA expression of T1R1, T1R3, SLC7A9 SLC6A19, SLC7A7 and SLC1A1 were observed between treatments.
Table 6. Effect of dietary L-glutamate supplementation on Relative mRNA expression of jejunal mucosa amino acid receptors and transporters in weaning piglets (n = 6).
| Items | Dietary treatments | SEM | P value | |
| Ala | Glu | |||
| Receptors | ||||
| CaR | 1.19 | 1.63 | 0.10 | 0.017 |
| T1R1 | 1.16 | 1.20 | 0.11 | 0.870 |
| T1R3 | 1.06 | 1.04 | 0.09 | 0.885 |
| mGluR1 | 0.84 | 1.23 | 0.10 | 0.031 |
| mGluR4 | 0.97 | 1.28 | 0.08 | 0.046 |
| Transporters | ||||
| SLC6A19 | 1.14 | 3.44 | 0.35 | <0.001 |
| SLC7A9 | 1.26 | 1.14 | 0.07 | 0.399 |
| SLC1A5 | 1.02 | 0.91 | 0.05 | 0.346 |
| SLC7A7 | 1.13 | 1.02 | 0.06 | 0.447 |
| SLC1A1 | 1.19 | 1.01 | 0.06 | 0.774 |
Ala alanine, Glu L-glutamate, SEM standard error of the mean.
Ala was used as the control of isonitrogen balance.
mRNA expression levels of CaR, T1R1, T1R3, mGluR1, mGluR4, SLC6A19, SLC7A9, SLC1A5, SLC7A7 and SLC1A1 were normalized using β-actin as an internal control.
CaR Ca2+-sensing receptor, T1R1 taste receptor type 1 member 1, T1R3 taste receptor type 1 member 3, mGluR1 metabotropic glutamate receptor 1, mGluR4 metabotropic glutamate receptor 4, SLC1A1 solute carrier family 1 (neuronal/epithelial high affinity glutamate transporter, system Xag), member 1, SLC6A19 solute carrier family 6 (neutral amino acid transporter), member 19, SLC1A5 neutral amino acid transporter B0-like, SLC7A9 solute carrier family 7 (glycoprotein-associated amino acid transporter light chain, bo,+ system), member 9, SLC7A7 solute carrier family 7 (amino acid transporter light chain, y+L system), member 7.
Discussion
Previous studies in piglets and preterm infants have shown that dietary L-glutamate is the most important contributor to mucosal oxidative metabolism [4]–[6]. L-Glutamate catabolism occurs in the intestinal enterocyte by GOT, GPT and GLUD1 to produce L-alanine, L-aspartate and α-ketoglutarate [26]. α-Ketoglutarate produced by transamination can then enter the mitochondria, and its metabolism via the tricarboxylic acid (TCA) cycle produces reduced coenzymes (NADH, FADH2) used by the mitochondria for ATP synthesis. The intestinal epithelial cells have high ATP utilization, which is in rapid renewal and responsible for the nutrient absorption process [6], [10]. In this study, L-glutamate supplementation beneficially increased the activity of GOT and the mRNA expression of jejunal mucosa GS (Table 3; Fig. 1). These findings suggest that L-glutamate supplementation may increase the content of glutamine, α-ketoglutarate and L-aspartate in the jejunal mucosa of weaning piglets. L-Aspartate produced by L-glutamate transamination can enter mitochondria and can also be oxidized in the TCA cycle, thus representing another oxidative fuel for enterocytes [27]. Therefore, the central importance of L-glutamate as an oxidative fuel is that it can improve digestion and absorption function of weaning piglets.
The small intestine of weaning piglets is likely to be damaged if it cannot obtain sufficient nutrients to meet the demands for mucosal protein synthesis and growth. The results of the macroscopic observation and architecture evaluation of the small intestine further demonstrated that dietary L-glutamate supplementation improved intestinal abnormalities (Table 4). In accordance, a recent study has reported that dietary supplementation 1% to 4% monosodium glutamate increased jejunal villus height and crypt depth in weanling piglets [21]. More recently, Xiao et al., [28] found that glutamate supplementation could significantly prevent intestinal mucosal atrophy via promotion of intestine epithelial cell proliferation in total parenteral (TPN) mice. In addition, some previous studies demonstrated that the glutamate is also involved in the mucosal healing process by mediating proliferation of intestinal epithelial cells [7],[29]. Therefore, the improvement of small intestinal morphology in weanling piglets could be associated with the L-glutamate supplementation.
The cells of the intestinal epithelium are linked by several unique proteins, including the transmembrane protein occludin, junctional adhesion molecule [30], members of the claudin family [31], linker proteins such as ZO-1 [32], and others. The foremost and critical components in the structural and functional organ of the tight junctions are occludin, ZO-1 and claudin-1 [33]. Occludin is an integral membrane protein of the epithelial tight junction, having functional importance in maintaining the integrity and barrier function of the tight junction [34]. ZO-1 is an important linker protein in tight junctions and acts as a bridge between the plasma membrane and cytoskeleton proteins [33]. Claudin-1 appears fairly tightly localized to the expression of ZO-1 in the small intestine [35]. To better clarify the molecular mechanism for intestine architecture in weaning piglets fed L-glutamate, we determined the changes in mRNA expression of occludin, ZO-1 and claudin-1 in jejunum mucosa. Our results showed that L-glutamate supplementation significantly increased jejunum mucosa occludin and ZO-1 mRNA expression (Fig. 2). Evidence from animals and cell studies shows that glutamine is important for intestinal barrier function and regulation of tight junction protein [36], [37]. As well, prohibiting the conversion from glutamine to glutamate has been shown inhibit the tight junction protein expression enhance effect of glutamine [38], [39]. Thus, the addition of L-glutamate to diet provided sufficient energy for the jejunum to facilitate the cell-cell contact associated genes, including tight junction protein. In contrast, Xiao et al., [28] showed that L-glutamate supplementation significantly decreased occludin and ZO-1 protein expression in the total parenteral (TPN) mice. The difference if the results could be ascribed to the method of L-glutamate administration (feed intake VS oral administration). More investigations in vitro and in vivo are needed to further determine the effects of L-glutamate on tight junction protein expression and to clarify the exact role of L-glutamate in epithelial barrier function.
Amino acids are essential nutrients for protein synthesis and other metabolic functions in animals. An interesting observation from this study was that dietary supplementation with L-glutamate increased the plasma concentrations of a number of amino acids (Table 5). These amino acids included arginine, histidine, isoleucine, leucine, methionine, phenylalanine, threonine, valine, alanine, glutamate, glyine, proline, serine, tyrosine and ornithine. In accordance with some previous studies in animals and humans, the intake of L-glutamate increased the concentrations of certain essential amino acids in plasma [21], [40]. A study showed that 30–50% of nutritionally essential amino acids in the diet were degraded by the pig small intestine in the first pass, leading to a reduced efficiency of utilization of dietary protein [5]. It is possible that L-glutamate reduces the catabolism of these amino acids in the small intestine, enhancing their entry into the portal circulation.
The sensing of luminal contents is important in initiating the appropriate response of digestion and absorption of nutrients and plays an important role in host defense mechanisms [41]. It has been shown that the taste-sensing system plays a critical role in luminal sensing in the small intestinal tract, especially for luminal nutrient signals [16], [42]. The interaction between luminal nutrients and taste receptor through G-coupled proteins initiates various downstream pathways and exerts different effects on the body that depends on the combination of 3 taste receptors [43]–[45]. In our study, the higher expression of CaR, mGluR1 and mGluR4 in the jejunal mucosa of the dietary L-glutamate supplemented group (Table 6) was likely resulted from glutamate receptors which detected the ingested glutamate and transmitted this information to intestinal cells. Some studies have indicated that GPCRs, including T1R1/T1R3 and mGluR, regulate intestinal hormone secretion and intestinal nutrient absorption [46], [47]. Therefore, this process indicated that the high expression of glutamate receptor is involved in the jejunal control of protein digestion and absorption. However, changes in the up-regulated expression of glutamate receptors may participate in the intestinal hormone secretion in the piglets, but further experiments are required to test this hypothesis.
Dietary amino acid is absorbed via their transporter systems on the mucosal side of the small intestine. Different amino acid transporters have unique substrate specificities [48]. For example, transporters have been characterized that are specific for basic amino acids (SLC7A7), neutral amino acids (SLC6A19, SLC1A5 and SLC7A9) and acidic amino acids (SLC1A1) [17]. To date, the exact role of L-glutamate in modulating AA transporters expression has yet to be defined by the limited available data. In this research, dietary L-glutamate supplementation increased SLC1A5 mRNA expression in the jejunal mucosa (Table 6). L-Glutamate improved small intestine villi height and enhanced tight junction protein expression. Therefore, L-glutamate regulates the expression of amino acid transporters in the jejunal mucosa of weaning piglets by facilitating the repair of intestinal architecture. In addition, L-glutamate increased small intestine mucosa utilization of certain amino acid, such as arginine, lysine and branched-chain amino acids. Recently, some studies have reported that dietary supplementation with lysine or arginine regulated the intestine expression of amino acid transporters in weanling pig [49]–[51]. Therefore, whether L-glutamate directly or indirectly regulates amino acid transporters in jejunal mucosa is still unknown; further research will be required to elucidate the underlying mechanisms.
In conclusion, dietary L-glutamate supplementation improved intestinal integrity and increased amino acid receptors and transporters, including CaR, mGluR1, mGluR4 and SLC1A5, in the jejunal mucosa of weaning piglets, these changes are beneficial for the improvement of jejunal nutrient digestion and the function absorption process. Therefore, the findings of this study could help advance our knowledge of L-glutamate function but further research will be required to elucidate the underlying mechanisms.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
The present study was supported by National Key Basic Research Program of China (no. 2013CB127306). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Li X, Rezaei R, Li P, Wu G (2011) Composition of amino acids in feed ingredients for animal diets. Amino Acids 40: 1159–1168. [DOI] [PubMed] [Google Scholar]
- 2. Wu G, Knabe DA (1994) Free and protein-bound amino acids in sow's colostrum and milk. J Nutr 124: 415. [DOI] [PubMed] [Google Scholar]
- 3. Haynes TE, Li P, Li X, Shimotori K, Sato H, et al. (2009) L-Glutamine or L-alanyl-L-glutamine prevents oxidant-or endotoxin-induced death of neonatal enterocytes. Amino Acids 37: 131–142. [DOI] [PubMed] [Google Scholar]
- 4. Brosnan JT, Brosnan ME (2013) Glutamate: a truly functional amino acid. Amino Acids 45: 413–418. [DOI] [PubMed] [Google Scholar]
- 5. Burrin DG, Stoll B (2009) Metabolic fate and function of dietary glutamate in the gut. Am J Clin Nutr 90: 850S–856S. [DOI] [PubMed] [Google Scholar]
- 6. Blachier F, Boutry C, Bos C, Tomé D (2009) Metabolism and functions of L-glutamate in the epithelial cells of the small and large intestines. Am J Clin Nutr 90: 814S–821S. [DOI] [PubMed] [Google Scholar]
- 7. Reeds PJ, Burrin DG, Stoll B, Jahoor F (2000) Intestinal glutamate metabolism. J Nutr 130: 978S–982S. [DOI] [PubMed] [Google Scholar]
- 8. Beyreuther K, Biesalski HK, Fernstrom JD, Grimm P, Hammes WP, et al. (2007) Consensus meeting: monosodium glutamate–an update. Eur J Clin Nutr 61: 304–313. [DOI] [PubMed] [Google Scholar]
- 9. Riedijk MA, de Gast-Bakker DH, Wattimena JL, Van Goudoever JB (2007) Splanchnic oxidation is the major metabolic fate of dietary glutamate in enterally fed preterm infants. Pediat Res 62: 468–473. [DOI] [PubMed] [Google Scholar]
- 10. Grossmann J, Mohr S, Lapentina EG, Fiocchi C, Levine AD (1998) Sequential and rapid activation of select caspases during apoptosis of normal intestinal epithelial cells. Am J Physiol 274: G1117–G1124. [DOI] [PubMed] [Google Scholar]
- 11. Cartmell J, Schoepp DD (2000) Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem 75: 889–907. [DOI] [PubMed] [Google Scholar]
- 12. Kirchgessner AL (2001) Glutamate in the enteric nervous system. Curr Opin Pharmacol 1: 591–596. [DOI] [PubMed] [Google Scholar]
- 13. San Gabriel A, Nakamura E, Uneyama H, Torii K (2009) Taste, visceral information and exocrine reflexes with glutamate through umami receptors. J Med Invest 56: 209–217. [DOI] [PubMed] [Google Scholar]
- 14. Zhang J, Yin Y, Shu XG, Li T, Li F, et al. (2013) Oral administration of MSG increases expression of glutamate receptors and transporters in the gastrointestinal tract of young piglets. Amino Acids 45: 1169–1177. [DOI] [PubMed] [Google Scholar]
- 15. Akiba Y, Watanabe C, Mizumori M, Kaunitz JD (2009) Luminal L-glutamate enhances duodenal mucosal defense mechanisms via multiple glutamate receptors in rats. Am J Physil-Gastr L 297: G781–G791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Akiba Y, Kaunitz JD (2009) Luminal chemosensing and upper gastrointestinal mucosal defenses. Am J Clin Nutr 90: 826S–831S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kanai Y, Hediger MA (2003) The glutamate and neutral amino acid transporter family: physiological and pharmacological implications. Eur J Pharmacol 479: 237–247. [DOI] [PubMed] [Google Scholar]
- 18. Bröer S (2008) Amino acid transport across mammalian intestinal and renal epithelia. Physiol Rev 88: 249–286. [DOI] [PubMed] [Google Scholar]
- 19. Broer A, Wagner C, Lang F, Broer S (2000) The heterodimeric amino acid transporter 4F2hc/y+LAT2 mediates arginine efflux in exchange with glutamine. Biochem. J 349: 787–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thomas S, Prabhu R, Balasubramanian KA (2005) Surgical manipulation of the intestine and distant organ damage-protection by oral glutamine supplementation. Surgery 137: 48–55. [DOI] [PubMed] [Google Scholar]
- 21. Rezaei R, Knabe DA, Tekwe CD, Dahanayaka S, Ficken MD, et al. (2013) Dietary supplementation with monosodium glutamate is safe and improves growth performance in postweaning pigs. Amino Acids 44: 911–923. [DOI] [PubMed] [Google Scholar]
- 22. Feng Z, Zhou X, Wu F, Yao K, Kong X, et al. (2014) Both dietary supplementation with monosodium L-glutamate and fat modify circulating and tissue amino acid pools in growing pigs, but with little interactive effect. Plos One 9: e84533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.National Research Council (2012) Nutrient Requirements of Swine, 10th ed. 373 Washington, DC: National Academy Press.
- 24. Wu G, Knabe DA (1995) Arginine synthesis in enterocytes of neonatal pigs. Am J Physiol 269: R621–R629. [DOI] [PubMed] [Google Scholar]
- 25. Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and 2−ΔΔCT method.Methods. 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 26. Suryawan A, Hawes JW, Harris RA, Shimomura Y, Jenkins AE, et al. (1998) A molecular model of human branched-chain amino acid metabolism. Am J Clin Nutr 68: 72–81. [DOI] [PubMed] [Google Scholar]
- 27. Windmueller HG, Spaeth AE (1976) Metabolism of absorbed aspartate, asparagine, and arginine by rat small intestine in vivo. Arch Biochem Biophys 175: 670–676. [DOI] [PubMed] [Google Scholar]
- 28. Xiao W, Feng Y, Holst JJ, Hartmann B, Yang H, et al. (2014) Glutamate prevents intestinal atrophy via luminal nutrient sensing in a mouse model of total parenteral nutrition. Faseb J 28: 2073–2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Amagase K, Ochi A, Kojo A, Mizunoe A, Taue M, et al. (2011) New therapeutic strategy for amino acid medicine: prophylactic and healing promoting effect of monosodium glutamate against NSAID-induced enteropathy. J Pharmacol Sci 118: 131–137. [DOI] [PubMed] [Google Scholar]
- 30. Liu Y, Nusrat A, Schnell FJ, Reaves TA, Walsh S, et al. (2000) Human junction adhesion molecule regulates tight junction resealing in epithelia. J Cell Sci 113: 2363–2374. [DOI] [PubMed] [Google Scholar]
- 31. Morita K, Furuse M, Fujimoto K, Tsukita S (1999) Claudin multigene family encoding four-transmembrane domain protein components of tight junction strands. Proc Natl Acad Sci U S A 96: 511–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Stevenson BR, Siliciano JD, Mooseker MS, Goodenough DA (1986) Identification of ZO-1: a high molecular weight polypeptide associated with the tight junction (zonula occludens) in a variety of epithelia. J Cell Biol 103: 755–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM (1998) The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem 273: 29745–29753. [DOI] [PubMed] [Google Scholar]
- 34. McCarthy KM, Skare IB, Stankewich MC, Furuse M, Tsukita S, et al. (1996) Occludin is a functional component of the tight junction. J Cell Sci 109: 2287–2298. [DOI] [PubMed] [Google Scholar]
- 35. Holmes JL, Van Itallie CM, Rasmussen JE, Anderson JM (2006) Claudin profiling in the mouse during postnatal intestinal development and along the gastrointestinal tract reveals complex expression patterns. Gene Expr Patterns 6: 581–588. [DOI] [PubMed] [Google Scholar]
- 36. Li N, Neu J (2009) Glutamine deprivation alters intestinal tight junctions via a PI3-K/Akt mediated pathway in Caco-2 cells. J Nutr 139: 710–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Beutheu S, Ouelaa W, Guerin C, Belmonte L, Aziz M, et al. (2014) Glutamine supplementation, but not combined glutamine and arginine supplementation, improves gut barrier function during chemotherapy-induced intestinal mucositis in rats. Clin Nutr 33: 694–701. [DOI] [PubMed] [Google Scholar]
- 38. Nose K, Yang H, Sun X, Nose S, Koga H, et al. (2010) Glutamine prevents total parenteral nutrition-associated changes to intraepithelial lymphocyte phenotype and function: a potential mechanism for the preservation of epithelial barrier function. J Interf Cytok Res 30: 67–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vermeulen MA, de Jong J, Vaessen MJ, van Leeuwen PA, Houdijk AP (2011) Glutamate reduces experimental intestinal hyperpermeability and facilitates glutamine support of gut integrity. World J Gastroenterol 17: 1569–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Boutry C, Matsumoto H, Airinei G, Benamouzig R, Tomé D, et al. (2011) Monosodium glutamate raises antral distension and plasma amino acid after a standard meal in humans. Am J Physil-Gastr L 300: G137–G145. [DOI] [PubMed] [Google Scholar]
- 41. Nguyen CA, Akiba Y, Kaunitz JD (2012) Recent advances in gut nutrient chemosensing. Curr Med Chem 19: 28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Dockray GJ (2003) Luminal sensing in the gut: an overview. J Physiol Pharmacol 54: 9–17. [PubMed] [Google Scholar]
- 43. Conigrave AD, Brown EM (2006) Taste Receptors in the Gastrointestinal Tract II. l-Amino acid sensing by calcium-sensing receptors: implications for GI physiology. Am J Physil-Gastr L 291: G753–G761. [DOI] [PubMed] [Google Scholar]
- 44. Sternini C (2007) Taste receptors in the gastrointestinal tract. IV. Functional implications of bitter taste receptors in gastrointestinal chemosensing. Am J Physil-Gastr L 292: G457–G461. [DOI] [PubMed] [Google Scholar]
- 45. Mace OJ, Lister N, Morgan E, Shepherd E, Affleck J, et al. (2009) An energy supply network of nutrient absorption coordinated by calcium and T1R taste receptors in rat small intestine. J Physiol 587: 195–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liou AP (2013) Digestive physiology of the pig symposium: G protein-coupled receptors in nutrient chemosensation and gastrointestinal hormone secretion. J Anim Sci 91: 1946–1956. [DOI] [PubMed] [Google Scholar]
- 47. Mace OJ, Marshall F (2013) Digestive physiology of the pig symposium: gut chemosensing and the regulation of nutrient absorption and energy supply. J Anim Sci 91: 1932–1945. [DOI] [PubMed] [Google Scholar]
- 48.Wu G (2013) Amino acids: biochemistry and nutrition. CRC Press, Boca Raton.
- 49. He L, Yang H, Hou Y, Li T, Fang J, et al. (2013) Effects of dietary l-lysine intake on the intestinal mucosa and expression of CAT genes in weaned piglets. Amino Acids 45: 383–391. [DOI] [PubMed] [Google Scholar]
- 50. Yin J, Ren W, Duan J, Wu L, Chen S, et al. (2013) Dietary arginine supplementation enhances intestinal expression of SLC7A7 and SLC7A1 and ameliorates growth depression in mycotoxin-challenged pigs. Amino Acids 46: 883–892. [DOI] [PubMed] [Google Scholar]
- 51. Zhang S, Qiao S, Ren M, Zeng X, Ma X, et al. (2013) Supplementation with branched-chain amino acids to a low-protein diet regulates intestinal expression of amino acid and peptide transporters in weanling pigs. Amino Acids 45: 1191–1205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.