Abstract
Objective
The proinflammatory cytokine interleukin-18 (IL-18) putatively modulates food intake and energy metabolism, but the effects of IL-18 in high-fat diet fed animals are unknown. Whether IL-18 alters basal metabolic rate or metabolic processes of living is unknown. Here, we tested the hypothesis that IL-18 modulates weight gain, energy intake, whole-body energy expenditure, and utilization of lipid as a fuel substrate in high-fat diet fed mice.
Methods
Food intake, whole-body metabolism, and motor activity of IL-18 knockout mice were compared to those of wildtype littermates; anorectic effects of intracerebroventricular IL-18 administration were compared between IL-18 receptor knockout, IL-18/IL-18R knockout and wildtype mice.
Results
Chow-reared IL-18 knockout mice were overweight at 6 months of age and then gained excess weight on both low-fat and high-fat diets, ate more high-fat diet, and showed reduced whole-body energy expenditure and increased respiratory exchange ratios. Reductions in energy expenditure of IL-18 knockout mice were seen across fasting vs. feeding conditions, low- vs. high-fat diets, high vs. low levels of physical activity and times of day, suggesting actions on basal metabolic rate. The circadian amplitude of energy expenditure, but not respiratory exchange ratio, food intake, or motor activity, also was blunted in IL-18 knockout mice. Central IL-18 administration reduced high-fat diet intake in wildtype mice, but not in mice lacking the IL-18 receptor.
Conclusion
The loss-of-function results support the hypothesis that endogenous IL-18 suppresses appetite and promote energy expenditure and lipid fuel substrate utilization not only during sickness, but also in healthy adults consuming high-fat diets.
Keywords: obesity or overweight, energy expenditure or metabolism, food intake or appetite, proinflammatory cytokine, body weight, high-fat diet, interleukin-18, cosinor analysis or circadian rhythm, indirect calorimetry, locomotor or physical activity
Introduction
Better understanding the molecular controls of energy metabolism may inform the treatment of obesity. Interleukin-18 (IL-18), an 18 kDa multifunctional cytokine discovered for its proinflammatory and interferon-γ-inducing properties (Okamura et al., 1995), produces diverse effects via activation of the IL-18 receptor complex (Born et al., 2000; Torigoe et al., 1997), an IL-1/Toll-like superfamily receptor. Recent findings suggest that IL-18 may be a physiologic modulator of food intake and energy metabolism. Unlike classic proinflammtory cytokines that mediate the sickness response (e.g., IL-1β, IL-6, TNF-α), IL-18 also is constitutively expressed in non-immune cells and derived partly from adipocytes. Similar to other adipocytokines, its circulating levels relate to metabolic state, including fat mass, weight loss, hyperglycemia, and dietary fat intake (Esposito et al., 2002a; Esposito et al., 2002b; Esposito et al., 2003). Intracebrebroventricular or peripheral administration of IL-18 potently suppressed chow intake, feed efficiency and weight regain in fasted mice, without promoting sickness-like behavior (Zorrilla et al., 2007). Conversely, mice partially (Il18+/−) or totally deficient (IL18−/−) in IL-18 overate chow and purified low-fat diet by young adulthood (Zorrilla et al., 2007). Adult Il18−/− mice showed increased feed efficiency; indirect calorimetry revealed reduced energy expenditure in low-fat diet-fed female Il18−/− mice and increased respiratory exchange ratios (RER) (VCO2/VO2) in mutants of both sexes (Zorrilla et al., 2007). By mid-adulthood, Il18−/− mice became obese (Netea et al., 2006; Zorrilla et al., 2007). Similar delayed-onset obesity phenotypes were observed in IL-18 receptor knockout (KO) mice and in IL-18-binding protein overexpressing mice (Netea et al., 2006).
The present studies sought to determine the effects of the IL-18 null genotype in mice fed high-fat diet. Previous calorimetry studies in IL-18 KO mice were performed using low-fat diet (Zorrilla et al., 2007). Few humans eat low-fat diets, however, and the indirect calorimetric profile of IL-18 null mice is unknown. High-fat diets can produce different rates of energy expenditure as compared with low-fat diets (Bandini et al., 1994; Ebbeling et al., 2012), in relation to the different energy and macronutrient intakes elicited by each. High-fat diets also promote greater relative utilization of lipids as a fuel substrate vs. low-fat diets (McNeill et al., 1988; Rumpler et al., 1991; Verboeket-van de Venne et al., 1994). As a result of these differences, many studies of transgenic mice have observed strikingly different metabolic phenotypes with high-fat diet exposure (Gordon et al., 2008; Klockener et al., 2011; Kusudo et al., 2012; Lee et al., 2007; Paula et al., 2010; Strader et al., 2004; Sutton et al., 2006; Wortley et al., 2004; Zigman et al., 2005). Potentially consistent with a role for IL-18 in metabolic adaptations to high-fat diet, high-fat meals increase circulating IL-18 levels. Therefore, the present study tested the hypothesis that IL-18 null mutation also reduces whole-body energy expenditure and utilization of lipid as a fuel substrate in high-fat diet fed mice.
Energy expenditure can be subdivided into components that reflect the basal metabolic rate of minimally maintaining the organism as compared to phasic components of energy expenditure related to activities of living, including physical activity, thermic effects of food intake and adaptive thermogenesis (Even and Nadkarni, 2012). In our previous study of IL-18 KO mice, whole-body energy metabolism was studied in free-feeding mice, and the genotypes exhibited differences in food intake and motor activity (Zorrilla et al., 2007). Thus, it remains unclear whether phasic components of energy expenditure are responsible for the observed differences in total daily energy expenditure or whether IL-18 KO mice may exhibit a reduced basal metabolic rate. To differentiate between the hypotheses that basal metabolic processes vs. phasic metabolic processes (e.g., activity, absorptive phase of feeding) contribute to IL-18 genotype effects on total daily energy expenditure, the present study measured whole-body energy expenditure of IL-18 KO and wildtype mice under both fasting and feeding conditions within each of the dark cycle and light cycle. Concurrent motor activity was measured.
A third goal was to determine the circadian-dependence of the IL-18 phenotype on food intake and energy expenditure. In our initial study, hyperphagia of low-fat diet was most evident during the mid-to-late dark cycle and respiratory exchange ratios were observed at some, but not other, times of day. On the other hand, genotype differences in energy expenditure of low-fat diet-fed mice and circulating IL-18 levels were consistent across a 24-hr period (Zorrilla et al., 2007). Still, circadian variations in sensitivity to IL-18 might exist, as has been seen for IL-1β and IFN-γ (Opp and Toth, 1997). We therefore performed a cosinor analysis of chronobiologic differences in the food intake, energy expenditure, respiratory exchange ratios and motor activity of high-fat diet-fed IL-18 KO vs. wildtype mice.
A final pharmacological study sought to determine whether brain IL-18 systems also modulate the control of high-fat diet intake and the mediating role of the IL-18R therein.
Methods
Ethical approval
Procedures adhered to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of The Scripps Research Institute.
Subjects
Subjects were IL-18 knockout (KO; Il18−/−) mice generated on a C57BL/6J background (Takeda et al., 1998) (from Arturo Zychlinsky, New York University, NY), IL-18 receptor α-subunit KO mice (Il18R−/−), double IL-18/IL-18R KO mice (Il18−/−/Il18R−/−), and their wildtype (WT) littermates. Mature (24-26 weeks) female mice were studied because IL-18 KO mice do not show increased feed efficiency, weight gain and adiposity until maturity and because genotype effects observed previously were slightly greater in females than in males (Netea et al., 2006; Zorrilla et al., 2007). Before studies, mice were group-housed in a 12:12 hr light cycle, humidity- (60%) and temperature-controlled (22°C) vivarium with chow and water available ad libitum (Harlan Teklad LM-485, 3.1 kcal/g, 17% [kcal] fat, 58% carbohydrates, 25% protein, Harlan, Indianapolis, IN).
Experimental diets
During indirect calorimetry, mice received a powdered purified low-fat diet (Research Diets D12450B; 10% [kcal] fat, 70% carbohydrate, 20% protein, 3.85 kcal/g) or its matched, high-fat/high-energy purified diet (D12492; 60% fat, 20% carbohydrate, 20% protein, 5.24 kcal/g).
Indirect calorimetry
Indirect calorimetry was performed using a computer-controlled, open-circuit system (Oxymax; Comprehensive Lab Animal Monitoring System; Columbus Instruments, Columbus, OH). Mice were tested in individual clear chambers (20 × 10 × 12.5 cm) with a stainless steel elevated wire floor. Each chamber contained a sipper tube delivering water, food tray connected to a balance, and 16 photobeams at 1.3 cm intervals situated in rows 3.3 and 7.3 cm above the floor to detect motor activity along the x- and z-axes, respectively. Room air was passed through chambers at ~0.5 L/min. The chamber exhaust was sampled for 1 min (at 16-min intervals) and passed through O2 and CO2 sensors for estimation of oxygen consumption (VO2) and carbon dioxide production (VCO2). Outdoor air reference values were sampled every 8 measurements. Gas sensors were calibrated before experiments with gas standards containing known concentrations of O2, CO2, and N2 (Airgas Puritan Medical, Ontario, CA). Respiratory exchange ratio (RER) was calculated as the ratio of carbon dioxide production (VCO2) to oxygen consumption (VO2). Energy expenditure (heat formation [(3.815 + 1.232*RER)*VO2 (in liters)]) was corrected for estimated metabolic mass per Kleiber’s power function (Kleiber, 1975), as were differences in food intake. As an alternative correction, raw energy expenditure estimates also were subjected to analysis of covariance (ANCOVA), covarying for body weight (Arch et al., 2006).
Study design
Mice were acclimated in the calorimetry apparatus with low-fat diet and water for 5 days before the present studies. Then, mice were fasted within the calorimetry apparatus for 3 hr beginning from the onset of the dark cycle, after which low-fat diet was returned and food intake, locomotor activity and metabolism were monitored for another 3 hr. Following refeeding and normalization of intake, the fast-refeed procedure was repeated, but beginning instead from the light cycle onset. Subsequently, mice were acclimated to the high-fat diet for 4 days, after which 24-hr of uninterrupted data were collected beginning from the dark cycle onset. Thereafter, dark- and light-cycle data were collected using the fast-refeed protocol as per the low-fat diet studies. Studies were performed under ambient room temperature (~24-26°C) with water available. Mice were weighed using a scale of 0.1 g precision every 1-2 days during the study.
Intracerebroventricular IL-18 infusion and high-fat diet intake
The present study sought to determine whether IL-18 might centrally modulate the control of high-fat intake and the requirement for the IL-18R in these effects. Mice lacking the IL-18R (Il18R−/− and Il18−/−/Il18R−/−) and their wildtype littermates were acclimated to high-fat diet and then, under isoflurane anesthesia, stereotactically implanted with a unilateral cannula targeting the lateral ventricle as described previously (Zorrilla et al., 2007). After 1 week recovery, individually-housed mice were food-deprived for 24 hr and then received vehicle (2 μl saline) or IL-18 (2 nmol/2 μl, injected over 4 minutes) 5 min before they received renewed access to pre-weighed high-fat diet access at the dark cycle onset. Treatments were given in a counterbalanced, within-subjects design, spaced by 11 days.
Statistics
Analyses of variance (ANOVA) or Student’s t-tests were used for comparisons involving >2 or exactly 2 levels, respectively. To analyze energy expenditure, RER and motor activity in the fast-refeed studies, a 4-way repeated-measures ANOVA was used. Within-subject factors were Feeding Condition (2: Fasting vs. Feeding), Diet (2: Low-fat vs. High-fat), and Cycle (2: Dark vs. Light). Genotype (2: IL-18 KO vs. WT) was a between-subjects factor. Energy intake during refeeding was analyzed by 3-way repeated-measures ANOVA with Diet and Cycle as within-subject factors and Genotype as a between-subjects factor. For the 23-hr ad lib high-fat diet study, t-tests were used to assess genotype differences in daily (23-hr) energy intake, energy expenditure, RER and motor activity. To compare the circadian time course of these measures, 3-way repeated measures ANOVA was used on 3-hr bin averages; Cycle (Dark vs. Light) and Time Bin (1-3, 4-6, 7-9 and 10-12 hr of each cycle) were within-subject factors, and Genotype was a between-subjects factor. Cosinor analysis was used to determine how genotype altered the daily chronobiological rhythms of energy intake and metabolism (Chen et al., 2006). The following circadian attributes were calculated: the midline estimating statistic of rhythm (MESOR; mean level around which the cosine function oscillates), amplitude [the distance from the MESOR to the extremes [peak and nadir] of the oscillation], and the acrophase (the time at which the ci peak occurs relative to a time of interest, in this case the start of the session/dark cycle). A predefined period of 24 h was used per the following equation:
Supplemental Figure 1 illustrates the cosinor analysis measures. Cosinor functions were fit individually to obtain the MESOR, amplitude, acrophase, and goodness of fit (r) for each mouse. Peaks were calculated as the MESOR + amplitude; nadirs were calculated as the MESOR − amplitude. Genotype differences in these parameters were evaluated by t-test. Parameters were cumulated into 3-h bins to facilitate modeling of the hypothesized underlying circadian rhythm. Mixed design, two-way ANOVA was used to determine the effects of IL-18 treatment (within-subjects factor), in interaction with Genotype (between-subjects factor), on 6-hr high-fat diet intake. The software used was Systat 12.0 (SPSS, Chicago, IL) and DataFit 9.0 (Oakdale Engineering, Oakdale, PA).
Results
Body weight
As Table 1 shows, age-matched, chow-reared female Il18−/− mice weighed 32% (7.5 g) more than WT mice at the study onset. By completion of the high-fat diet studies, IL-18 KO weighed 50% (11.4 g) more than WT controls. A Genotype main effect indicated that IL-18 KO mice gained more weight than WT mice (F1,11=10.04, p< 0.01), irrespective of the diet available (Genotype × Diet: F1,11=0.76, p>0.40). A Diet main effect reflected that mice of both genotypes gained weight faster during the high-fat diet, than low-fat diet, phase of the study (F1,11=33.38, p<0.0001) (see Table 1).
Table 1.
Interleukin-18 genotype differences in body weight and weight change across the study period
| Body weight measurement | IL-18 knockout (n = 7) | Wildtype control (n = 6) |
|---|---|---|
| Low-fat diet phase | ||
| Onset (g) | 30.6 ± 2.2 * | 23.1 ± 1.1 |
| Completion (g) | 30.3 ± 2.2 ** | 21.4 ± 0.6 |
| Net daily change (dg/day) | −0.6 ± 0.8 ## | −3.5 ± 1.2 |
| High-fat diet phase | ||
| Onset (g) | 32.0 ± 2.0 ** | 23.1 ± 0.8 |
| Completion (g) | 34.1 ± 2.3 *** | 22.7 ± 0.7 |
| Net daily change (dg/day) | 3.5 ± 0.7 ##, 1 | −0.5 ± 0.8 1 |
Body weight progression of adult female Il18−/− and wildtype littermate mice across the 5-day low-fat diet phase and 6-day high-fat diet phase of the study. Data are expressed as M ± SEM. Superscripts denote significant differences from wildtype controls
p < 0.05,
p < 0.005,
p = 0.001 (Student’s t-test),
p < 0.01 (main effect of Genotype) and from respective low-fat diet measure
p < 0.001 (main effect of Diet).
Fast-refeed protocol
Food intake
Energy intake during the 3-hour refeeding periods did not differ per main effects or interactions that involved Genotype or Diet (all ps>0.1; Genotype vs. WT [M±SEM kcal] for low-fat dark cycle, 2.61±0.11 vs. 3.06±0.33, light cycle, 2.84±0.26 vs. 2.89±0.28; for high-fat dark cycle, 2.79±0.56 vs. 3.05±0.45, light cycle, 2.71±0.34 vs. 3.44±0.50).
Energy expenditure
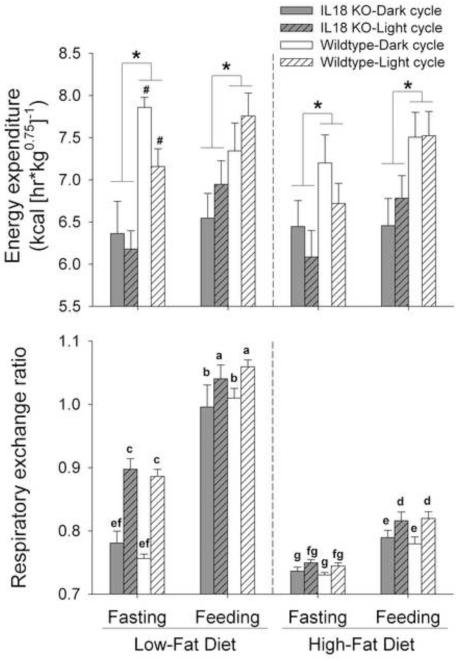
Figure 1 shows that, across times of day, diets and feeding state, female Il18−/− mice expended less energy than WT littermates (Genotype: F1,11=6.01, p<0.05). A trend for a Genotype × Diet × Fasted interaction (F1,11=4.51, p=0.057) indicated that the magnitude of IL-18 genotype differences in heat tended to vary in relation to the diet and feeding state of the mice. Genotype differences in energy expenditure tended to be even greater in mice fasted from low-fat diet, than in those fasted from high-fat diet (Genotype × Diet in fasted mice, p=0.07). Specifically, WT mice showed greater energy expenditure when fasted from low-fat diet, than from high-fat diet (p=0.05), a difference not evident in IL-18 KO mice (p=0.97). In contrast, genotype differences were of similar magnitude in the fed state between diet conditions. No other interactions involving Genotype approached significance (all ps>0.11). Main effects indicated that fasting (F1,11=19.62, p<0.001) and high-fat diet (F1,11=4.75, p=0.05) independently reduced whole-body heat expenditure vs. their respective fed and low-fat diet conditions (see Figure 1).
Figure 1.
Energy expenditure and respiratory exchange ratios in interleukin-18 knockout (IL-18 KO) and wildtype (WT) mice during brief fast-refeed conditions. Data express M (+SEM) weight-normalized energy expenditure (Panel A) and respiratory exchange ratios (B) of mature female mice measured across a 3-hr fasting period and a subsequent 3-hr refeeding period. In each subject, the fast-refeed protocol was performed during both the dark and light cycle and while receiving low-fat or high-fat diet. Panel A shows that IL-18 KO mice (n = 7) expended less energy than WT mice (n = 6) across all conditions, indicated by a main effect of Genotype; a Genotype × Diet × Fasted interaction reflected that the difference was most pronounced when mice were fasting from low-fat diet. Not denoted with symbols, main effects indicated that mice expended less energy when fasting (than feeding) and when maintained on high-fat (than low-fat) diet. Panel B shows that Genotype differences in respiratory exchange ratios were not evident in the context of large group differences that resulted from main and interaction effects of Fasting, Cycle, and Diet (see Results). *p < 0.05, main effect of Genotype, #p < 0.05 vs. respective high-fat condition. In panel B, groups with different letter symbols differ from another, as determined via post hoc Fisher’s protected least significant difference (LSD) tests.
Respiratory exchange ratio
Figure 1 shows the large main effects of Diet (F1,11=339.57, p<0.001), Light Cycle (F1,11=64.46, p<0.001) and Fasting (F1,11=334.00, p<0.001) on respiratory exchange ratios (RER). Consistent with greater relative utilization of fat as a fuel substrate, lower RERs were seen during fasting, the high-fat diet condition, and the early dark cycle (i.e., after the light cycle, during which comparatively little is eaten). Diet × Cycle (F1,11=36.16, p<0.001), Diet × Fasting (F1,11=165.94, p<0.001), Fasting × Cycle (F1,11=4.65, p=0.05) and Diet × Fasting × Cycle interactions (F1,11=19.15, p<0.001), reflected that: 1) refeeding on low-fat diet increased RER more than did refeeding on high-fat diet, 2) RERs of mice fasting from low-fat diet were not as low as those of mice fasting from high-fat diet, and 3) the latter difference was greater at the beginning of the light cycle, when mice had just completed their active (feeding) cycle. Genotype-related differences in RER were not detected in the context of these large effects (all ps>0.15) (see Figure 1).
Motor activity
Table 2 shows horizontal and vertical motor activity of mice during calorimetry testing. Motor activity in both dimensions was greater during the dark cycle, than light cycle, and especially during the fasting (vs. feeding) phases of testing (Fasting × Cycle: Fs1,11>8.40, ps<0.015; Cycle: Fs1,11>20.37, ps<0.001). However, a substantial Diet × Fasting interaction reflected that motor activity was greater during the feeding than fasting period in high fat diet-fed mice, whereas the reverse was true in low fat diet-fed mice (Fs1,11>20.94, ps<0.001); this pattern was significantly more prominent in WT mice than in IL-18 KO mice (Genotype × Diet × Fasting: Fs1,11>5.08, ps<0.05). Genotype effects on motor activity could not (fully) account for differences in energy expenditure, because the latter were still present even during time bins that IL-18 KO mice moved as much as or more than WT mice (see Table 2).
Table 2.
Motor activity of interleukin-18 knockout (IL-18 KO) and wildtype mice during the fast-refeed protocol
| Horizontal Activity | Vertical Activity | |||||||
|---|---|---|---|---|---|---|---|---|
| Fasting | Feeding | Fasting | Feeding | |||||
| Low-Fat | High-Fat | Low-Fat | High-Fat | Low-Fat | High-Fat | Low-Fat | High-Fat | |
| Dark cycle | ||||||||
| IL-18 KO | 25.3 ± 2.9 | 20.0 ± 2.5 | 17.5 ± 2.5 | 18.1 ± 1.9 | 5.2 ± 1.3 | 4.5 ± 1.7 | 3.3 ± 1.0 | 4.0 ± 1.5 |
| Wildtype | 31.8 ± 5.8*** | 19.4 ± 2.7 | 13.2 ± 2.3 | 23.9 ± 2.2## | 6.5 ± 1.5** | 3.6 ± 0.7 | 2.0 ± 0.6 | 4.5 ± 0.5# |
| Light cycle | ||||||||
| IL-18 KO | 6.0 ± 1.2 | 9.7 ± 0.9 | 6.0 ± 0.9 | 15.3 ± 1.7 | 1.4 ± 0.3 | 1.4 ± 0.4 | 1.6 ± 0.3 | 1.8 ± 0.4 |
| Wildtype | 6.1 ± 1.7*** | 10.6 ± 1.2 | 6.0 ± 1.0 | 16.7 ± 2.4## | 2.0 ± 0.7** | 0.9 ± 0.2 | 2.2 ± 0.6 | 2.9 ± 0.8 |
Data express M (±SEM) photocell interruptions (x100) in the x-dimension (horizontal activity) or z-dimension (vertical activity) in mature female IL-18 knockout (KO) mice (n = 7) and their wildtype controls (n = 6) during a 3-hr fasting period and a subsequent 3-hr refeeding period. In each subject, the fast-refeed protocol was performed during both the dark and light cycle and while receiving low-fat or high-fat diet. Potential genotype effects on motor activity could not account for differences in energy expenditure, because mice expended less energy even during periods when IL-18 KO mice moved as much as or more than WT mice. The only significant effect involving Genotype was a Genotype × Diet × Fasting interaction. Post hoc deconvolution of this interaction, shown as symbols in the Table, indicated that wildtype mice moved significantly less during the feeding vs. fasting period in low-fat diet testing, but more in the feeding vs. fasting period during high-fat diet testing, and the corresponding differences were smaller or absent in IL-18 KO mice. Irrespective of genotype, mice moved significantly more during the dark cycle (than light cycle), especially during the fasting (vs. feeding) phase of testing (see Results for statistics). Symbols represent
p < 0.01,
p < 0.001 greater than wildtype mice fasting from high-fat diet or feeding on low-fat diet;
p < 0.05,
p < 0.005 greater than wildtype mice feeding on low-fat diet or fasting from high-fat diet.
23-hr ad libitum high-fat diet protocol
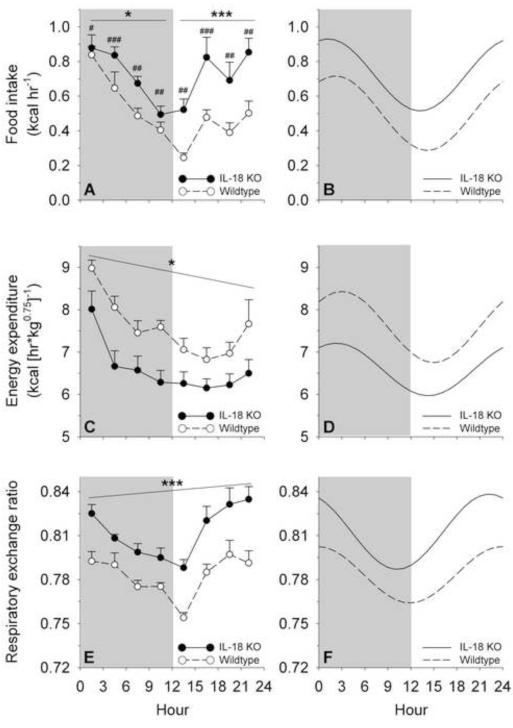
Table 3 shows that during the 23-hr ad libitum high-fat diet study, female Il18−/− mice ate 44% more high-fat diet (t11=4.18, p<0.005), expended 18% less energy (t11=−2.47, p<0.05), and showed a significantly increased mean RER (t11=5.17, p<0.0005) vs. WT mice. Il18−/− mice still ate more even after controlling for differences in non-fat body weight (1.47+0.08 vs. 1.23+0.06 kcal/g0.75, p<0.05). There were no genotype differences in total horizontal or vertical motor activity (t11=−0.88 and 0.29, respectively; ps>0.4) (see Table 3). Figure 2 shows the observed (panels A, C, E) and cosinor-fitted (B, D, F) time course of food intake and energy metabolism measures during the study. Table 3 shows the parameters of the cosinor functions, which fit the observed data well, especially beginning ~2 hr into the session, by when effects of initiating the session had dissipated.
Table 3.
Interleukin-18 (IL-18) knockout and high-fat diet intake, metabolism and motor activity
| Measure | IL-18 knockout (n = 7) | Wildtype control (n = 6) |
|---|---|---|
|
| ||
| High-fat diet intake, kcal | 16.5 ± 1.0 ** | 11.5 ± 0.6 |
| Cosinor parameters | ||
| MESOR, kcal hr−1 | 0.72 ± 0.04 ** | 0.50 ± 0.03 |
| Amplitude, kcal hr−1 | 0.21 ± 0.01 | 0.21 ± 0.02 |
| Peak, kcal hr−1 | 0.93 ± 0.05 ** | 0.72 ± 0.03 |
| Nadir, kcal hr−1 | 0.51 ± 0.05 ** | 0.29 ± 0.03 |
| Acrophase, hr | 1.2 ± 1.2 | 2.1 ± 0.5 |
| Goodness-of-fit, r | 0.70 ± 0.07 | 0.76 ± 0.03 |
|
| ||
| Total energy expenditure, kcal/kg0.75 | 151.5 ± 6.9 * | 174.1 ± 5.7 |
| Cosinor parameters | ||
| MESOR, kcal (hr*kg0.75)−1 | 6.59 ± 0.30 * | 7.59 ± 0.26 |
| Amplitude, kcal (hr*kg0.75)−1 | 0.62 ± 0.08 * | 0.84 ± 0.05 |
| Peak, kcal (hr*kg0.75)−1 | 7.22 ± 0.38 * | 8.42 ± 0.28 |
| Nadir, kcal (hr*kg0.75)−1 | 5.97 ± 0.23 * | 6.75 ± 0.25 |
| Acrophase, hr | 2.3 ± 0.5 | 3.0 ± 0.5 |
| Goodness-of-fit, r | 0.72 ± 0.04 | 0.78 ± 0.02 |
|
| ||
| Respiratory exchange ratio | 0.812 ± 0.005 *** | 0.783 ± 0.003 |
| Cosinor parameters | ||
| MESOR | 0.813 ± 0.005 *** | 0.783 ± 0.004 |
| 0.783 ± 0.004 | 0.026 ± 0.003 | 0.019 ± 0.002 |
| Peak | 0.838 ± 0.006 *** | 0.803 ± 0.006 |
| Nadir | 0.787 ± 0.006 *** | 0.764 ± 0.002 |
| Acrophase, hr | −1.8 ± 0.8 | −0.1 ± 0.6 |
| Goodness-of-fit, r | 0.86 ± 0.04 * | 0.73 ± 0.03 |
|
| ||
| Horizontal motor activity, beam breaks |
57,879 ± 5189 | 64,189 ± 4879 |
|
| ||
| Vertical motor activity, beam breaks | 15,337 ± 3340 | 14,215 ± 1566 |
Daily and cosinor parameters of food intake, energy expenditure, respiratory exchange ratio and motor activity of female Il18−/− mice and wildtype littermate tested across 23 hr in a CLAMS indirect calorimetry system. Please see Methods for definition of cosinor parameters. Data express M ± SEM. Symbols denote differences from wildtype controls
p < 0.05,
p < 0.005,
p < 0.0005 (Student’s t-test).
Figure 2.
Circadian time course of food intake, energy expenditure and respiratory exchange ratio in high-fat diet-fed interleukin-18 knockout (IL-18 KO) and wildtype (WT) mice. Data express M (+SEM) rates of food intake (Panel A), weight-normalized energy expenditure (C) and average respiratory exchange ratios (E) of mature female mice averaged across 3-hr bins during a daily observation period in indirect calorimetry chambers. Panels B, D and F show the respective cosinor functions, interpolated from the observed data; cosinor parameters are presented in Table 3. The grey shading indicates the dark cycle. Panels A and B illustrate that IL-18 KO mice ate significantly more high-fat diet than WT mice; this difference was even greater during the light cycle than dark cycle and was reflected in an increased circadian MESOR, peak and nadir. Genotype differences pooled across the cycle *p = 0.06, ***p < 0.0001 (Genotype × Cycle interaction followed by Fisher’s protected least significance difference tests); pooled across corresponding bins #p < 0.05, ##p < 0.005, ###p < 0.001 (Genotype × Bin interaction followed by Fisher’s protected least significance difference tests). Panels C and D show that IL-18 KO mice expended significantly less energy than WT mice across the entire day and also had a blunted circadian rhythm; these differences were reflected in a reduced circadian amplitude, MESOR, peak and nadir. *p < 0.05, Genotype main effect. Panels E and F show that IL-18 KO mice had greater mean respiratory exchange ratios than WT mice across the entire light/dark cycle, differences that were reflected in an increased circadian MESOR, peak and nadir. ***p < 0.001, Genotype main effect. Genotypes did not differ in the acrophase of rhythms.
Food intake
Time course analysis showed that IL-18 KO mice ate more high-fat diet than WT mice (Genotype: F1,11=16.52, p<0.002), especially during the light cycle (Genotype × Cycle: F1,11=7.74, p<0.02) and at particular times within a cycle (Genotype × Bin: F3,33=5.42, p<0.005). IL-18 KO mice had a greater MESOR, peak and nadir of their circadian rhythm for high-fat diet intake vs WT mice (ts11=4.08, 3.86 and 3.47, respectively, ps<0.005). No genotype difference was seen in the amplitude of the food intake rhythm (t11=−0.25, p>0.80). As expected, mice ate less during the light cycle than dark cycle (F1,11=7.44, p=0.02), and intake declined across the dark cycle and increased across the light cycle (linear contrast Cycle × Bin: F1,11=81.28, p<0.001). The feeding rhythm of both genotypes was adequately fit by the cosinor function and had a circadian peak (acrophase) ~1-2 hr into the dark cycle (see Figure 2A-B and Table 3).
Energy expenditure
IL-18 KO mice expended less energy than WT mice (Genotype: F1,11=5.97, p<0.05), irrespective of the cycle (Genotype × Cycle: F1,11=1.90, p>0.19), the time bin within the cycle (Genotype × Bin: F3,33=2.30, p>0.09), and their 3-way interaction (Genotype × Cycle × Bin: F3,33=1.33, p>0.28). IL-18 KO mice not only had a reduced MESOR (t11=−2.45, p<0.05), peak (t11=−2.32, p<0.05), and nadir (t11=−2.16, p=0.05) of their circadian rhythm for energy expenditure, but also a reduced amplitude (t11=−2.16, p=0.05). Genotype differences were not seen in the timing of the circadian peak for heat, which occurred ~2-3 hr into the dark cycle for both genotypes (acrophase: t11=−1.00, p>0.3), or in the goodness-of-fit of the cosinor function (t11=−0.99, p>0.3) (see Figure 2C-D and Table 3). As expected, energy expenditure was lower during the light cycle than dark cycle (F1,11=48.89, p<0.001) and decreased across the dark cycle and increased across the light cycle (linear contrast Cycle × Bin: F1,11=110.83, p<0.001).
IL-18 KO mice also expended less energy if ANCOVA, covarying for body weight, was used as the method of analysis. Differences manifested in Genotype × Cycle × Bin (F3,30=5.27, p<0.005) and Genotype × Cycle interactions (F1,10=4.54, p=0.05). The interactions again reflected the blunted circadian amplitude of energy expenditure of IL-18 KO mice, with reduced energy expenditure rate evident throughout the dark cycle (weight-adjusted lsmeans + standard error for KO vs.WT, Hr 1-3: 0.61+0.06 vs. 0.47+0.06, Hr 4-6: 0.54+0.05 vs. 0.40+0.05, Hr 7-9: 0.50+0.05 vs. 0.39+0.05, Hr 10-12: 0.52+0.05 vs. 0.36+0.05 kcal hr−1).
Respiratory exchange ratio
IL-18 KO mice also consistently had higher RERs than WT mice (Genotype: F1,11=24.88, p<0.001), irrespective of the cycle (Genotype × Cycle: F1,11=1.89, p>0.19), the time bin within the cycle (Genotype × Bin: F3,33=0.28, p>0.83), or their 3-way interaction (Genotype × Cycle × Bin: F3,33=0.85, p>0.47). IL-18 KO mice had a greater MESOR, peak and nadir of their circadian rhythm for RERs as compared with WT mice (ts11=4.68, 4.32 and 3.51, respectively, ps<0.005). However, unlike the rhythm for heat, no genotype difference was seen in the amplitude of RER rhythm (t11=1.53, p>0.15). The RER rhythm of both genotypes was adequately fit by the cosinor function and had an acrophase just before the onset of the dark cycle (see Figure 2E-F and Table 3). As expected, RERs declined across the dark cycle and increased across the light cycle (linear contrast Cycle × Bin: F1,11=86.48, p<0.001).
Motor activity
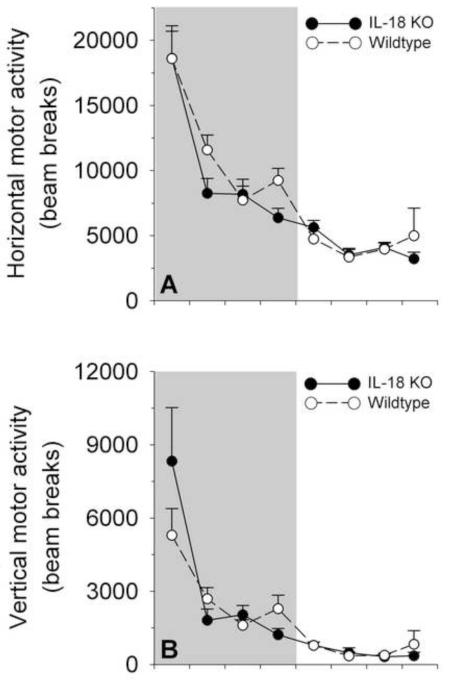
Figure 3 shows that IL-18 KO mice did not differ reliably from WT mice in horizontal or vertical motor activity, as evidenced by nonsignificant main and interaction effects that involved genotype (all ps>0.07). As expected, motor activity was greater during the dark (vs. light) cycle (Cycle: F1,11=97.76, p<0.001), and varied within cycles as a function of time (Bin: F1,11=97.76, p<0.001). Because genotype effects were not seen, cosinor analyses were not performed.
Figure 3.
Circadian time course of motor activity in high-fat diet-fed interleukin-18 knockout (IL-18 KO) and wildtype (WT) mice. Data express M (+SEM) photocell interruptions in the x-dimension (horizontal activity, Panel A) or z-dimension (vertical activity, Panel B) by mature female mice averaged across 3-hr bins during a daily observation period in indirect calorimetry chambers. The grey shading indicates the dark cycle. Panels A and B illustrate that no significant main or interaction effects that involved Genotype were observed on motor activity measures. As expected, irrespective of genotype, motor activity was greater during the dark (vs. light) cycle and decreased across the dark cycle, but not light cycle (see Results for statistics).
Central IL-18 infusion reduces high-fat diet intake via an IL-18R dependent mechanism
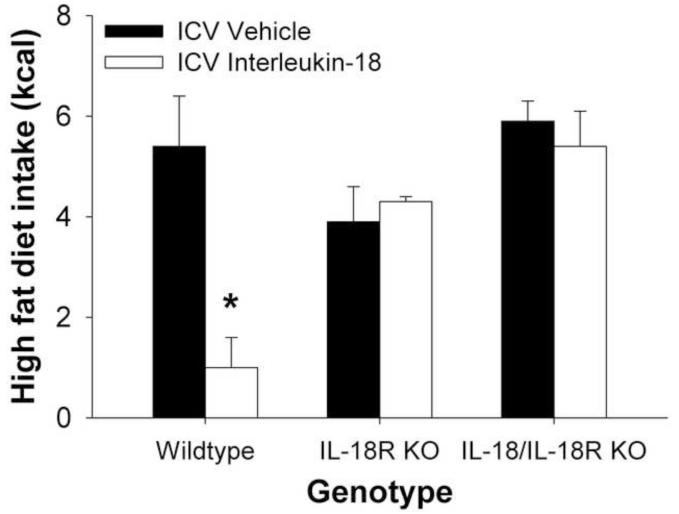
Finally, as shown in Figure 4, acute intracerebroventricular infusion of IL-18 reduced 6-hr high-fat diet intake in fasted wildtype mice, but not in Il18R−/− mice or Il18−/−/Il18R−/− mice, as reflected in significant Genotype × Dose (F2,12=4.03, p<0.05) and Genotype (F2,12=8.83, p<0.005) effects.
Figure 4.
Acute intracerebroventricular administration of interleukin-18 (2 nmol/2 μl) reduced 6-hr, nocturnal high-fat diet intake in 24-hr fasted wildtype mice, but not in transgenic knockout mice that lacked the interleukin-18 receptor alpha subunit (Il18R−/−−/− or Il18−/−/Il18R−/− mice), yielding a significant Genotype × Dose effect. Data express M (+SEM) high-fat diet energy intake. *p < 0.05, differs from respective vehicle-treated condition.
Discussion
The present study confirms that IL-18 deficiency promotes positive energy balance in healthy female mice across times of day and dietary feeding conditions. Chow-reared female IL-18 KO mice were overweight at ~6 months of age, gained excess weight on both low-fat and high-fat purified diets, ate more high-fat diet, and showed reduced whole-body energy expenditure and decreased relative utilization of lipid as a fuel substrate vs. WT mice. The results further implicate endogenous IL-18 as a catabolic regulator of food intake and energy metabolism during adulthood.
Reductions in energy expenditure of IL-18 KO mice were seen across feeding conditions (fasting, refeeding, ad libitum) and diets (low-fat, high-fat), newly demonstrating that at least some component of the reductions are independent of thermic effects of feeding. Differences in energy expenditure were evident using Kleiber power-function (Kleiber, 1975) or ANCOVA-based analysis to correct for differences in metabolic mass (Arch et al., 2006). IL-18 KO mice expended less energy during physically active (dark cycle) and less active (light cycle) phases of the day and also showed reduced energy output even when the genotypes exhibited similar levels of physical activity. Genotype differences were seen even while mice were fasting during the light cycle, a period with similarly low physical activity. Collectively, the findings extend our previous calorimetry studies in chow-fed mice by providing strong evidence for a reduction in basal metabolic rate. Accordingly, IL-18 deficiency led to a reduced MESOR of the circadian function for energy expenditure and an increased MESOR of the circadian function for respiratory exchange ratio, consistent with positive energy balance and decreased lipid substrate utilization, respectively. IFN-γ, which is induced by IL-18, pharmacologically increases resting metabolic rate in humans (de Metz et al., 1999) and mediates not only anorectic, but also hypermetabolic responses to inflammatory stimuli (Arsenijevic et al., 2000), making IFN-γ a potential mechanism by which IL-18 alters metabolism.
Additionally, several genotype differences in non-basal metabolic processes were newly revealed and merit mention. First, IL-18 KO mice failed to show the heightened level of energy expenditure when fasting from low-fat diet vs. high-fat diet that was seen in wildtype mice. This increase in wildtype mice was accompanied by a greater increase in motor activity as compared with IL-18 null mutants. Second, IL-18 KO mice only showed increased RERs when provided high-fat diet ad libitum, during which they overate relative to wildtype controls. No genotype differences in RER were seen when mice were studied during fasting or refeeding conditions, during which intake levels were equivalent across genotypes. Third, the amplitude of the circadian rhythm of energy expenditure was blunted in IL-18 KO mice, and the amplitude of this rhythm reflects non-maintenance metabolic processes (Berger and Phillips, 1988). An increased frequency or quantity of energy intake, as was observed here, has been associated with a blunted amplitude of energy expenditure rhythms (Rashotte et al., 1995). Thus, the present study revealed that IL-18 KO mice differ not only in basal metabolic rate, but also in phasic aspects of energy metabolism.
While peripheral sites of IL-18 action also are possible, (Conti et al., 1997; Penttila et al., 2003; Sugama et al., 2000; Wang et al., 2006; Zorrilla et al., 2007), several previous and current findings implicate central actions of IL-18. Previously we showed that central IL-18 administration suppressed low-fat chow intake, feed efficiency and weight regain in fasted mice with an anorectic potency (≤1 nmol; (Zorrilla et al., 2007)) on the same order as many anorexigenic molecules with central modes of action (Barrachina et al., 1997; Mounien et al., 2009; Semjonous et al., 2009). IL-18 itself is constitutively expressed in third ventricle ependymal cells, which may function as bidirectional intermediaries between the CSF and periventricular energy balance-regulating hypothalamic neurocircuitry, as occurs for leptin (Amat et al., 1999; Cheunsuang et al., 2006). IL-18 modulates neuronal activity (Cumiskey et al., 2007; Curran and O’Connor, 2001; Kanno et al., 2004) and is also centrally produced in medial habenula, cortex, striatum, and glia (Alboni et al., 2010; Conti et al., 1999; Culhane et al., 1998; Sugama et al., 2002). Peripheral immune challenge elicits the hypothalamic synthesis of IL-18 and its receptor, suggesting that central IL-18 activation might contribute to anorectic and metabolic components of the “sickness syndrome.” (Alboni et al., 2010; Alboni et al., 2011).
Also consistent with a central mode of action, IL-18 receptor complex subunits are constitutively expressed in mouse hypothalamus (Alboni et al., 2009; Alboni et al., 2010; Alboni et al., 2011; Wheeler et al., 2000), notably in the paraventricular nucleus and ventromedial hypothalamic nucleus. Both the IL-18Rα and Rβ subunits also are present in the hindbrain, including the nucleus tractus solitarius. The reviewed appetite-regulatory brain regions express not only the canonical IL-18Rs but also three additional isoforms predicted to be either decoy receptors or negative regulators of IL-18 action (Alboni et al., 2010; Alboni et al., 2011). Consistent with this distribution, here we found that central IL-18 administration also suppressed high-fat diet intake and did so via an IL-18R-dependent mechanism. Additional transgenic models, including conditional and region-specific IL-18Rs KO under development in the laboratory, will hopefully prove helpful in determining the exact sites and mechanisms of action.
Mice deficient in proinflammatory cytokines (or their receptors) other than IL-18 also display an obese phenotype, including those null for IL-1R1 (Garcia et al., 2006), IL-6 (Wallenius et al., 2002) and leptin (Zhang et al., 1994). With the exception of leptin, however, the mechanisms by which IL-6, IL-18 and IL-1β systems modulate energy homeostasis remain poorly understood from both physiological and molecular perspectives. The present manuscript demonstrates a key metabolic role for IL-18 that is still present during high-fat diet exposure. When considering the specificity vs. generality of IL-18 actions with respect to other proinflammatory cytokines, several differences and similarities bear discussion. First, through distinct receptors, both IL-18 and IL-1 activate MyD88-IRAK-NF-kB signaling, whereas IL-6 and leptin both activate JAKSTAT signaling, indicating the convergence of different cytokines to two pathways. Second, despite their apparent similarities, IL-1 and IL-18 are molecularly dissociable and present differences that may be exploited pharmacologically. From a translational perspective, two key differences are that unlike IL-1β, IL-18 does not potently produce fever (Gatti et al., 2002) or malaise (Zorrilla et al., 2007), raising the hypothesis that the IL-18/IL-18R system might be targeted to modulate energy homeostasis without inducing as many “sickness syndrome” side effects that limited targeting of the IL-1 system.
In humans, circulating IL-18 levels positively correlate with body mass index, adiposity, and metabolic syndrome disorders (Bruun et al., 2007; Esposito et al., 2002b; Hung et al., 2005; Leick et al., 2007; Sun et al., 2011). Such a finding is consistent with the increased adipocytokine release from adipose tissue in obesity (Fain, 2006). Accordingly, fat-resident monocyte/macrophage lineage cells are major sources of circulating IL-18 (Fain, 2006), adipocytes from obese humans secrete 3-fold more IL-18 than those from lean donors (Skurk et al., 2005), and subcutaneous adipose tissue expression of IL-18 is elevated in obesity and metabolic syndrome disorders (Bruun et al., 2007; Leick et al., 2007; Membrez et al., 2009; Weiss et al., 2011). Consistent with a signal of metabolic state, circulating IL-18 levels are increased by hyperglycemia or a high-fat meal (Esposito et al., 2002a; Esposito et al., 2003), and intermittent glucose exposure increases the secretion of IL-18 by adipocytes (Sun et al., 2009). Conversely, weight loss and exercise decrease IL-18 levels (Bruun et al., 2007; Esposito et al., 2003; Leick et al., 2007; Madsen et al., 2009; Vilarrasa et al., 2007). Some adipocytokines whose levels increase with obesity (e.g, leptin) oppose positive energy balance in negative feedback fashion (Plata-Salaman, 2001; Wong and Pinkney, 2004). Perhaps reminiscent of obesity-related leptin resistance, leukocytes from patients with obesity or type 2 diabetes are resistant to IL-18 (Zilverschoon et al., 2008). Polymorphisms of the IL-18 gene or its receptor have been associated with obesity (Melen et al., 2010) and metabolic syndrome disorders (Evans et al., 2007; Koch et al., 2011; Presta et al., 2009) in humans. The present and previous (Netea et al., 2006; Zorrilla et al., 2007) loss-of-function and pharmacological results support the hypothesis that endogenous IL-18 suppresses appetite and promotes energy expenditure and lipid fuel substrate utilization and may do so not only during sickness, but also in healthy adults.
Supplementary Material
Highlight.
Results from interleukin-18 knockout mice suggest that it may curb appetite and promote lipid utilization in healthy adults consuming high-fat diets.
Acknowledgements
We thank Amanda Roberts for help with indirect calorimetry. The work was supported by National Institute of Health grants DK094026, NS43501, and AG28040 as well as The Ellison Medical Foundation. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Neurological Disorders and Stroke, the National Institute of Aging, or The Ellison Medical Foundation.
Footnotes
Supplementary material is available at Brain, Behavior and Immunity’s website.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Statement The authors have no conflicts of interest with the present work.
References
- Alboni S, Cervia D, Ross B, Montanari C, Gonzalez AS, Sanchez-Alavez M, Marcondes MC, De Vries D, Sugama S, Brunello N, Blom J, Tascedda F, Conti B. Mapping of the full length and the truncated interleukin-18 receptor alpha in the mouse brain. J Neuroimmunol. 2009;214:43–54. doi: 10.1016/j.jneuroim.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alboni S, Cervia D, Sugama S, Conti B. Interleukin 18 in the CNS. J Neuroinflammation. 2010;7:9. doi: 10.1186/1742-2094-7-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alboni S, Montanari C, Benatti C, Blom JM, Simone ML, Brunello N, Caggia F, Guidotti G, Marcondes MC, Sanchez-Alavez M, Conti B, Tascedda F. Constitutive and LPS-regulated expression of interleukin-18 receptor beta variants in the mouse brain. Brain Behav Immun. 2011;25:483–493. doi: 10.1016/j.bbi.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amat P, Pastor FE, Blazquez JL, Pelaez B, Sanchez A, Alvarez-Morujo AJ, Toranzo D, Amat-Peral G. Lateral evaginations from the third ventricle into the rat mediobasal hypothalamus: an amplification of the ventricular route. Neuroscience. 1999;88:673–677. doi: 10.1016/s0306-4522(98)00470-9. [DOI] [PubMed] [Google Scholar]
- Arch JR, Hislop D, Wang SJ, Speakman JR. Some mathematical and technical issues in the measurement and interpretation of open-circuit indirect calorimetry in small animals. Int J Obes (Lond) 2006;30:1322–1331. doi: 10.1038/sj.ijo.0803280. [DOI] [PubMed] [Google Scholar]
- Arsenijevic D, Garcia I, Vesin C, Vesin D, Arsenijevic Y, Seydoux J, Girardier L, Ryffel B, Dulloo A, Richard D. Differential roles of tumor necrosis factor-alpha and interferon-gamma in mouse hypermetabolic and anorectic responses induced by LPS. Eur Cytokine Netw. 2000;11:662–668. [PubMed] [Google Scholar]
- Bandini LG, Schoeller DA, Dietz WH. Metabolic differences in response to a high-fat vs. a high-carbohydrate diet. Obes Res. 1994;2:348–354. doi: 10.1002/j.1550-8528.1994.tb00074.x. [DOI] [PubMed] [Google Scholar]
- Barrachina MD, Martinez V, Wang L, Wei JY, Tache Y. Synergistic interaction between leptin and cholecystokinin to reduce short-term food intake in lean mice. Proc Natl Acad Sci U S A. 1997;94:10455–10460. doi: 10.1073/pnas.94.19.10455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger RJ, Phillips NH. Comparative aspects of energy metabolism, body temperature and sleep. Acta Physiol Scand Suppl. 1988;574:21–27. [PubMed] [Google Scholar]
- Born TL, Morrison LA, Esteban DJ, VandenBos T, Thebeau LG, Chen N, Spriggs MK, Sims JE, Buller RM. A poxvirus protein that binds to and inactivates IL-18, and inhibits NK cell response. J Immunol. 2000;164:3246–3254. doi: 10.4049/jimmunol.164.6.3246. [DOI] [PubMed] [Google Scholar]
- Bruun JM, Stallknecht B, Helge JW, Richelsen B. Interleukin-18 in plasma and adipose tissue: effects of obesity, insulin resistance, and weight loss. Eur J Endocrinol. 2007;157:465–471. doi: 10.1530/EJE-07-0206. [DOI] [PubMed] [Google Scholar]
- Chen A, Zorrilla E, Smith S, Rousso D, Levy C, Vaughan J, Donaldson C, Roberts A, Lee KF, Vale W. Urocortin 2-deficient mice exhibit gender-specific alterations in circadian hypothalamus-pituitary-adrenal axis and depressive-like behavior. J Neurosci. 2006;26:5500–5510. doi: 10.1523/JNEUROSCI.3955-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheunsuang O, Stewart AL, Morris R. Differential uptake of molecules from the circulation and CSF reveals regional and cellular specialisation in CNS detection of homeostatic signals. Cell Tissue Res. 2006;325:397–402. doi: 10.1007/s00441-006-0162-z. [DOI] [PubMed] [Google Scholar]
- Conti B, Jahng JW, Tinti C, Son JH, Joh TH. Induction of interferon-gamma inducing factor in the adrenal cortex. J Biol Chem. 1997;272:2035–2037. doi: 10.1074/jbc.272.4.2035. [DOI] [PubMed] [Google Scholar]
- Conti B, Park LC, Calingasan NY, Kim Y, Kim H, Bae Y, Gibson GE, Joh TH. Cultures of astrocytes and microglia express interleukin 18. Brain Res Mol Brain Res. 1999;67:46–52. doi: 10.1016/s0169-328x(99)00034-0. [DOI] [PubMed] [Google Scholar]
- Culhane AC, Hall MD, Rothwell NJ, Luheshi GN. Cloning of rat brain interleukin-18 cDNA. Mol Psychiatry. 1998;3:362–366. doi: 10.1038/sj.mp.4000389. [DOI] [PubMed] [Google Scholar]
- Cumiskey D, Pickering M, O’Connor JJ. Interleukin-18 mediated inhibition of LTP in the rat dentate gyrus is attenuated in the presence of mGluR antagonists. Neuroscience Letters. 2007;412:206–210. doi: 10.1016/j.neulet.2006.11.007. [DOI] [PubMed] [Google Scholar]
- Curran B, O’Connor JJ. The pro-inflammatory cytokine interleukin-18 impairs long-term potentiation and NMDA receptor-mediated transmission in the rat hippocampus in vitro. Neuroscience. 2001;108:83–90. doi: 10.1016/s0306-4522(01)00405-5. [DOI] [PubMed] [Google Scholar]
- de Metz J, Sprangers F, Endert E, Ackermans MT, ten Berge IJ, Sauerwein HP, Romijn JA. Interferon-gamma has immunomodulatory effects with minor endocrine and metabolic effects in humans. J Appl Physiol. 1999;86:517–522. doi: 10.1152/jappl.1999.86.2.517. [DOI] [PubMed] [Google Scholar]
- Ebbeling CB, Swain JF, Feldman HA, Wong WW, Hachey DL, Garcia-Lago E, Ludwig DS. Effects of dietary composition on energy expenditure during weight-loss maintenance. JAMA. 2012;307:2627–2634. doi: 10.1001/jama.2012.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, Quagliaro L, Ceriello A, Giugliano D. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002a;106:2067–2072. doi: 10.1161/01.cir.0000034509.14906.ae. [DOI] [PubMed] [Google Scholar]
- Esposito K, Pontillo A, Ciotola M, Di Palo C, Grella E, Nicoletti G, Giugliano D. Weight loss reduces interleukin-18 levels in obese women. J Clin Endocrinol Metab. 2002b;87:3864–3866. doi: 10.1210/jcem.87.8.8781. [DOI] [PubMed] [Google Scholar]
- Esposito K, Pontillo A, Di Palo C, Giugliano G, Masella M, Marfella R, Giugliano D. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: a randomized trial. Jama. 2003;289:1799–1804. doi: 10.1001/jama.289.14.1799. [DOI] [PubMed] [Google Scholar]
- Evans J, Collins M, Jennings C, van der Merwe L, Soderstrom I, Olsson T, Levitt NS, Lambert EV, Goedecke JH. The association of interleukin-18 genotype and serum levels with metabolic risk factors for cardiovascular disease. Eur J Endocrinol. 2007;157:633–640. doi: 10.1530/EJE-07-0463. [DOI] [PubMed] [Google Scholar]
- Even PC, Nadkarni NA. Indirect calorimetry in laboratory mice and rats: principles, practical considerations, interpretation and perspectives. Am J Physiol Regul Integr Comp Physiol. 2012;303:R459–476. doi: 10.1152/ajpregu.00137.2012. [DOI] [PubMed] [Google Scholar]
- Fain JN. Release of interleukins and other inflammatory cytokines by human adipose tissue is enhanced in obesity and primarily due to the nonfat cells. Vitam Horm. 2006;74:443–477. doi: 10.1016/S0083-6729(06)74018-3. [DOI] [PubMed] [Google Scholar]
- Garcia MC, Wernstedt I, Berndtsson A, Enge M, Bell M, Hultgren O, Horn M, Ahren B, Enerback S, Ohlsson C, Wallenius V, Jansson JO. Mature-onset obesity in interleukin-1 receptor I knockout mice. Diabetes. 2006;55:1205–1213. doi: 10.2337/db05-1304. [DOI] [PubMed] [Google Scholar]
- Gatti S, Beck J, Fantuzzi G, Bartfai T, Dinarello CA. Effect of interleukin-18 on mouse core body temperature. Am J Physiol Regul Integr Comp Physiol. 2002;282:R702–709. doi: 10.1152/ajpregu.00393.2001. [DOI] [PubMed] [Google Scholar]
- Gordon RR, Hunter KW, Sorensen P, Pomp D. Genotype × diet interactions in mice predisposed to mammary cancer. I. Body weight and fat. Mamm Genome. 2008;19:163–178. doi: 10.1007/s00335-008-9095-z. [DOI] [PubMed] [Google Scholar]
- Hung J, McQuillan BM, Chapman CM, Thompson PL, Beilby JP. Elevated interleukin-18 levels are associated with the metabolic syndrome independent of obesity and insulin resistance. Arterioscler Thromb Vasc Biol. 2005;25:1268–1273. doi: 10.1161/01.ATV.0000163843.70369.12. [DOI] [PubMed] [Google Scholar]
- Kanno T, Nagata T, Yamamoto S, Okamura H, Nishizaki T. Interleukin-18 stimulates synaptically released glutamate and enhances postsynaptic AMPA receptor responses in the CA1 region of mouse hippocampal slices. Brain Research. 2004;1012:190–193. doi: 10.1016/j.brainres.2004.03.065. [DOI] [PubMed] [Google Scholar]
- Kleiber M. The fire of life: an introduction to animal energetics. Krieger Publishing Company; New York: 1975. [Google Scholar]
- Klockener T, Hess S, Belgardt BF, Paeger L, Verhagen LA, Husch A, Sohn JW, Hampel B, Dhillon H, Zigman JM, Lowell BB, Williams KW, Elmquist JK, Horvath TL, Kloppenburg P, Bruning JC. High-fat feeding promotes obesity via insulin receptor/PI3K-dependent inhibition of SF-1 VMH neurons. Nat Neurosci. 2011;14:911–918. doi: 10.1038/nn.2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch W, Wolferstetter H, Schatke A, Schomig A, Kastrati A. Interleukin 18 gene variation and risk of acute myocardial infarction. Cytokine. 2011 doi: 10.1016/j.cyto.2011.09.006. [DOI] [PubMed] [Google Scholar]
- Kusudo T, Wang Z, Mizuno A, Suzuki M, Yamashita H. TRPV4 deficiency increases skeletal muscle metabolic capacity and resistance against diet-induced obesity. J Appl Physiol. 2012;112:1223–1232. doi: 10.1152/japplphysiol.01070.2011. [DOI] [PubMed] [Google Scholar]
- Lee M, Kim A, Chua SC, Jr., Obici S, Wardlaw SL. Transgenic MSH overexpression attenuates the metabolic effects of a high-fat diet. Am J Physiol Endocrinol Metab. 2007;293:E121–131. doi: 10.1152/ajpendo.00555.2006. [DOI] [PubMed] [Google Scholar]
- Leick L, Lindegaard B, Stensvold D, Plomgaard P, Saltin B, Pilegaard H. Adipose tissue interleukin-18 mRNA and plasma interleukin-18: effect of obesity and exercise. Obesity (Silver Spring) 2007;15:356–363. doi: 10.1038/oby.2007.528. [DOI] [PubMed] [Google Scholar]
- Madsen EL, Bruun JM, Skogstrand K, Hougaard DM, Christiansen T, Richelsen B. Long-term weight loss decreases the nontraditional cardiovascular risk factors interleukin-18 and matrix metalloproteinase-9 in obese subjects. Metabolism. 2009;58:946–953. doi: 10.1016/j.metabol.2009.02.031. [DOI] [PubMed] [Google Scholar]
- McNeill G, Bruce AC, Ralph A, James WP. Inter-individual differences in fasting nutrient oxidation and the influence of diet composition. Int J Obes. 1988;12:455–463. [PubMed] [Google Scholar]
- Melen E, Himes BE, Brehm JM, Boutaoui N, Klanderman BJ, Sylvia JS, Lasky-Su J. Analyses of shared genetic factors between asthma and obesity in children. J Allergy Clin Immunol. 2010;126:631–637. e631–638. doi: 10.1016/j.jaci.2010.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Membrez M, Ammon-Zufferey C, Philippe D, Aprikian O, Monnard I, Mace K, Darimont C. Interleukin-18 protein level is upregulated in adipose tissue of obese mice. Obesity (Silver Spring) 2009;17:393–395. doi: 10.1038/oby.2008.535. [DOI] [PubMed] [Google Scholar]
- Mounien L, Do Rego JC, Bizet P, Boutelet I, Gourcerol G, Fournier A, Brabet P, Costentin J, Vaudry H, Jegou S. Pituitary adenylate cyclase-activating polypeptide inhibits food intake in mice through activation of the hypothalamic melanocortin system. Neuropsychopharmacology. 2009;34:424–435. doi: 10.1038/npp.2008.73. [DOI] [PubMed] [Google Scholar]
- Netea MG, Joosten LA, Lewis E, Jensen DR, Voshol PJ, Kullberg BJ, Tack CJ, van Krieken H, Kim SH, Stalenhoef AF, van de Loo FA, Verschueren I, Pulawa L, Akira S, Eckel RH, Dinarello CA, van den Berg W, van der Meer JW. Deficiency of interleukin-18 in mice leads to hyperphagia, obesity and insulin resistance. Nat Med. 2006;12:650–656. doi: 10.1038/nm1415. [DOI] [PubMed] [Google Scholar]
- Okamura H, Tsutsi H, Komatsu T, Yutsudo M, Hakura A, Tanimoto T, Torigoe K, Okura T, Nukada Y, Hattori K, et al. Cloning of a new cytokine that induces IFN-gamma production by T cells. Nature. 1995;378:88–91. doi: 10.1038/378088a0. [DOI] [PubMed] [Google Scholar]
- Opp MR, Toth LA. Circadian modulation of interleukin-1-induced fever in intact and vagotomized rats. Ann N Y Acad Sci. 1997;813:435–436. doi: 10.1111/j.1749-6632.1997.tb51729.x. [DOI] [PubMed] [Google Scholar]
- Paula GS, Souza LL, Cabanelas A, Bloise FF, Mello-Coelho V, Wada E, Ortiga-Carvalho TM, Oliveira KJ, Pazos-Moura CC. Female mice target deleted for the neuromedin B receptor have partial resistance to diet-induced obesity. J Physiol. 2010;588:1635–1645. doi: 10.1113/jphysiol.2009.185322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penttila IA, Flesch IE, McCue AL, Powell BC, Zhou FH, Read LC, Zola H. Maternal milk regulation of cell infiltration and interleukin 18 in the intestine of suckling rat pups. Gut. 2003;52:1579–1586. doi: 10.1136/gut.52.11.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plata-Salaman CR. Cytokines and feeding. Int J Obes Relat Metab Disord. 2001;25(Suppl 5):S48–52. doi: 10.1038/sj.ijo.0801911. [DOI] [PubMed] [Google Scholar]
- Presta I, Andreozzi F, Succurro E, Marini MA, Laratta E, Lauro R, Hribal ML, Perticone F, Sesti G. IL-18 gene polymorphism and metabolic syndrome. Nutr Metab Cardiovasc Dis. 2009;19:e5–6. doi: 10.1016/j.numecd.2008.10.002. [DOI] [PubMed] [Google Scholar]
- Rashotte ME, Basco PS, Henderson RP. Daily cycles in body temperature, metabolic rate, and substrate utilization in pigeons: influence of amount and timing of food consumption. Physiol Behav. 1995;57:731–746. doi: 10.1016/0031-9384(94)00315-7. [DOI] [PubMed] [Google Scholar]
- Rumpler WV, Seale JL, Miles CW, Bodwell CE. Energy-intake restriction and diet-composition effects on energy expenditure in men. Am J Clin Nutr. 1991;53:430–436. doi: 10.1093/ajcn/53.2.430. [DOI] [PubMed] [Google Scholar]
- Semjonous NM, Smith KL, Parkinson JR, Gunner DJ, Liu YL, Murphy KG, Ghatei MA, Bloom SR, Small CJ. Coordinated changes in energy intake and expenditure following hypothalamic administration of neuropeptides involved in energy balance. Int J Obes (Lond) 2009;33:775–785. doi: 10.1038/ijo.2009.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skurk T, Kolb H, Muller-Scholze S, Rohrig K, Hauner H, Herder C. The proatherogenic cytokine interleukin-18 is secreted by human adipocytes. Eur J Endocrinol. 2005;152:863–868. doi: 10.1530/eje.1.01897. [DOI] [PubMed] [Google Scholar]
- Strader AD, Reizes O, Woods SC, Benoit SC, Seeley RJ. Mice lacking the syndecan-3 gene are resistant to diet-induced obesity. J Clin Invest. 2004;114:1354–1360. doi: 10.1172/JCI20631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugama S, Cho BP, Baker H, Joh TH, Lucero J, Conti B. Neurons of the superior nucleus of the medial habenula and ependymal cells express IL-18 in rat CNS. Brain Res. 2002;958:1–9. doi: 10.1016/s0006-8993(02)03363-2. [DOI] [PubMed] [Google Scholar]
- Sugama S, Kim Y, Baker H, Tinti C, Kim H, Joh TH, Conti B. Tissue-specific expression of rat IL-18 gene and response to adrenocorticotropic hormone treatment. J Immunol. 2000;165:6287–6292. doi: 10.4049/jimmunol.165.11.6287. [DOI] [PubMed] [Google Scholar]
- Sun J, Xu Y, Dai Z, Sun Y. Intermittent high glucose stimulate MCP-l, IL-18, and PAI-1, but inhibit adiponectin expression and secretion in adipocytes dependent of ROS. Cell Biochem Biophys. 2009;55:173–180. doi: 10.1007/s12013-009-9066-3. [DOI] [PubMed] [Google Scholar]
- Sun L, Hu FB, Yu Z, Li H, Liu H, Wang X, Yu D, Wu H, Zhang G, Zong G, Liu Y, Lin X. Lean body mass, interleukin 18, and metabolic syndrome in apparently healthy Chinese. PLoS One. 2011;6:e18104. doi: 10.1371/journal.pone.0018104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton GM, Trevaskis JL, Hulver MW, McMillan RP, Markward NJ, Babin MJ, Meyer EA, Butler AA. Diet-genotype interactions in the development of the obese, insulin-resistant phenotype of C57BL/6J mice lacking melanocortin-3 or -4 receptors. Endocrinology. 2006;147:2183–2196. doi: 10.1210/en.2005-1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda K, Tsutsui H, Yoshimoto T, Adachi O, Yoshida N, Kishimoto T, Okamura H, Nakanishi K, Akira S. Defective NK cell activity and Th1 response in IL-18-deficient mice. Immunity. 1998;8:383–390. doi: 10.1016/s1074-7613(00)80543-9. [DOI] [PubMed] [Google Scholar]
- Torigoe K, Ushio S, Okura T, Kobayashi S, Taniai M, Kunikata T, Murakami T, Sanou O, Kojima H, Fujii M, Ohta T, Ikeda M, Ikegami H, Kurimoto M. Purification and characterization of the human interleukin-18 receptor. J Biol Chem. 1997;272:25737–25742. doi: 10.1074/jbc.272.41.25737. [DOI] [PubMed] [Google Scholar]
- Verboeket-van de Venne WP, Westerterp KR, ten Hoor F. Substrate utilization in man: effects of dietary fat and carbohydrate. Metabolism. 1994;43:152–156. doi: 10.1016/0026-0495(94)90237-2. [DOI] [PubMed] [Google Scholar]
- Vilarrasa N, Vendrell J, Sanchez-Santos R, Broch M, Megia A, Masdevall C, Gomez N, Soler J, Pujol J, Bettonica C, Aranda H, Gomez JM. Effect of weight loss induced by gastric bypass on proinflammatory interleukin-18, soluble tumour necrosis factor-alpha receptors, C-reactive protein and adiponectin in morbidly obese patients. Clin Endocrinol (Oxf) 2007;67:679–686. doi: 10.1111/j.1365-2265.2007.02945.x. [DOI] [PubMed] [Google Scholar]
- Wallenius V, Wallenius K, Ahren B, Rudling M, Carlsten H, Dickson SL, Ohlsson C, Jansson JO. Interleukin-6-deficient mice develop mature-onset obesity. Nat Med. 2002;8:75–79. doi: 10.1038/nm0102-75. [DOI] [PubMed] [Google Scholar]
- Wang N, Sugama S, Conti B, Teramoto A, Shibasaki T. Interleukin-18 mRNA expression in the rat pituitary gland. J Neuroimmunol. 2006;173:117–125. doi: 10.1016/j.jneuroim.2005.12.009. [DOI] [PubMed] [Google Scholar]
- Weiss TW, Arnesen H, Troseid M, Kaun C, Hjerkinn EM, Huber K, Wojta J, Seljeflot I. Adipose tissue expression of interleukin-18 mRNA is elevated in subjects with metabolic syndrome and independently associated with fasting glucose. Wien Klin Wochenschr. 2011 doi: 10.1007/s00508-011-0028-6. [DOI] [PubMed] [Google Scholar]
- Wheeler RD, Culhane AC, Hall MD, Pickering-Brown S, Rothwell NJ, Luheshi GN. Detection of the interleukin 18 family in rat brain by RT-PCR. Brain Research. Molecular Brain Research. 2000;77:290–293. doi: 10.1016/s0169-328x(00)00069-3. [DOI] [PubMed] [Google Scholar]
- Wong S, Pinkney J. Role of cytokines in regulating feeding behaviour. Curr Drug Targets. 2004;5:251–263. doi: 10.2174/1389450043490532. [DOI] [PubMed] [Google Scholar]
- Wortley KE, Anderson KD, Garcia K, Murray JD, Malinova L, Liu R, Moncrieffe M, Thabet K, Cox HJ, Yancopoulos GD, Wiegand SJ, Sleeman MW. Genetic deletion of ghrelin does not decrease food intake but influences metabolic fuel preference. Proc Natl Acad Sci U S A. 2004;101:8227–8232. doi: 10.1073/pnas.0402763101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]
- Zigman JM, Nakano Y, Coppari R, Balthasar N, Marcus JN, Lee CE, Jones JE, Deysher AE, Waxman AR, White RD, Williams TD, Lachey JL, Seeley RJ, Lowell BB, Elmquist JK. Mice lacking ghrelin receptors resist the development of diet-induced obesity. J Clin Invest. 2005;115:3564–3572. doi: 10.1172/JCI26002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilverschoon GR, Tack CJ, Joosten LA, Kullberg BJ, van der Meer JW, Netea MG. Interleukin-18 resistance in patients with obesity and type 2 diabetes mellitus. Int J Obes (Lond) 2008;32:1407–1414. doi: 10.1038/ijo.2008.109. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Sanchez-Alavez M, Sugama S, Brennan M, Fernandez R, Bartfai T, Conti B. Interleukin-18 controls energy homeostasis by suppressing appetite and feed efficiency. Proc Natl Acad Sci U S A. 2007;104:11097–11102. doi: 10.1073/pnas.0611523104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.