Abstract
During Time-Place Learning (TPL), animals link biological significant events (e.g. encountering predators, food, mates) with the location and time of occurrence in the environment. This allows animals to anticipate which locations to visit or avoid based on previous experience and knowledge of the current time of day. The TPL task applied in this study consists of three daily sessions in a three-arm maze, with a food reward at the end of each arm. During each session, mice should avoid one specific arm to avoid a foot-shock. We previously demonstrated that, rather than using external cue-based strategies, mice use an internal clock (circadian strategy) for TPL, referred to as circadian TPL (cTPL). It is unknown in which brain region(s) or peripheral organ(s) the consulted clock underlying cTPL resides. Three candidates were examined in this study: (a) the suprachiasmatic nucleus (SCN), a light entrainable oscillator (LEO) and considered the master circadian clock in the brain, (b) the food entrainable oscillator (FEO), entrained by restricted food availability, and (c) the adrenal glands, harboring an important peripheral oscillator. cTPL performance should be affected if the underlying oscillator system is abruptly phase-shifted. Therefore, we first investigated cTPL sensitivity to abrupt light and food shifts. Next we investigated cTPL in SCN-lesioned- and adrenalectomized mice. Abrupt FEO phase-shifts (induced by advancing and delaying feeding time) affected TPL performance in specific test sessions while a LEO phase-shift (induced by a light pulse) more severely affected TPL performance in all three daily test sessions. SCN-lesioned mice showed no TPL deficiencies compared to SHAM-lesioned mice. Moreover, both SHAM- and SCN-lesioned mice showed unaffected cTPL performance when re-tested after bilateral adrenalectomy. We conclude that, although cTPL is sensitive to timing manipulations with light as well as food, neither the SCN nor the adrenals are required for cTPL in mice.
Keywords: Behavior, cognition, corticosterone, entrainment, food, light, memory, oscillator
Introduction
Field studies have shown that many animals live in situations in which the locations of prey (food sources), mates, or predators vary predictably over time (Becker et al., 1993; Daan & Koene, 1981; Rijnsdorp et al., 1981; Gill, 1988; Silver & Bittman, 1984; Wahl, 1932; Wilkie et al., 1996). The ability to encode spatiotemporal reoccurring events and to exploit this information by efficiently organized daily activities, is believed to constitute a significant fitness advantage which has likely shaped the architecture of cognitive and circadian systems over the course of evolution (Aschoff, 1989; Beugnon et al., 1995; Carr & Wilkie, 1997; Carr et al., 1999; Daan, 1981; Enright, 1970; Gallistel, 1990; Mistlberger, 1994; Mistlberger et al., 1996; Reebs, 1996). Indeed, Time-Place Learning (TPL), the process in which animals link events with the spatial location and the time of day (TOD), has been demonstrated in many species (Mulder et al., 2013a).
Several studies have confirmed the use of an internal clock for TPL (Biebach, 1989; Falk, 1992; Mistlberger et al., 1996; Mulder et al., 2013c; Pizzo & Crystal, 2002; Saksida & Wilkie, 1994; Van der Zee et al., 2008; Wenger et al., 1991). However, alternative to such a circadian strategy, animals have also been shown to (conditionally) use non-circadian strategies, based on external cues, like ordinal- or interval timing (Carr & Wilkie, 1997, 1999; Carr et al., 1999; Pizzo & Crystal, 2002, 2004; Thorpe et al., 2003). With an ordinal timing strategy, animals respectively remember the sequence of events (e.g. first test session, avoid location A; second test session, avoid location B, etc.). With an interval timing strategy, animals remember the passage of time relative to a zeitgeber (e.g. shortly after light onset, avoid location A; longer after light onset, avoid location B, etc.). Because animals may use different strategies for TPL, the use of a circadian strategy (using an internal circadian timing mechanism independent of external cues) has to be identified by showing stable TPL performance after skipping the first daily test session(s) (excluding an ordinal strategy) and by testing in zeitgeber deprived conditions (excluding an interval timing strategy) (Carr & Wilkie, 1997; Crystal, 2009). While TPL refers to the behavioral output of visiting correct locations at the correct TOD (using any possible strategy), we refer to circadian TPL (cTPL) when the use of a circadian strategy is confirmed or implied. cTPL demonstrates that circadian oscillators can serve as a consulted clock for brain areas involved in cognition to recognize and record TOD, so that the timing of specific behaviors can be regulated in accordance with prior experience (Mistlberger et al., 1996). Time stamping refers to the process underlying the encoding of a specific TOD (the time stamp). Presumably, biological significant events induce an internal clock-derived time stamp that is stored in memory as a contextual cue and associated with place- and event-specific information. Such a mechanism can only function if a circadian oscillator is continuously monitored by brain areas engaged in cognitive tasks (i.e. learning and memory, decision making) to check if previously recorded time stamps match the current TOD (Mulder et al., 2013a). However, yet the locus and neural substrates of the clock mechanism utilized in cTPL remain elusive.
The circadian system can be described as a complex hierarchical network of circadian clocks in the brain and periphery, which together influence many behavioral and physiological rhythms (Dibner et al., 2010). In mammals, the hypothalamic suprachiasmatic nucleus (SCN), situated directly above the optic chiasm, is recognized as the “master clock” (Groos & Hendriks, 1982; Ralph et al., 1990; Stephan & Zucker, 1972). The SCN entrains to the environmental light/dark cycle and in turn synchronizes many subordinate clocks in the brain and periphery. Because the SCN entrains to photic environmental cues (zeitgebers), it is classified as a light-entrainable oscillator (LEO). In addition, brief periods of food availability form a second major zeitgeber known to entrain circadian rhythms. SCN lesions abolish light-entrainable rhythms, but do not affect the circadian properties of feeding-entrainable rhythms (Boulos et al., 1980; Marchant & Mistlberger, 1997; Stephan et al., 1979b; Stephan, 1981, 1989). Therefore, a separate (anatomically and functionally distinct) Food Entrainable Oscillator (FEO) is distinguished, although the locus and neural substrates of the FEO have not been identified conclusively. Possibly, the FEO properties emerge from a distributed system of brain areas (Mistlberger, 2011). On a cellular level, circadian rhythms are predominantly controlled by clock genes and their protein products, which are expressed in virtually all cells in the body. In short, CLOCK (Circadian Locomotor Output Cycles Kaput) and BMAL1 (Brain and Muscle ARNT-like protein 1) form a heterodimeric complex which acts as a transcription activator for PER (Period) and CRY (Cryptochrome) proteins. PER and CRY dimerize and translocate back into the nucleus to inhibit the CLOCK-BMAL1 transcription factor, forming a closed transcriptional-translational feedback loop (Ko & Takahashi, 2006).
Previously we demonstrated cTPL for the first time in mice (Van der Zee et al., 2008), using a paradigm that emulates the natural situation in which hungry animals seek food while different feeding locations can be predictably unsafe to visit, depending on the TOD. Young wild-type C57Bl6 mice readily acquired this task and control experiments indicated that they used a circadian strategy. We further confirmed the circadian nature of TPL by showing that Cry1/Cry2 double knockout mice were unable to acquire the test (Van der Zee et al., 2008). Conversely, we found that Per1/Per2 double mutant mice acquired cTPL similarly as wild-type controls, devaluating the role of Per as core clock genes in cTPL (Mulder et al., 2013c). It remains unclear in which brain area or peripheral organ Cry (but not Per) expression is critical.
As the master circadian pacemaker, the SCN is a reasonable candidate to either play a crucial or modulatory role in cTPL. The SCN may function as the main consulted clock in cTPL. Interestingly, salient events have been shown to induce a circadian rhythm in the expression of muscarinic acetylcholine receptors in the SCN, with peak expression levels coinciding with the event-specific TOD (Van der Zee, 2004). It has therefore been proposed that the SCN may function as a programmable “alarm clock”, using the neuropeptide vasopressin (AVP) as an output to transfer the specific TOD information to other brain regions (Biemans et al., 2003; Hut & Van der Zee, 2011; van der Veen et al., 2008, 2009). With such a mechanism (in which the SCN produces or gates a circadian modulated output at relevant TOD’s), continuous monitoring of a circadian oscillator is not necessary (the presence of the output signal will provide go/no go information, while the amplitude can be associated with place and event information). A less crucial role of the SCN may be expected if SCN output is one of multiple temporal signals to brain areas engaged in cognitive tasks, or when the SCN merely serves to entrain non-SCN oscillators that underlie cTPL. In line with this, Gritton et al. (2013) and coworkers recently reported significant impairments of task acquisition in SCN-lesioned rats, and hypothesized that non-SCN oscillators take much longer to become synchronized to each other and to external zeitgebers in absence of a functional SCN.
The role of the SCN has been investigated in paradigms in which animals show memory for TOD, but in which TOD is not a discriminative cue. Such circadian retention paradigms (e.g. fear conditioning, passive avoidance, conditioned place preference/avoidance) involve a training (positive or negative stimulus encounter) followed by a retention test. Animals will usually show optimal retention 24 h (or multiples thereof) after training (independent of the time of training), indicating memory for the time of training (Mulder et al., 2013a). However, this pattern is not always shown (Oklejewicz et al., 2001; McDonald, 2002), indicating that this phenomenon may be species specific and/or task dependent (Ralph, 2002). Circadian retention was found to be lost in rats with hypothalamic lesions including the SCN (Stephan & Kovecevic 1978), but repeatedly found to persist in SCN-lesioned hamsters (Ko et al., 2003; Cain & Ralph, 2009; Cain et al., 2012). In hamsters, the SCN was found to play a role as a weak zeitgeber, entraining potentially involved extra-SCN oscillators (Ralph, 2013). Similarly, although food anticipation persists in SCN-lesioned animals (Mistlberger, 1994; Stephan, 1979b), it has been reported that the SCN participates actively during food entrainment. It modulates the response of hypothalamic and corticolimbic structures, resulting in an increased anticipatory response (Angeles-Castellanos, 2010). Although food anticipation, circadian retention paradigms, and cTPL all demonstrate memory for TOD, it is currently unclear whether the same neurobiological mechanisms underlie these behaviors. Theoretically, food anticipation and circadian retention behavior can be explained by an entrained oscillator which induces a certain behavior when a set phase angle is reached. With TPL, animals are trained to go to different places at different TOD’s. They must therefore discriminate between different TOD’s and link each TOD with a different location choice. Theoretically, this requires a decision-making process based on associative memory, and a consulted clock (Biebach, 1989; Carr et al., 1999; Mistlberger et al., 1996; Mulder et al., 2013a). One TPL study showed that SCN-lesioned rats still acquired cTPL in a simple TPL task involving lever-pressing for food at two locations, while each lever provided food at a different TOD (Mistlberger et al., 1996). The authors concluded that TOD cues can be provided by a circadian oscillator other than the light-entrainable SCN, likely food-entrainable. However, Widman et al. (2004) showed TPL in a paradigm which did not include a food reward, and concluded that, either the SCN or the FEO may be conditionally used for cTPL. Despite the potential for knockout studies, the role of the SCN in cTPL has not been investigated before in mice. Moreover, cTPL has not been investigated before in our more complex TPL setup which requires discrimination between three locations and TOD’s. Such a complex task may require more accurate SCN governed entrainment of oscillators in cTPL-involved brain regions.
The adrenal glands harbor an important peripheral oscillator to consider in relation to cTPL. The SCN interconnects with the adrenal cortex through SCN governed ACTH (Adrenocorticotropic hormone) secretion from the anterior pituitary gland, but also via automatic nervous system pathways, which can directly modulate adrenal ACTH sensitivity (Kalsbeek et al., 2012). In response to ACTH, the adrenal cortex produces glucocorticoids, while this production is gated by the local adrenal clock (Oster et al., 2006). Glucocorticoids regulate a wide variety of functions, including arousal, stress response, energy metabolism and cognition. Glucocorticoid receptors are widely expressed in the hippocampus and corticosterone is known to modulate processes underlying learning and memory (Dana & Martinez Jr, 1984; Smriga et al., 1996). Importantly, with intact behavior rhythms present (e.g. induced through masking via the light cycle or daily testing), the adrenal clock can sustain corticosterone rhythmicity in absence of a functional SCN pacemaker (Oster et al., 2006). Likewise, food anticipatory activity (FAA) is preceded by a corticosterone (CORT) peak (Honma et al., 1992; Nelson et al., 1975), which is still present in SCN-lesioned animals (Krieger et al., 1977). Adrenal outputs may therefore serve as an internal time-signal used in cTPL even in the absence of a functional SCN. Similar to the SCN, the adrenal clock may either play a crucial or modulatory role in cTPL, functioning as the main consulted clock or as an output of a yet undisclosed clock system.
Here we first set out to investigate whether a LEO or a FEO underlies cTPL in mice, by abruptly phase-shifting these oscillators separately while monitoring TPL performance. Because these results strengthened our hypothesis that the SCN may be involved when mice master our (complex) TPL task, we then investigated cTPL in SCN-lesioned mice. Similarly, we hypothesized a potential role of adrenal corticosterone rhythms providing TOD information to cTPL involved brain areas engaged in cognitive tasks. Therefore we investigated whether TPL-trained mice showed increased CORT levels at the first daily TPL test session, compared to homecage control mice. These results validated further investigation of cTPL in adrenalectomized mice.
Materials and methods
Animals and housing
All experiments were performed using male C57BL6/J mice (Harlan, Horst, the Netherlands). A detailed overview of the groups and group sizes in the performed experiments is provided in Table 1. All mice were housed individually in macrolon type II cages (length 35 cm, width 15 cm, height 13.5 cm, Bayer, Germany), with sawdust as bedding and shredded cardboard as nesting material. The mice were kept in a climate room with controlled temperature (22 ± 1 °C) and humidity (55 ± 10%). A light/dark (LD) schedule (12h light - 12h dark; lights on at 07:00 h GMT + 1h) was maintained, except in the constant light (LL) or constant dark (DD) period. Light intensity was always 20–50 lux measured between the cages. Food (standard rodent chow: RMHB/ 2180, Arie Block BV, Woerden, NL) was available ad libitum, except during food deprivation. Normal tap water was available ad libitum. Cages were enriched with a plastic running-wheel (diameter 13.5 cm) and were cleaned at least once every two weeks. All mice were checked daily for food/water/health/activity/abnormal behavior. All procedures were in accordance with the regulation of the ethical committee for the use of experimental animals of the University of Groningen, The Netherlands (License number DEC 5583D) and meet the ethical standards of the journal as outlined in Portaluppi et al. (2010). All efforts were made to minimize the number of animals used and their discomfort.
Table 1.
Overview of experiments and groups.
| Experiment | (SCN lesion Batch) | groups | N | age (months) |
|---|---|---|---|---|
| 1. CORT | HCC | 9 | 4 | |
| TPL | 8 | 4 | ||
| 2. LEO/FEO | HCC* | 2 | 11 | |
| TPL | 7 | 11 | ||
| 3. SCN lesion | Batch1 | HCC | 8 | 4 |
| SHAM | 4 | 4 | ||
| SCNx | 5 | 4 | ||
| Batch2 | HCC | 5 | 4 | |
| SHAM | 4 | 4 | ||
| SCNx | 5 | 4 | ||
| 4. Adrenalectomy | Batch2 (re-tested) | ADX | 7** | 7 |
Not food deprived.
3 SHAM, 4 SCNx.
The LEO/FEO experiment was performed with a separate group of animals, including TPL tested mice (TPL) and homecage control mice (HCC, not food deprived). The SCN lesion experiment was performed in two separate batches, including TPL tested SHAM- and SCN-lesioned mice. Animals with incomplete SCN lesions were not further used and are not shown. SHAM and SCNx mice from batch2 were re-tested after bilateral adrenalectomy (ADX group). In experiments 2 and 3, HCC animals were housed and food deprived together the TPL tested animals. The number of animals (N) and ages (at the beginning of TPL testing) are indicated for each group.
Experiments and experimental outline
A detailed overview of the experiments and experimental groups is provided in Table 1. CORT measurements at TPL training times were performed on animals from experiments 1 and 4. Experiment 1 included intact mice, which had successfully mastered cTPL (TPL, n = 9), and homecage control mice (HCC, n = 8). Next to investigating differences between TPL-trained and HCC mice, the measurements from experiment 1 serve as a positive control for the measurements of the adrenalectomized mice from experiment 4 (Figure 7). The light pulse and food shift manipulations, to investigated LEO/FEO involvement in cTPL, were performed in experiment 2, including mice which had successfully mastered cTPL (n = 7) and two HCC mice, which were not food-deprived in contrast to all other HCC groups (so that we could clearly distinguish the effect size of the light pulse in these mice). The SCN lesion experiment (experiment 3) was performed in two separate batches. Mice of the first batch, three months old at reception, were habituated to the climate room and housing conditions for 1 month before receiving bilateral SCN lesions (n = 14) or SHAM lesions (n = 4). After recovery for at least 10 days, mice were phenotyped for arrhythmic running-wheel behavior in constant darkness (DD) over a 2 week period. Based on a visual and statistical rhythmicity assessment, five completely arrhythmic SCN-lesioned (SCNx) and all four SHAM-lesioned (SHAM) mice were selected for TPL testing (the maximal number of mice supported by the protocol). One week later mice were put back on LD and the spontaneous alternation (SA) test was performed. Ad libitum body weights were determined after the SA test and on the two following days, after which food deprivation (timed feeding) was initiated on the SCNx, SHAM and HCC mice (mice received minimally 1.5 g food per day). TPL testing was started the next day. Animals were tested daily during 38 days, starting with 10 days of habituation steps in LD, followed by 20 days of testing in LD, 3 days of testing in DD and 5 days of testing in LD. Session skips were performed on following days, with the number between brackets indicating which session was skipped: 14(1); 16(2); 21(1); 22(1,2,3); 35(1). Animals were sacrificed the day after their last TPL test day, at the time of their first daily test session (deviation ± 5 minutes).
Figure 7.
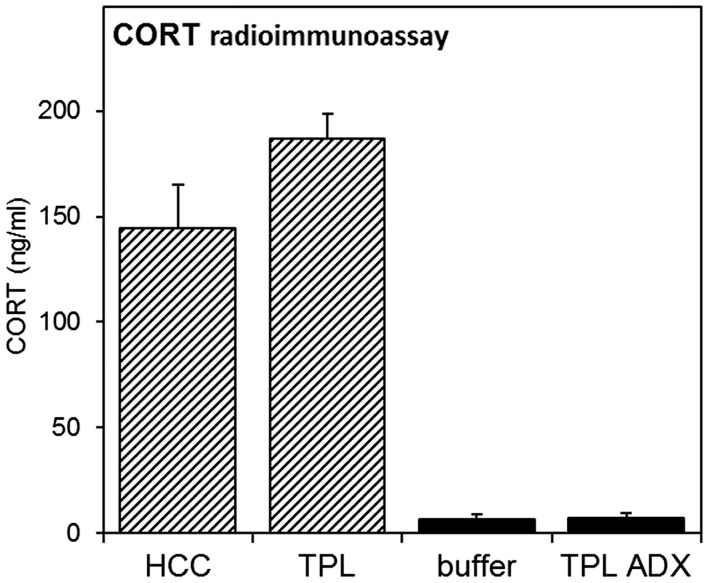
Corticosterone radioimmunoassay. CORT measurements were performed on animals from experiments 1 (striped bars) and 4 (black bars). Animals were sacrificed between ZT2-3.5, when animals expected to be tested in the first TPL session. Blood samples were taken from the heart prior to transcardial perfusion and CORT was measured by radioimmunoassay. Error bars represent SEM.
A similar schema was followed for the second batch. These animals were tested for 31 days, starting with 10 days of habituation steps in LD, followed by 10 days of testing in LD, 5 days of testing in LL and 6 days of testing in DD. Session skips were performed on following days, with the number between brackets indicating which session was skipped: 14(1); 16(2); 22(1); 24(2); 27(1). Two days after testing, animals were put back on LD with ad libitum food. Two weeks later, animals received bilateral adrenalectomy surgery (Table 1, experiment 4). Unfortunately, we lost one SCNx mouse during this surgery, and later excluded one SHAM mouse based on too high remaining CORT levels. One month later, animals were re-tested during 13 days (starting with 8 days of testing in LD, followed by 5 days of testing in LL (habituation steps were not repeated). Session skips were performed on following days, with the number between brackets indicating which session was skipped: 5(2); 7(1); 11(1); 13(2). Similar to the animals from the first batch, the mice were sacrificed the day after their last TPL test day, at the time of their first daily test session (deviation ± 5 min).
TPL testing procedure
The used TPL test apparatus and testing procedures were described before (Mulder et al., 2013c; Van der Zee et al., 2008). Briefly, to induce food-seeking behavior and voluntary location-choices, mice were food deprived to 85% of their ad libitum body weight, as individually determined by the average of three daily measurements prior to initiating food deprivation. To monitor bodyweight during testing, mice were weighed before being tested in each daily session and received an individual amount of food at the end of the light-phase (ZT10.5). Homecage control (HCC) mice were not TPL tested, but similarly food deprived (unless stated otherwise). Mice were tested in their inactive (light-) phase. In each of three daily sessions (lasting maximally 10 minutes per mouse), TPL test mice had to learn to avoid one of the three presented feeding locations (bated with powdered standard rodent chow, <0.1g), depending on the TOD (i.e. session). On visiting the non-target location, mice received a mild but aversive foot-shock (set to 620 volts; 0.09 mA; <1 s). A session was considered correct, on an individual level, only when the two target locations were visited first, avoiding the non-target location or visiting it lastly. Daily performance was calculated for each animal as the percentage of correct sessions (e.g. 0, 33, 67 or 100%) and these performances were averaged and plotted per group, forming a learning curve over multiple testing days. Mice from the two groups were alternated in the testing sequence. Actual testing was preceded by habituation steps as described previously (Mulder et al., 2013c; Van der Zee et al., 2008). See supplemental data in Van der Zee et al. (2008), for a graphical representation of the habituation steps. In short, target locations were always baited. During the first four days (1–4), the non-target location was also baited so that all locations were safe to explore freely (no foot-shock delivery). During the next three days (5–7), the non-target location was kept unbaited, but still safe to visit (no foot-shock delivery). During the following three days (8–10), the shock was introduced at the non-target location, while still kept unbaited, so that mice could identify the non-target location based on sight and smell. On day 8, mice were habituated to first-time foot-shock exposure. The non-target location was kept inaccessible until the mice had first consumed the food rewards in the two target locations. This way, in each session all mice received both the positive food experience, followed by the negative foot-shock experience. Because of the manipulation, day 8 was excluded from further analysis. After these habituation steps, actual testing started with all locations baited and foot-shock delivery in the non-target location. Hence, mice could not identify the non-target / target location(s) based on sight/smell and had to use knowledge of circadian phase to discriminate the hazardous non-target location. A schematic overview of the daily protocol is provided below (Figure 1).
Figure 1.
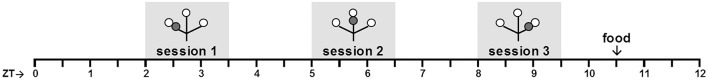
Schematic overview of the daily TPL testing protocol. Open circles indicate food (powdered standard rodent chow, <0.1 g) at the end of an arm of the maze; grey circles indicate the non-target shock location. Mice were tested individually three times a day in 10 minute trials, with an intersession time of 3 hours. Bodyweights were taken before each trial. Mice received an individual amount of food at the end of each day in order to maintain body weight at 85–87% of ad libitum feeding weight. Testing was performed in the light phase. ZT0 (zeitgeber time zero) indicates lights on.
Spontaneous alternation test
Short-term spatial memory performance (working memory) was assessed by recording SA behavior in a Y-maze paradigm, as described before (Mulder et al., 2013c). The Y maze consisted of tree tubular and transparent Plexiglas arms (Evonik Industries AG, Essen, Germany) forming the Y. All three arms were 4.4 cm in internal diameter, 29 cm long, and at a 120 ° angle from each other. The experimental room contained visual cues, which served as distal spatial cues. Mice (naive to the maze) were placed in the center of the Y-maze (5 cm internal diameter) and allowed to explore the maze freely during an eight-minute session. The series of arm entries was recorded visually. An arm entry was considered to be complete when all four limbs of the animal had entered a Y-maze arm. The maze was cleaned between each test with water and paper towels. An alternation is defined as successive entries into the three different maze arms. The alternation percentage (SA performance) was calculated as the ratio of actual to possible alternations (defined as the total number of arm entries minus two) (Anisman, 1975). Exploratory behavior was assessed by counting the number of arm entries.
SCN lesions
Mice were anaesthetized with an isoflurane/oxygen gas mixture and received 60 μl finadyne s.c. (1mg/ml, Schering-Plough NV/SA, Brussels, Belgium). Mice were then placed in a stereotact (secured with earbars and a tooth-bar/nose clamp) equipped to support maintenance of the anesthesia. Eyes were protected against dehydration by applying Vita-Pos® salve (Ursapharm, Saarbrücken, Germany) and the shaved skin was disinfected with 70% EtOH. A small medial incision was made starting from behind the eyes (just above bregma) to just below lambda. The periost was gently scraped away with a scalpel and the scalp was cleaned using cotton swaps. Dorsoventral top-of-skull coordinates were measured at several locations to ensure the head was level and adjustments were made if necessary. Bregma and lambda coordinates were accurately determined. A small hole (about 1.5 mm in diameter) was drilled just below bregma, wide enough to support both bilateral lesion sites. The dura was punctured and remaining skull fragments were gently removed with a needle. At this point, mice received 0.5 ml warm (±25 °C) saline/glucose (0.45% NaCl + 2.5% glucose) i.p. After bleeding from the superior sagittal sinus was stopped with cotton swaps, a Teflon-coated tungsten wire electrode (A-M Systems, Carlsborg, WA), with a 0.3 mm exposed tip, was slowly lowered in the brain to general SCN coordinates: AP 0.3; ML 0.2; DV -5.3 (in mm, relative to bregma and skull top). Coordinates were slightly adjusted to individual bregma-lambda distance. The DV coordinate for SHAM-lesioned mice was −4.8 mm. A ground needle was injected i.p. and bilateral electrolytic lesions were made by passing 1.1 mA DC current for 20 seconds. The electrode was left in the brain to cool down for one minute before it was slowly (0.1 mm/s) taken out. After the contralateral lesion, mice were immediately removed from the stereotact. The head wound was sutured (Ethicon perma-hand N266 5–0), disinfected with Povidine-iodine (Betadine®) and mice received another i.p. injection of 0.5 ml warm (±25 °C) saline/glucose. Mice were placed back in their homecage, remained under a UV heat lamp for 24h, and recovered for at least 2 weeks (first 5 days without running-wheel).
Bilateral adrenalectomy
Mice were anaesthetized with isoflurane, placed on their ventral side on a heating mat, and received 60 μl finadyne s.c. (1 mg/ml) (Schering-Plough NV/SA, Brussels, Belgium). Bilaterally, after fur was trimmed locally, a dorsal medial-lateral incision was made just below the ribcage (1–1.5 cm towards the spine). Each fat encapsulated adrenal was gently pulled up with small tweezers and cut out with scissors. Bleeding was reduced by cutting close to the adrenals and further stopped with cotton swaps. The muscle layers were closed with absorbable sutures (Safil® DS19 4/0, B. Braun Medical AG, Emmenbrücke, Switzerland), the skin layer was closed with silk sutures (Ethicon perma-hand N266 5-0) using an inverse knot. The wound was disinfected with Povidone-iodine (Betadine®) and mice received 1 ml warm (±25 °C) saline/glucose i.p. (0.45% NaCl + 2.5% glucose) to compensate for fluid loss. Mice were put back in their homecage, placed under a UV heat lamp for 24h and recovered further in their homecage (without running-wheel) for at least 2 weeks. After adrenalectomy, mice were given 1% sodium chloride in their drinking water to compensate adrenal regulation of bodily salt content. Complete removal of the adrenals was verified post-mortem by eye. In addition, blood samples were taken from the heart before transcardial perfusion and collected in microcentrifuge tubes containing EDTA as the anticoagulant, and kept on ice. Blood samples were centrifuged at 2600g for 15 min and the supernatant was stored at −80 °C until radioimmunoassay for corticosterone (CORT) (MP Biomedicals, Orangeburg, NY ImmuChem™ Double Antibody Corticosterone 125I RIA Kit, catalog No. 07–120102). The average intra-assay C.V. for this kit is 7%. Further details can be found in the kit manual, which can be requested at MP Biomedicals.
Post mortem verification of SCN lesion position
Under deep pentobarbital anaesthesia, mice were perfused transcardially for 1 minute with 0.9% NaCl + 0.5% heparin (400 U) in H2O (15ml/min), followed by 150 ml 4% paraformaldehyde (PF) in 0.1 M phosphate buffer (PB) for fixation. Brains were collected, postfixated for 24h in 4% PF in 0.1 M PB, rinsed for one day in 0.01 M phosphate buffered saline (PBS, pH 7.4) and then kept overnight in 30% sucrose in PBS cryoprotectant at 4 °C. Brains were frozen using liquid nitrogen and stored at −80 °C until further processing. The brains were cut in 30 μm coronal sections (bregma 0.26 to −1.58) using a cryotome and stored in 4% PF at 4 °C for at least two weeks before silver staining. Brain sections were rinsed 3 × 5 min in H2O, followed by 5 × 5 min in pre-treatment solution (0.45% NaOH + 0.6% NH4NO3 in H2O) and silver impregnated for 10 min in 0.3% AgNO3 + 5.4% NaOH + 6.4% NH4NO3 in H2O. After washing 3×5 min in 0.5% Na2CO3 + 29.7% EtOH + 0.012% NH4NO3 in H2O, slices were developed for 4 min in 0.056% citric acid (C6H8O7 ċ H2O) + 0.549% formaline + 10% EtOH + 0.012% NH4NO3 in H2O (PH adjusted to 5.9), fixated for 4 min in 37.5% Sodium thiosulfate (Na2O3S2 ċ 5H2O), and finally rinsed 3×5 min in H2O. The next day, slices were mounted on glass from a 1% gelatin + 0.01% Aluin solution, dried overnight and defatted/dehydrated through respectively 100% EtOH, 100% EtOH, 70% EtOH + 30% Xylol, 30% EtOH + 70% Xylol, 100% Xylol, 100% Xylol, 100% Xylol. Glass preparations were cover slipped using DPX mountant, dried for two days and then cleaned. Digital images of lesion sites were taken using a macro lens. For each mouse, the damaged area was mapped as a 50% transparent black layer into three coronal template sections: one anterior-, one in the middle-, and one posterior of the SCN (bregma coordinates −0.22; −0.46; −0.94 respectively). From these images, the most saturated area (covering the areas damaged in all subjects) and all area covered (covering areas damaged in at least one subject) were remapped to new corresponding template sections (Figure 2).
Figure 2.
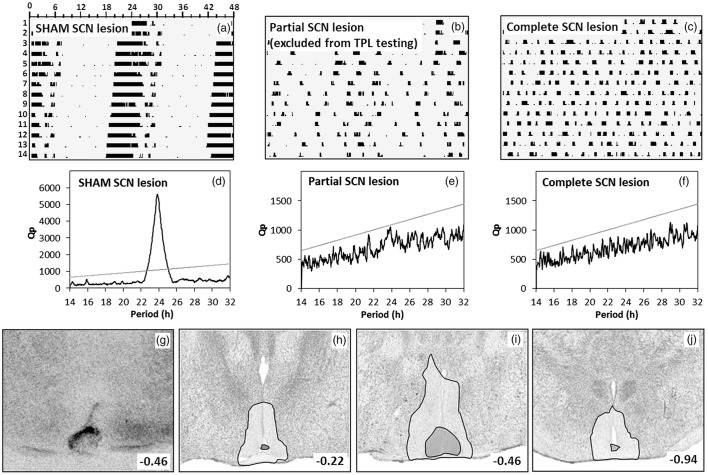
Phenotypic and post-mortem assessment of SCN lesions. (a–c) Sample double plotted quantitative actograms of a SHAM-lesioned mouse (a), a partial arrhythmic SCN-lesioned mouse (b), and a completely arrhythmic SCN-lesioned mouse (c), during a two week DD period. Running wheel revolutions (counted per two minute bins) are plotted with a maximum of 100 revolutions per bin. Time is marked in hours along the horizontal axis. Successive days are stacked on the vertical axis starting at the top. (d–f) Periodogram analysis of the corresponding (upper) running wheel data. A period range of 14 to 32 hours (x-axis) was analyzed with a single bin resolution. Prevalence of each period is expressed as a Qp value (y-axis). The linear grey line represents the Chi-square significance threshold (p < 0.05). Peaks extending above this threshold indicate that the corresponding period is significantly present in the data. (g–j) SCN lesion damage/extend of selected animals were verified post-mortem by silver staining. (g) Typical lesion of a TPL selected animal. Panels (h–j) summarize damage extend in all selected TPL animals. Damage extend is shown at the rostral SCN (−0.22 AP to bregma) (h), at the central SCN (−0.46 to bregma) (i) and at the caudal SCN (−0.94 to bregma) (j). The white transparent area represents maximal damage extend (area damaged in at least one animal), the dark grey transparent area represents minimal damage extend (area damaged in all TPL selected animals).
Activity recording and analysis
Activity, measured by running-wheel revolutions, was recorded continuously throughout all experiments, by a Circadian Activity Monitor System (CAMS by H.M. Cooper, JA Cooper, INSERM U846, Department of Chronobiology, Bron, France). Revolutions were counted per two minute bins and processed into double plotted qualitative actograms and activity profiles. FAA, normalized for general activity, was calculated by FAA/[DA-FAA], where FAA is the average activity over the period one hour before mealtime until mealtime, and DA is the total average daily activity.
Statistics
Statistical analyses were performed using GraphPad Prism 5.01 (GraphPad software, San Diego, CA). Rhythmicity was assessed by Chi-square periodogram analysis (Refinetti, 2004; Sokolove & Bushell, 1978) using ACTOVIEW for Excel 2010, programmed by C. Mulder, University of Groningen (freely available on request), which was also used to create the actograms. Differences between groups were tested by two-tailed unpaired t-tests or one-way ANOVA with Bonferroni posttests. Pre-post differences were tested by two-tailed paired t-test. The chi-square test was used to test for any location preference during the first habituation step. Differences from chance level were tested by two-tailed one-sample t-test. Differences between groups over multiple testing days were assessed by repeated measures ANOVA with Bonferroni posttest. p < 0.05 was considered significant.
Results
Investigating LEO/FEO involvement in cTPL
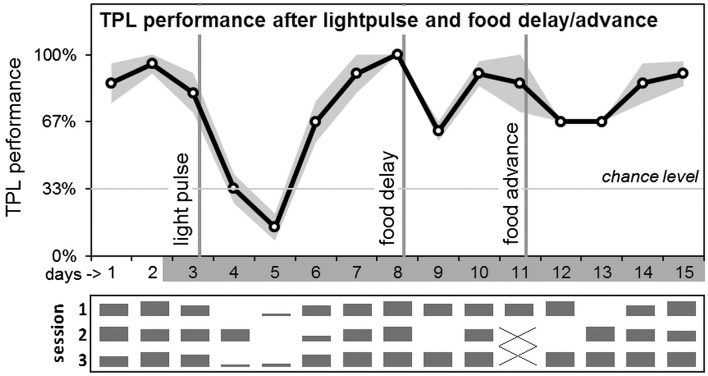
Results are shown in Figure 3. Manipulations were performed on a cohort of seven mice, which had successfully mastered cTPL. High intensity light pulses can phase delay SCN/LEO mediated circadian rhythms when applied at the beginning of the dark phase. We applied a 3h light pulse of 400–800 lux, according to an Aschoff type II protocol (Albrecht et al., 2001). On day 2, lights went out on the regular time (ZT12). On following days, mice remained housed- and were tested in darkness (under a constant dim red light <1 lux measured at the bottom of the cages and the level of the mice in the TPL paradigm). After TPL testing on day 3, the light pulse was applied at the beginning of the subjective dark phase, from circadian time (CT) 12 to CT15. In agreement with the known mouse phase response curve (PRC; Comas et al., 2006), the light pulse induced a 2.5–3 h phase delay in the activity onset of the two HCC animals (the effect size was most clearly distinguishable in these animals because their behavioral rhythms were not influenced/masked by food deprivation and TPL testing procedures). The intervention resulted in a markedly decline in TPL performance lasting for 2–3 days. Daily performances were compared by two-tailed paired t-test. Compared to day 3, performance was dropped significantly on days 4 (p = 0.0004) and 5 (p = 0.0006), but was recovered on day 6 (p = 0.45). Next, a 6 h food delay was performed. Instead of receiving food at CT10.5, mice received food at CT16.5, after TPL testing on day 8. Compared to day 8, this intervention resulted in a significant performance loss on day 9 (p = 0.0002), while performance was recovered on day 10 (p = 0.17). Subsequently a food advance was performed. Instead of receiving food at CT10.5, mice received food at CT4.5, after the first TPL test session on day 11 (test sessions 2 and 3 omitted). Compared to day 10 (day 11 was an incomplete test day), this resulted in a significant performance loss on days 12 (p = 0.008) and 13 (p = 0.008), while performance was recovered on day 14 (p = 0.60). Although test sessions 2 and 3 were omitted on day 11, performance loss does not normally occur after omitting multiple sessions or even complete test days (Mulder et al., 2013c). Daily session-specific performance is shown below the average daily performance graph, in relative bar charts (Figure 3). The light pulse mainly affected performance in sessions 1 and 3 on day 4, while affecting all three daily sessions on day 5. The food delay mainly affected performance only in session 2 on day 9 (all mice wrongly avoided the right-side location instead of the middle location, i.e. mice reacted as if the TOD was later, closer to the third session TOD). The food advance mainly affected performance in session 2 on day 12 (all mice wrongly avoided the left-side location instead of the middle, i.e. mice reacted as if the TOD was earlier, closer to the first session TOD) and session 1 on day 13 (all mice wrongly avoided the right-side location instead of the left-side location, i.e. mice reacted as if the TOD was later, closer to the third session TOD).
Figure 3.
Average daily TPL performance after abrupt LEO and FEO phase-shifts. After testing on day 3, in the beginning of the subjective dark phase, a 3h light pulse (400–800 lux) was applied according to an Aschoff type II protocol. After performance recovered, food was delayed by 6 hours (after testing on day 8). After performance recovered, food was advanced by 6h on day 11. Days are shown on the x-axis (non-shaded days indicate testing in LD; shaded days indicate testing in DD). The grey area around the black performance curve indicates SEM. Vertical lines indicate the interventions. Chance level is indicated by the horizontal line. Daily session-specific performance is shown in bar charts underneath the average daily performance graph (x-axis days are aligned; vertical height of the bars represent relative performance).
Visual/statistical assessment (before TPL testing) and post-mortem verification of SCN lesions
Results are summarized in Figure 2. After SCN/SHAM lesion surgery and the recovery period, animals were put into constant darkness (DD) for 2 weeks, to phenotypically assess behavioral running wheel rhythmicity. Data was processed into double plotted qualitative actograms (Figure 2a–c). Shown are typical actograms of a visually assessed SHAM-lesioned mouse (a), a partial SCN-lesioned mouse (b), and a complete SCN-lesioned mouse (c).
Next to a visual inspection of the actograms, the activity data of the DD period was assessed by Chi-square periodogram analysis. Representative periodograms corresponding to the upper actograms are shown in Figure 2(d–f). Only SCN-lesioned animals that were assessed as arrhythmic by both visual actogram inspection and periodogram analysis were selected for TPL behavioral testing, together with the SHAM-lesioned mice. Exact period and DQp values of the TPL selected mice are given in Table 2. For all SCN-lesioned animals selected for TPL testing (SCNx), complete SCN lesions were confirmed post-mortem (SCNx) by histological inspection of lesion position/-extend after silver staining. Figure 2(g) shows a typical complete SCN lesion. Figure 2(h–j) summarizes damage extend in all SCN-lesioned animals selected for TPL testing. Damage extend is shown at the anterior/rostral SCN (h), mid SCN (i) and at the posterior/caudal SCN (j). The white transparent area represents maximal damage extend (areas damaged in at least one animal), the dark grey transparent area represents minimal damage extend (areas damaged in all SCN-lesioned animals selected for TPL).
Table 2.
Post lesion rhythmicity assessment in DD.
| Batch 1 |
Batch 2 |
||||||
|---|---|---|---|---|---|---|---|
| Mouse | Visual | Period | DQp | Mouse | Visual | Period | DQp |
| SCN lesion | |||||||
| 1 | A | – | −217 | 10 | A | – | −30 |
| 2 | A | – | −211 | 11 | A | – | −226 |
| 3 | A | – | −162 | 12 | A | – | −394 |
| 4 | A | – | −42 | 13 | A | – | −310 |
| 5 | A | – | −289 | 14 | A | – | −125 |
| SHAM | |||||||
| 6 | R | 23.83 | 3961 | 15 | R | 24.00 | 881 |
| 7 | R | 24.03 | 1882 | 16 | R | 23.93 | 804 |
| 8 | R | 23.80 | 2265 | 17 | R | 23.97 | 1688 |
| 9 | R | 23.80 | 4535 | 18 | R | 23.80 | 1609 |
Results from the visual and statistical rhythmicity assessment of mice selected for TPL testing. Visual assessment is based on the pattern of activity apparent in double-plotted actograms. Animals assessed as rhythmic are indicated with an ‘R’; animals assessed as arrhythmic are indicated with an ‘A’. Rhythmicity was statistically assessed by Chi-square periodogram analysis in which a period range of 14 to 32 hours was analyzed over 13 DD days. The DQp value is the difference between the Qp value and the Chi-square significance threshold. The most pronounced period (with the highest positive DQp value) is expressed in hours.
Spontaneous alternation
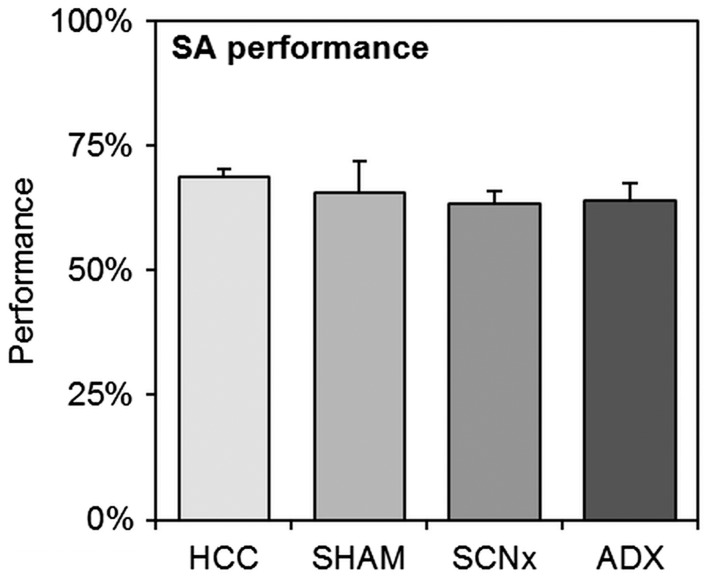
Prior to each TPL test, a SA test was performed. The SA test is a behavioral paradigm to investigate short-term spatial working memory (by assessing alternation percentage, i.e. SA performance) and general exploration behavior (by assessing the number of entries). Results are shown in Figure 4. We found no differences in SA performance between homecage control mice (HCC, n = 13), SHAM-lesioned mice (SHAM, n = 8), SCN-lesioned mice (SCNx, n = 10) (pooled data from both batches), or mice from the second batch after adrenalectomy (ADX, n = 7; SHAM and SCNx mice from the ADX group were statistically tested as separate groups): One-way ANOVA: F = 1.14, dF = 4, p = 0.36. Bonferroni posttests showed no significant differences between groups (p ≥ 0.1 for all group comparisons). Also, we found no differences in the number of entries between the groups: One-way ANOVA: F = 0.83, dF = 4, p = 0.52. Bonferroni posttests showed no significant differences between groups (p ≥ 0.1 for all group comparisons).
Figure 4.
Spontaneous alternation (SA) results of homecage control mice (HCC, n = 13), SHAM-lesioned mice (SHAM, n = 8), SCN-lesioned mice (SCNx, n = 10) (pooled data from both batches), or mice from the second batch after adrenalectomy (ADX, n = 7). No statistical differences were found between any of the groups. Error bars represent SEM.
Habituation to time-place learning
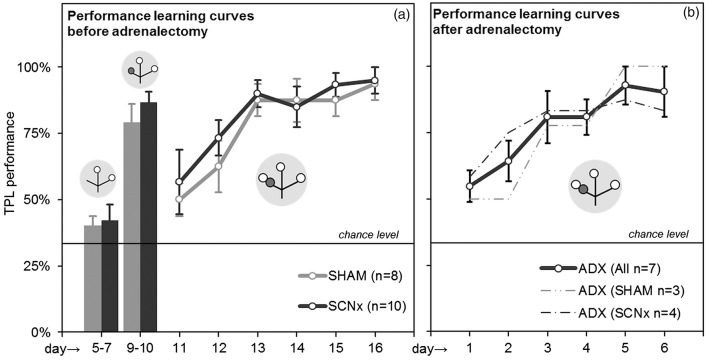
Results are shown in Figure 5(a) (pooled data from the two batches). During the first habituation step (days 1 through 4, not shown), mice could freely explore the three baited locations. No significant preference for a single (first choice) location was found (chi-square p = 0.93, no significant group/batch differences). During the second habituation step (days 5 through 7, test situation with target locations baited; non-target location unbaited without foot-shock delivery), performance of SHAM and SCNx mice did not significantly differ from chance level (two-tailed one-sample t-test: p = 0.10 and p = 0.17 respectively), nor from each other (two-tailed unpaired t-test: p = 0.80). On day 8, mice were habituated to first time foot-shock exposure (see materials and methods, excluded from analyses). During the third habituation step (days 9 and 10, test situation with target locations baited, non-target location unbaited with foot-shock delivery), both SHAM and SCNx mice significantly learned to avoid the non-target location, showing performance significantly different from (above) chance level (p < 0.001 for both SHAM and SCNx mice), with no significant difference between the groups (p = 0.34). High performance is common in this habituation step because mice can identify the non-target/target location(s) based on sight/smell of the absence/presence of food. No significant differences were found between the two batches in any of the habituation steps.
Figure 5.
Habituation results and TPL learning curves. (a) Average performances of SHAM and SCNx mice during the last two habituation steps (left bar graphs) and the first 6 days of TPL testing (learning curve). (b) Combined and separate learning curves of ADX (SHAM) and ADX (SCNx) mice. Grey circular symbols represent the maze. Within, small open circles indicate food at the end of an arm of the maze and small dark grey circles indicate the application of the foot-shock. Note that only the 1st session test situations are depicted. The non-target location (non-baited and non-shock reinforced during habituation days 5–7, non-baited and shock-reinforced during habituation days 9–10, baited and shock-reinforced during actual testing on following days), changes with the TOD (i.e. session). The horizontal lines represent chance level (33%).
Time-place learning
After the habituation steps, testing was performed with food in all locations and foot-shock delivery in the non-target location, which changed according to the TOD. Hence, mice could not identify the non-target/target location(s) based on sight/smell and had to use knowledge of circadian phase to discriminate the hazardous non-target location. Figure 5(a) shows TPL performances of the first six experimental days (testing in LD, pooled data from both batches). On the first experimental day (11), performance of both groups started above chance level, suggesting that the mice already learned TPL in some degree from the habituation steps. Learning curves were formed mainly over the first six days (11–16), during which all mice gradually learned to avoid the non-target location and reached a performance platform around 85–90% (two-way RM ANOVA effect of days: F = 10.42, DF = 5, p < 0.0001; no effect of groups: F = 0.62, dF = 1, p = 0.44). Bonferroni posttests showed no significant differences between groups on any of days 11-16 (p > 0.05 on each day), indicating that learning curves were similar. In fact, for each batch tested separately, no significant differences were found between SHAM and SCNx mice on any of all the experimental days (first batch days 11–38: two-way RM ANOVA effect of groups F = 0.77, dF = 1, p = 0.41, Bonferroni posttests: p > 0.05 for all days; second batch days 11–31: F = 0.53, dF = 1, p = 0.49, Bonferroni posttests: p > 0.05 for all days). In both batches, all individual mice performed significantly above chance level (average over all experimental days: one sample t-test (p < 0.0001 for each mouse).
Animals from the second batch were re-tested after bilateral adrenalectomy surgery. Unfortunately, one SCNx mouse was lost in ADX surgery and one SHAM mouse was excluded because measured CORT levels were too high, suggesting an incomplete adrenalectomy. The ADX group thus includes 4 SCNx and 3 SHAM mice (Table 1). Figure 5(b) shows TPL performances of the first six days (testing in LD). Habituation steps were not repeated. On the first experimental day, average performance started above chance level, indicating that the mice still remembered the time-place associations to some degree. Again, ADX mice formed a learning curve over the first six days (two-way RM ANOVA effect of days: F = 3.69, df = 5, p = 0.01. SHAM and SCNx mice within the ADX group did not significantly differ on any of the experimental days (days 1–13: two-way RM ANOVA effect of groups F = 0.25, df = 1, p = 0.63; Bonferroni posttests: p > 0.05 for all days), indicating similar learning curves. All individual mice performed significantly above chance level (average over all experimental days 1–13: one sample t-test (p < 0.0001 for each mouse).
Investigating circadian characteristics of TPL behavior
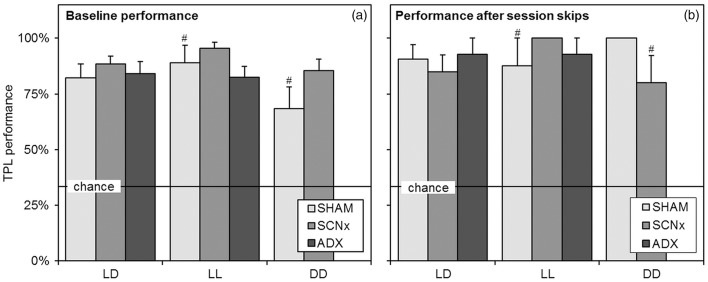
The potential use of non-circadian strategies can be identified by skipping sessions and testing in absence of a LD cycle (Mulder et al., 2013a, 2013c). Baseline TPL performances in the different light regimes are shown in Figure 6(a). Baseline performance is defined as average performance on normal testing days, excluding the first three days of the learning curve and days on which manipulations (sessions skip) were performed. Batches were pooled for data from the same group/light regime). In the upcoming statistical comparisons to chance level (by two tailed one sample t-tests), the number of included subjects (N) is indicated per batch (N = Nbatch1 + Nbatch2). The same format is applied for the number of included days. In LD, performance of all groups was significantly different from chance level (SHAM: n = 4 + 4, days = 7 + 4, p < 0.0001; SCNx: n = 5 + 5, days = 7 + 4, p < 0.0001; ADX: n = 0 + 7, days = 0 + 3, p < 0.0001. Also in LL, all groups performed significantly above chance level (SHAM: n = 0 + 4, days = 0 + 3, p = 0.006; SCNx: n = 0 + 5, days = 0 + 3, p < 0.0001; ADX: n = 0 + 7, days = 0 + 3, p < 0.0001. Also in DD, both tested groups performed significantly above chance level (SHAM: n = 4 + 4, days = 3 + 5, p = 0.009; SCNx: n = 5 + 5, days = 3 + 5, p < 0.0001). We did observe that SHAM mice showed a small decline (but not significant) in average performance during testing in DD, as is reflected in a slightly lower average baseline performance for this group. No differences were found between the groups in any of the light regimes (one-way ANOVA F = 1.52, df = 9, p = 0.17; Bonferroni posttests p ≥ 0.1 for all comparisons). SHAM and SCNx mice from the ADX group were tested as separate groups, indicating no differences.
Figure 6.
Baseline TPL performance and session skipping results. (a) Baseline TPL performances of the different groups when tested in the different light regimes. Baseline performance is defined as average performance on normal testing days, excluding the learning phase (first three days) and days on which manipulations (sessions skips) were performed. Batches were pooled for data from the same group and light regime. (b) Average TPL performances of the groups after multiple (different) session skips in the different light regimes. Performance was measured in the single next session after the skipped session. In both panels, chance level is indicated by the horizontal line. Error bars represent SEM. All results were significantly above chance level (# indicates p < 0.01, for unmarked bars p < 0.001).
Session skipping results are shown in Figure 6(b). Performance was measured in the single next session after the skipped session. First- as well as second sessions were skipped. Over the two batches, data from multiple session skips were averaged per group/light regime. In LD, six sessions were skipped with the SHAM and SCNx groups and two sessions were skipped with the ADX group. In LL, two sessions were skipped with all groups and in DD one session was skipped for the SHAM and SCNx groups. A specification on which session was skipped on which days is provided in the materials and methods (experimental outline section). The number of included subjects (N) in each group/light regime is the same as provided in the description of baseline performances. We found no significant negatively affected performances after session skips compared to baseline performances in any of the groups/light regimes. No significant differences were found in LD (two-tailed paired t-test, SHAM p = 0.44, SCNx p = 0.45, ADX p = 0.45), or in LL (SHAM p = 0.93, SCNx p = 0.18, ADX p = 0.32). In DD, an almost significant performance increase after session skips was found for the SHAM group (SHAM p = 0.07, SCNx p = 0.84). In all light regimes, all groups performed significantly above chance level after session skips (two-tailed one-sample t-test: In LD: SHAM p < 0.0001, SCNx p < 0.0001, ADX p = 0.0002; in LL: SHAM p = 0.02, SCNx no variation, ADX p = 0.0002; in DD: SHAM no variation, SCNx p = 0.019). No differences were found between the groups in any of the light regimes (one-way ANOVA F = 0.70, df = 9, p = 0.71; Bonferroni posttests p ≥ 0.1 for all comparisons). SHAM and SCNx mice from the ADX group were tested as separate groups, indicating no differences. A complete test day was skipped with the animals from the first batch, not resulting in performance loss on the next day (performance SHAM: 91.7 ± 8%; SCNX: 93.3 ± 7%).
Corticosterone radioimmunoassay results
CORT measurements were performed on animals from experiments 1 and 4. Animals were sacrificed between ZT2-3.5, when animals expected to be tested in the first TPL session. Blood samples were taken from the heart prior to transcardial perfusion and CORT was measured by radioimmunoassay. Results are shown in Figure 7. Intact TPL-trained mice from experiment 1 showed a small trend for higher CORT levels compared to HCC mice (Figure 7, striped bars; two-tailed paired t-test: p = 0.09). CORT levels in ADX animals from experiment 4 did not differ from average measurements of three buffer samples (Figure 7, black bars; two-tailed paired t-test: p = 0.95), confirming successful removal of the adrenals.
Analysis of running-wheel activity
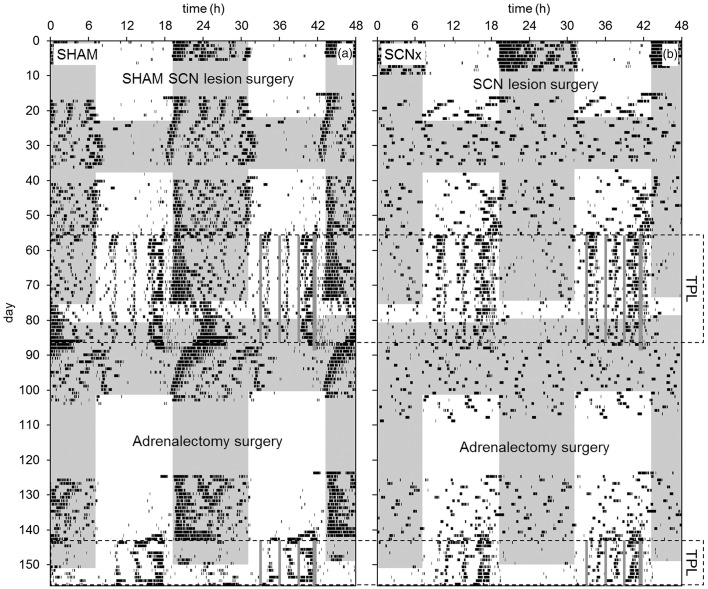
Representative actograms of a SHAM and an SCNx mouse (from the second batch) are shown in Figure 8. Just like the previously tested Cry and Per deficient mice (Mulder et al., 2013c; Van der Zee et al., 2008), SCNx mice showed rhythmic activity during TPL testing in both LD and in constant conditions (LL, DD). However, SCNx mice immediately became arrhythmic during phenotyping in DD (Figure 8b days 24–38), and after the first TPL test, when the mice were remained in DD (Figure 8b days 82–87). The absence of transient cycles indicates masking due to the testing procedures rather than a circadian clock that may have regained functionality due to the regularity of the testing procedures. SCNx mice became almost entirely diurnal during TPL testing. In contrast, SHAM mice showed a free running rhythm component during testing in LL and DD, which was not present in the SCNx mice. These observations agree with a dysfunctional circadian clock in SCNx mice.
Figure 8.
Representative double-plotted qualitative actograms of a SHAM SCN-lesioned mouse (a) and an SCN-lesioned mouse (b) from the second batch. TOD is plotted on the upper x-axis; days are plotted on the y-axis. Shaded areas indicate darkness. SCN lesion and adrenalectomy surgeries are indicated. Data recording failed on days 37–40. Periods in which animals were TPL tested are indicated by the dotted boxes. Within the boxes, on the right side of the actograms, grey vertical lines respectively indicate the three TPL test sessions and the time at which food was provided (thicker grey line: timed feeding/food deprivation was continued for two more days after TPL testing).
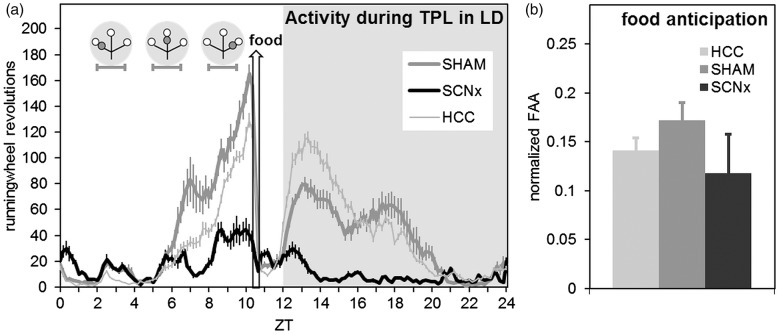
In agreement with other studies (Storch & Weitz, 2009), SCNx mice still showed FAA (Figure 9a). Although SCNx mice show generally lower activity, FAA normalized for general activity (Figure 9b) did not significantly differ from the SHAM mice (two-tailed unpaired t-tests, p = 0.25).
Figure 9.
Activity profiles and food anticipation during TPL testing in LD. (a) Activity profile over all 30 TPL test days in LD (batch1, representative for batch 2 as well), plotted in 10 min bins. Both SHAM and SCNx mice show food anticipation. Zeitgeber time is indicated on the horizontal axis. The shaded area indicates darkness. Gray circular symbols represent the daily TPL test session situations. Within the grey circular symbols, open circles indicate food at the end of an arm of the maze, and gray circles indicate the non-target (shock) location. Horizontal error bars below the circular symbols indicate TPL test session durations. The hollow vertical arrow indicates when food was provided (daily at ZT10.5). (b) Food anticipatory activity normalized for general activity. Activity one hour before mealtime (FAA) was divided by the total daily activity (DA) minus the FAA (FAA/[DA-FAA]). No significant difference was found between SHAM and SCNx mice. In both panels, error bars represent SEM.
Discussion
With TPL animals encode and anticipate the place and time of relevant events. The SCN and adrenal glands are potential candidates for being crucial components of the timing mechanism underlying cTPL. Here we show that although TPL performance is influenced by timing manipulations with light as well as food, the SCN and adrenal glands are not a prerequisite for cTPL in mice. Here we further discuss these findings, the role of the SCN in cognitive tasks, and the mechanism underlying cTPL.
Functional circadian timekeeping in SCN-lesioned and adrenalectomized mice
Several strategies for animals to master a TPL paradigm have been previously identified: a stimulus-response strategy, ordinal timing, interval timing, or using a circadian clock (Carr & Wilkie, 1997). The use of session-specific discrimination cues (a stimulus-response strategy) was experimentally ruled out by keeping testing procedures exactly the same for each daily session. With an ordinal or interval timing strategy, animals respectively remember the sequence of events, or the passage of time relative to a zeitgeber. These strategies were ruled out by showing that skipping sessions and testing in LL and/or DD did not affect TPL performance in any of the tested groups. Together, these results indicate the use of an internal timing mechanism. This leaves the possibility that mice used a circadian hourglass mechanism (a unidirectional internal process that depends on daily resetting by a zeitgeber). With intact performance after the session skips in LD, LL and DD, and a complete non-testing day, this possibility was ruled out for all potential zeitgebers except food. However, feeding time as a zeitgeber for an hourglass mechanism has been ruled out before in behaviorally arrhythmic mice, tested in our specific TPL paradigm (Mulder et al., 2013c). Together, these results strongly indicate that an intact oscillator or oscillator system is underlying cTPL in SCNx and ADX mice, as shown before in wild-type and Per1/Per2 mutant mice (Mulder et al., 2013c; Van der Zee et al., 2008). TPL also depends on spatial discrimination abilities. However, we found no differences between HCC, SHAM, SCNx and ADX mice in the SA test for spatial working memory and exploratory activity.
TPL sensitivity to LEO and FEO phase-shifts
TPL performance should be affected if the underlying oscillator (-system) is abruptly reset to a different phase, so that subjective testing times will mismatch with the previously acquired time stamps. We first investigated whether cTPL performance is sensitive to abrupt phase-shifts of light or food availability in SCN-intact mice. Although the animals were of older age than the animals used in the SCN lesion experiment, we recently found that mice show no age related cTPL deficiencies before the age of 17 months (Mulder et al., 2013b, publication in progress). We used a high intensity light pulse given at the beginning of the subjective night according to a protocol known to phase delay the LEO in the SCN and SCN mediated rhythms by 2.5–3 h (Comas et al., 2006). Similarly, we shifted the FEO by providing food 6 h earlier and 6 h later compared to the fixed time the mice were used to. Because the effectiveness of the induced FEO shifts could not be quantified precisely, a discrepancy between the induced degrees of LEO and FEO phase-shifts is likely, thus allowing only a relative comparison. Nevertheless, in line with the characteristics of an underlying circadian oscillator (-system), the light pulse and food advance negatively affected TPL performance for multiple days while performance gradually recovered. The light pulse and food shifting manipulations may have directly or indirectly (through coupling) affected the cTPL involved oscillators, or may have caused internal desynchrony within an otherwise entrained system of cTPL involved oscillators (i.e. oscillators within the system may have been differentially affected). The recovery can be explained by the input of TPL testing (on the days after the interventions), which either or both re-entrained the underlying clock system(s) and/or re-set the recorded time stamps relative to the phase-shifted oscillator(s). Note however that TPL performance was lower at the second day after the light pulse compared to the first day after the light pulse (Figure 3, day 5 versus day 4), indicating that involved non-SCN oscillators may take time to gradually adjust to the phase-shifted SCN. Interestingly, rather than making random choices, mice generally made the same mistakes after both the light pulse and food shifting manipulations. These results agree with an internal timing system that has been similarly affected in all mice as the cause for the performance effects, rather than any non-specific effects of the interventions, like stress, disturbed sleep or general performance impairment due to a jet lag. Moreover, mice appeared to be alert, motivated and responding as normal during testing after the interventions.
Another interesting finding is that the phase shifting interventions resulted in session-specific performance disturbances and alternative location choices that are not well explained by a single underlying oscillator that has phase-advanced or -delayed. Although it is difficult to predict the dynamic influence of a previous test session on the next (e.g. partial re-setting of timestamps and/or the underlying clock mechanism may occur after the first test session, affecting the second), the results suggest that the cTPL underlying timing mechanism is more complex than a single underlying LEO or FEO. Based on the current results, we postulate that cTPL may involve timekeeping at the level of the session-specific memory traces (see the final section of this discussion), which can thus be differentially affected and thereby result in session-specific disturbances of cTPL performance. Note that such memory integrated clocks may still require the setting and/or entrainment by a reference oscillator, or a system of oscillators, which may include the LEO and FEO as critical or modulatory components. Future research is required to unravel the exact underlying mechanism.
Taken together, the results suggest that a LEO can at least modulate cTPL behavior, while the underlying clock also seems sensitive to food as a zeitgeber. In line with this, Ralph et al. recently reported that time memory in golden hamsters involves the setting of a 24 h oscillator that is functionally and anatomically distinct from the SCN, but is entrained by the SCN acting as a weak (internal) zeitgeber (Ralph et al., 2013). Among other cues, feeding-entrained rhythms may similarly act as a weak zeitgeber to brain regions underlying cTPL. In line with this, we previously proposed that cTPL involved brain areas engaged in cognitive tasks may adapt to the most reliably available internal time signal, while receiving input from multiple oscillators (Mulder et al., 2013c).
The role of the SCN
The effects of a light pulse on cTPL performance strengthened our hypothesis of a role for the SCN in cTPL, either being crucial or modulatory. We hypothesized that in mice the SCN may be the main consulted reference clock, which may even function as a programmable alarm clock (see introduction). Moreover, given that the SCN serves to synchronize various non-photic oscillators, we hypothesized that cTPL in mice may require accurate SCN governed entrainment of local timekeeping in the presumably manifold brain areas involved in cTPL. This may be particularly important to master a TPL task in which three locations/time-points have to be discriminated. Indeed, many studies have shown that the SCN or SCN-mediated rhythms are important for task acquisition and performance (Antoniadis et al., 2000; Davies et al., 1974; Devan et al., 2001; Fekete et al., 1985; Gritton et al., 2013; Neto et al., 2008; Ruby et al., 2008; Stephan & Kovacevic, 1978; Stone et al., 1992; Tapp & Holloway, 1981). cTPL likely requires multiple brain systems involved in feeding, arousal, attention, reward, motivation, spatial orientation, memory, decision making, and time-keeping. For example, Aragona et al. (2002) correlated the expression of TPL behavior with dopamine turnover in the nucleus accumbens (NAcc) and paraventricular nucleus (PVN) of the hypothalamus, indicating involvement of the reward system. Similarly, daily rises in acetylcholine levels associated with task performance have been shown to become anticipatory and time-locked when training occurs at the same time every day, and this precise daily increase in acetylcholine persists for several days in the absence of cognitive training (Paolone et al., 2012). The implementation of a foot-shock in our TPL paradigm likely attributes additional systems related to fear processing (emotional content) and risk evaluation (Amir & Stewart, 2009; Lansink et al., 2012; McIntyre et al., 2012; Roozendaal et al., 2008; Wang et al., 2009).
Recently, Gritton et al. (2013) found evidence that the above hypothesized SCN functions are not mutually exclusive. The authors show that rats entrain to a cognitively demanding task, and that basal forebrain cholinergic projections to the SCN provide the principal signal allowing for the expression of this cognitive entrainment. The authors show that cognitive training also robustly entrains SCN-lesioned rats, indicating (primary) involvement of non-SCN oscillators. However, SCN lesions resulted in significant impairments in task acquisition, indicating that SCN-mediated timekeeping benefits new learning and cognitive performance. The authors conclude that cognitive training entrains non-photic oscillators, while cholinergic signaling to the SCN serves as a temporal timestamp attenuating SCN photic-driven rhythms, thereby permitting non-SCN oscillators to modulate behavior. It has been shown that this cholinergic signaling can induce lasting effects in the SCN, resulting in the circadian expression (coinciding with event TOD) of muscarinic acetylcholine receptors, fitting the view of the SCN as a programmable clock (Hut & Van der Zee, 2011; Van der Zee, 2004). Interestingly, the attenuation of SCN photic driven rhythms at training times suggests that photic driven rhythms will have more influence on behavior at non-training times. This may explain why the light pulse (not given at a TPL test time) induced such a large effect on TPL performance (compared to the food shifts). Although Gritton and coworkers reported significant impairments in task acquisition, and hypothesized that non-SCN oscillators take much longer to become synchronized to each other and to external zeitgebers in absence of a functional SCN, we did not observe such impairments in TPL tested SCN-lesioned mice. Presumably our TPL paradigm is not sensitive enough to detect (minor) positive effects of SCN entrainment. Nevertheless, our results suggest that SCN governed entrainment of non-SCN oscillators is not required for cTPL, even when three locations/time-points have to be discriminated. We consistently found (in all SCN-lesioned mice) that the SCN is not a prerequisite for cTPL acquisition and retention. If anything, SCNx mice performed better rather than worse compared to SHAM mice (Figure 5). We recently observed the same trend in Per1/Per2 mutant mice compared to wild-type mice (Mulder et al., 2013c). On the other hand, SHAM mice showed a small decline (but not significant) in average performance during testing in DD (Figure 6). These observations may be explained by the notion that non-SCN oscillators often interact or compete with the SCN to influence biological and behavioral rhythms (Acosta-Galvan et al., 2011; Angeles-Castellanos et al., 2010; Mendoza et al., 2005). Indeed, SCN ablation is often accompanied by enhanced anticipatory activity to non-photic cues (Angeles-Castellanos et al., 2010; Pezuk et al., 2010; Stephan et al., 1979a,b).
The role of the adrenals
In line with previous studies in SCN deficient (behaviorally arrhythmic) animals (Mistlberger et al., 1996; Mulder et al., 2013c; Van der Zee et al., 2008), SCNx mice in the current study still showed intact behavioral rhythms during TPL testing, even in constant light conditions. With intact behavior rhythms present, the adrenal clock can sustain corticosterone rhythmicity in absence of a functional SCN pacemaker (Oster et al., 2006). Moreover, food anticipation is preceded by a corticosterone peak, which is also still present in SCN-lesioned animals (Krieger et al., 1977; Stephan, 1981, 1989). In line with this, SCNx mice in the current study showed intact food anticipatory activity (FAA), although it should be mentioned that FAA measured in this study is likely influenced by the testing procedures. Extensive evidence indicates that stress hormones released from the adrenal glands are critically involved in memory consolidation of emotionally arousing experience by amygdala activation (Amir & Stewart, 2009; McIntyre et al., 2012; Wang et al., 2009; Roozendaal et al., 2008). Moreover, the rhythmic expression of Per1 in the dentate gyrus was found to be suppressed by corticosterone (Gilhooley et al., 2011). Therefore, we hypothesized that the internal timing mechanism utilized for cTPL may be driven by the adrenal clock. To investigate this, we first measured CORT in intact TPL-trained mice, who expected to be tested in their first TPL session, and HCC mice (experiment 1). We found a trend for higher CORT levels in TPL-trained mice. CORT measurements from both groups were relatively high compared to literature findings (Dalm et al., 2005), which may be explained by the different sacrifice procedure (we used an unaesthetic instead of immediate decapitation). Besides individual variation, this may have masked a significant effect between the groups. We next performed bilateral adrenalectomy on the SHAM and SCNx mice from the second batch, and re-tested them in the TPL paradigm (experiment 4). Because we did not find any differences between the SHAM and SCNx mice, these groups were pooled to one ADX group. None of the individual ADX animals showed cTPL deficiencies, indicating that neither the SCN nor the adrenals are required for cTPL. One point of discussion is that the ADX mice were re-tested. Therefore adrenal corticosterone signaling may still play an initial (enhancing) role in driving (an) underlying oscillator(s) that may become independent with training. Whether naïve adrenalectomized mice can acquire cTPL remains to be investigated. Again, our TPL paradigm may not be sensitive enough to detect minor learning/memory enhancing effects of corticosterone signaling. Nevertheless the current results do not support an essential role for the adrenals in cTPL.
A distributed memory integrated clock
The origin of the primary consulted clock in cTPL remains elusive. This clock may be localized in a single brain region, or emerge from a network of interconnected brain structures, as hypothesized for the FEO. Although the first option is not excluded by our findings, the latter option has gained likeliness. At the systems level, the elements participating in a distributed clock may be variable. For instance, the FEO clock network may conditionally take part in the cTPL clock network depending on whether a TPL task involves restricted feeding (Mulder et al., 2013c; Widman et al., 2004). A distributed clock network is likely complex in the sense that it may involve widespread brain regions, different types of oscillators (self-sustained, partially self-sustained, or hourglass mechanisms, sensitive to various zeitgebers), and an intricate coupling architecture. However, the memory system likely holds a central place in cTPL behavior, providing both essential associative memory input of previous experience, as well as receiving (encoding) specific representations of encountered biological significant events. Clock genes are expressed in all sub regions of the hippocampus and thought to support temporally regulated events underlying memory processes, such as acquisition, consolidation and retrieval (Eckel-Mahan & Storm, 2009; Gerstner & Yin, 2010; Gerstner et al., 2009; Jilg et al., 2010; Kondratova et al., 2010; Rawashdeh & Stehle, 2010). Recent findings suggest that hippocampal “time cells” in the CA1 region take part in episodic memory networks and include a code that can be used to distinguish time intervals on an extended scale of hours to days (Mankin et al., 2012; Shapiro, 2011; Yin & Troger, 2011). Therefore, experience-related cues may act as zeitgebers to a distributed network of cTPL involved brain regions, including the hippocampus, where local timekeeping mechanisms may be entrained. Previously we found that Cry1/Cry2 double knockout mice were unable to master TPL, while Per1/Per2 double mutant mice showed cTPL similar as wild-type mice (Mulder et al., 2013c; Van der Zee et al., 2008). Whether Cry, but not Per genes are essential for temporal coding in the hippocampus remains to be investigated, for example by using hippocampus specific clock gene knockout animals.
Taken together, our current findings contribute to the growing body of evidence that the brain harbors a memory integrated clock system, which includes the LEO, FEO and brain regions involved in associative memory formation. We suspect that the local hippocampal clock, entrained by the distributed network in which it participates, is pivotal regarding the input and output of time-place-event associated memory traces.
Acknowledgments
The authors thank Jennifer Mohawk for advice on the SCN lesions and Wanda Douwenga for help with the perfusions.
Footnotes
Declaration of interest Publication of this work was supported by ZonMW (part of NWO, Netherlands Organisation for Scientific Research), grant number 114024020. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Acosta-Galvan G, Yi CX, van der Vliet J, et al. Interaction between hypothalamic dorsomedial nucleus and the suprachiasmatic nucleus determines intensity of food anticipatory behavior. Proc Natl Acad Sci USA. 2011;108:5813–18. doi: 10.1073/pnas.1015551108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht U, Zheng B, Larkin D, et al. MPer1 and mper2 are essential for normal resetting of the circadian clock. J Biol Rhythms. 2001;16:100–4. doi: 10.1177/074873001129001791. [DOI] [PubMed] [Google Scholar]
- Amir S, Stewart J. Behavioral and hormonal regulation of expression of the clock protein, PER2, in the central extended amygdala. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:1321–8. doi: 10.1016/j.pnpbp.2009.04.003. [DOI] [PubMed] [Google Scholar]
- Angeles-Castellanos M, Salgado-Delgado R, Rodriguez K, et al. The suprachiasmatic nucleus participates in food entrainment: A lesion study. Neuroscience. 2010;165:1115–26. doi: 10.1016/j.neuroscience.2009.11.061. [DOI] [PubMed] [Google Scholar]
- Anisman H. Dissociation of disinhibitory effects of scopolamine: Strain and task factors. Pharmacol Biochem Behav. 1975;3:613–18. doi: 10.1016/0091-3057(75)90182-3. [DOI] [PubMed] [Google Scholar]
- Antoniadis EA, Ko CH, Ralph MR, McDonald RJ. Circadian rhythms, aging and memory. Behav Brain Res. 2000;111:25–37. doi: 10.1016/s0166-4328(00)00145-5. [DOI] [PubMed] [Google Scholar]
- Aragona BJ, Curtis JT, Davidson AJ, et al. Behavioral and neurochemical investigation of circadian time-place learning in the rat. J Biol Rhythms. 2002;17:330–44. doi: 10.1177/074873002129002636. [DOI] [PubMed] [Google Scholar]
- Aschoff J. Temporal orientation: Circadian clocks in animals and humans. Anim Behav. 1989;37:881–96. [Google Scholar]
- Becker PH, Frank D, Sudmann SR. Temporal and spatial pattern of common tern (Sterna hirundo) Foraging in the Wadden Sea. Oecologia. 1993;93:389–93. doi: 10.1007/BF00317883. [DOI] [PubMed] [Google Scholar]
- Beugnon G, Pastergue-Ruiz I, Schatz B, Lachaud JP. Cognitive approach of spatial and temporal information processing in insects. Behav Proc. 1995;35:55–62. doi: 10.1016/0376-6357(95)00061-5. [DOI] [PubMed] [Google Scholar]
- Biebach H. Time-and-place learning by garden warblers, Sylvia-Borin . Anim Behav. 1989;37:353–60. [Google Scholar]
- Biemans BA, Van der Zee EA, Daan S. Age-dependent effects of conditioning on cholinergic and vasopressin systems in the rat suprachiasmatic nucleus. Biol Chem. 2003;384:729–36. doi: 10.1515/BC.2003.081. [DOI] [PubMed] [Google Scholar]
- Boulos Z, Rosenwasser AM, Terman M. Feeding schedules and the circadian organization of behavior in the rat. Behav Brain Res. 1980;1:39–65. doi: 10.1016/0166-4328(80)90045-5. [DOI] [PubMed] [Google Scholar]
- Cain SW, Chalmers JA, Ralph MR. Circadian modulation of passive avoidance is not eliminated in arrhythmic hamsters with suprachiasmatic nucleus lesions. Behav Brain Res. 2012;230:288–90. doi: 10.1016/j.bbr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- Cain SW, Ralph MR. Circadian modulation of conditioned place avoidance in hamsters does not require the suprachiasmatic nucleus. Neurobiol Learn Mem. 2009;91:81–4. doi: 10.1016/j.nlm.2008.10.005. [DOI] [PubMed] [Google Scholar]
- Carr JAR, Tan AO, Wilkie DM. Further evidence that rats use ordinal timing in a daily time-place learning task. Behav Proc. 1999;48:35–48. doi: 10.1016/s0376-6357(99)00074-1. [DOI] [PubMed] [Google Scholar]
- Carr JAR, Wilkie DM. Rats use an ordinal timer in a daily time-place learning task. J Exp Psychol Anim Behav Process. 1997;23:232–47. doi: 10.1037//0097-7403.23.2.232. [DOI] [PubMed] [Google Scholar]
- Carr JAR, Wilkie DM. Rats are reluctant to use circadian timing in a daily time-place task. Behav Process. 1999;44:287–99. doi: 10.1016/s0376-6357(98)00036-9. [DOI] [PubMed] [Google Scholar]
- Comas M, Beersma DG, Spoelstra K, Daan S. Phase and period responses of the circadian system of mice (Mus musculus) to light stimuli of different duration. J Biol Rhythms. 2006;21:362–72. doi: 10.1177/0748730406292446. [DOI] [PubMed] [Google Scholar]
- Crystal JD. Theoretical and conceptual issues in time-place discrimination. Eur J Neurosci. 2009;30:1756–66. doi: 10.1111/j.1460-9568.2009.06968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daan S, Koene P. On the timing of foraging flights by oystercatchers, haematopus ostralegus, on tidal mudflats. Neth J Sea Res. 1981;15:1–22. [Google Scholar]
- Daan S. J Aschoff. Biological rhythms. Verlag, US: Springer; 1981. Adaptive daily strategies in behavior; pp. 275–98. [Google Scholar]
- Dalm S, Enthoven L, Meijer OC, et al. Age-related changes in hypothalamic-pituitary-adrenal axis activity of male C57BL/6J mice. Neuroendocrinology. 2005;81:372–80. doi: 10.1159/000089555. [DOI] [PubMed] [Google Scholar]
- Dana RC, Martinez JL., Jr Effect of adrenalectomy on the circadian rhythm of LTP. Brain Res. 1984;308:392–5. doi: 10.1016/0006-8993(84)91086-2. [DOI] [PubMed] [Google Scholar]
- Davies JA, Navaratnam V, Redfern PH. The effect of phase-shift on the passive avoidance response in rats and the modifying action of chlordiazepoxide. Br J Pharmacol. 1974;51:447–51. doi: 10.1111/j.1476-5381.1974.tb10681.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devan BD, Goad EH, Petri HL, et al. Circadian phase-shifted rats show normal acquisition but impaired long-term retention of place information in the water task. Neurobiol Learn Mem. 2001;75:51–62. doi: 10.1006/nlme.1999.3957. [DOI] [PubMed] [Google Scholar]
- Dibner C, Schibler U, Albrecht U. The mammalian circadian timing system: Organization and coordination of central and peripheral clocks. Annu Rev Physiol. 2010;72:517–49. doi: 10.1146/annurev-physiol-021909-135821. [DOI] [PubMed] [Google Scholar]
- Eckel-Mahan KL, Storm DR. Circadian rhythms and memory: Not so simple as cogs and gears. EMBO Rep. 2009;10:584–91. doi: 10.1038/embor.2009.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enright JT. Ecological aspects of endogenous rhythmicity. Ann Rev Ecol Syst. 1970;1:221–38. [Google Scholar]
- Falk H. Learning a time-place pattern of food availability. Behav Ecol Sociobiol. 1992;31:9–15. [Google Scholar]
- Fekete M, van Ree JM, Niesink RJ, de Wied D. Disrupting circadian rhythms in rats induces retrograde amnesia. Physiol Behav. 1985;34:883–7. doi: 10.1016/0031-9384(85)90008-3. [DOI] [PubMed] [Google Scholar]
- Gallistel CR. The organization of learning. Cambridge, MA: MIT Press; 1990. [Google Scholar]
- Gerstner JR, Lyons LC, Wright KP, Jr, et al. Cycling behavior and memory formation. J Neurosci. 2009:2912824–30. doi: 10.1523/JNEUROSCI.3353-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstner JR, Yin JC. Circadian rhythms and memory formation. Nat Rev Neurosci. 2010;11:577–88. doi: 10.1038/nrn2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilhooley MJ, Pinnock SB, Herbert J. Rhythmic expression of per1 in the dentate gyrus is suppressed by corticosterone: Implications for neurogenesis. Neurosci Lett. 2011;489:177–81. doi: 10.1016/j.neulet.2010.12.011. [DOI] [PubMed] [Google Scholar]
- Gill FB. Trapline foraging by hermit hummingbirds: Competition for an undefended, renewable resource. Ecology. 1988;69:1933–42. [Google Scholar]
- Gritton HJ, Stasiak AM, Sarter M, Lee TM. Cognitive performance as a zeitgeber: Cognitive oscillators and cholinergic modulation of the SCN entrain circadian rhythms. PLoS One. 2013;8:e56206. doi: 10.1371/journal.pone.0056206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groos G, Hendriks J. Circadian rhythms in electrical discharge of rat suprachiasmatic neurones recorded in vitro. Neurosci Lett. 1982;34:283–8. doi: 10.1016/0304-3940(82)90189-6. [DOI] [PubMed] [Google Scholar]
- Honma K, Noe Y, Honma S, et al. Roles of paraventricular catecholamines in feeding-associated corticosterone rhythm in rats. Am J Physiol. 1992;262:E948–55. doi: 10.1152/ajpendo.1992.262.6.E948. [DOI] [PubMed] [Google Scholar]
- Hut RA, Van der Zee EA. The cholinergic system, circadian rhythmicity, time memory. Behav Brain Res. 2011;221:466–80. doi: 10.1016/j.bbr.2010.11.039. [DOI] [PubMed] [Google Scholar]
- Jilg A, Lesny S, Peruzki N, et al. Temporal dynamics of mouse hippocampal clock gene expression support memory processing. Hippocampus. 2010;20:377–88. doi: 10.1002/hipo.20637. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, van der Spek R, Lei J, et al. Circadian rhythms in the hypothalamo-pituitary-adrenal (HPA) axis. Mol Cell Endocrinol. 2012;349:20–9. doi: 10.1016/j.mce.2011.06.042. [DOI] [PubMed] [Google Scholar]
- Ko CH, McDonald RJ, Ralph MR. The suprachiasmatic nucleus is not required for temporal gating of performance on a reward-based learning and memory task. Biol Rhythm Res. 2003;34:177–92. [Google Scholar]
- Ko CH, Takahashi JS. Molecular components of the mammalian circadian clock. Hum Mol Genet. 2006;15:R271–7. doi: 10.1093/hmg/ddl207. [DOI] [PubMed] [Google Scholar]
- Kondratova AA, Dubrovsky YV, Antoch MP, Kondratov RV. Circadian clock proteins control adaptation to novel environment and memory formation. Aging (Albany, NY) 2010;2:285–97. doi: 10.18632/aging.100142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger DT, Hauser H, Krey LC. Suprachiasmatic nuclear lesions do not abolish food-shifted circadian adrenal and temperature rhythmicity. Science. 1977;197:398–9. doi: 10.1126/science.877566. [DOI] [PubMed] [Google Scholar]
- Lansink CS, Jackson JC, Lankelma JV, et al. Reward cues in space: Commonalities and differences in neural coding by hippocampal and ventral striatal ensembles. J Neurosci. 2012;32:12444–59. doi: 10.1523/JNEUROSCI.0593-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankin EA, Sparks FT, Slayyeh B, et al. Neuronal code for extended time in the hippocampus. Proc Natl Acad Sci USA. 2012;109:19462–7. doi: 10.1073/pnas.1214107109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant EG, Mistlberger RE. Anticipation and entrainment to feeding time in intact and SCN-ablated C57BL/6j mice. Brain Res. 1997;765:273–82. doi: 10.1016/s0006-8993(97)00571-4. [DOI] [PubMed] [Google Scholar]
- McDonald RJ, Hong NS, Ray C, Ralph MR. No time of day modulation or time stamp on multiple memory tasks in rats. Learn Motiv. 2002;33:230–52. [Google Scholar]
- McIntyre CK, McGaugh JL, Williams CL. Interacting brain systems modulate memory consolidation. Neurosci Biobehav Rev. 2012;36:1750–62. doi: 10.1016/j.neubiorev.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza J, Angeles-Castellanos M, Escobar C. A daily palatable meal without food deprivation entrains the suprachiasmatic nucleus of rats. Eur J Neurosci. 2005;22:2855–62. doi: 10.1111/j.1460-9568.2005.04461.x. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE, de Groot MH, Bossert JM, Marchant EG. Discrimination of circadian phase in intact and suprachiasmatic nuclei-ablated rats. Brain Res. 1996;739:12–18. doi: 10.1016/s0006-8993(96)00466-0. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE. Circadian food-anticipatory activity: Formal models and physiological mechanisms. Neurosci Biobehav Rev. 1994;18:171–95. doi: 10.1016/0149-7634(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE. Neurobiology of food anticipatory circadian rhythms. Physiol Behav. 2011;104:535–45. doi: 10.1016/j.physbeh.2011.04.015. [DOI] [PubMed] [Google Scholar]
- Mulder CK, Gerkema MP, Van der Zee EA. Circadian clocks and memory: Time-place learning. Front Mol Neurosci. 2013a;6:8 (1–10). doi: 10.3389/fnmol.2013.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder CK, Gerkema MP, Van der Zee EA. International Conference on Individual Differences. 20th Benelux Congress of Zoology. Groningen, The Netherlands: 2013b. Individual differences in time-place learning: Comparison of life-long training with first-time training at old age. [Google Scholar]
- Mulder CK, Van der Zee EA, Hut RA, Gerkema MP. Time-place learning and memory persist in mice lacking functional per1 and per2 clock genes. J Biol Rhythms. 2013c;28:367–79. doi: 10.1177/0748730413512958. [DOI] [PubMed] [Google Scholar]
- Nelson W, Scheving L, Halberg F. Circadian rhythms in mice fed a single daily meal at different stages of lighting regimen. J Nutr. 1975;105:171–84. doi: 10.1093/jn/105.2.171. [DOI] [PubMed] [Google Scholar]
- Neto SP, Carneiro BT, Valentinuzzi VS, Araujo JF. Dissociation of the circadian rhythm of locomotor activity in a 22 h light-dark cycle impairs passive avoidance but not object recognition memory in rats. Physiol Behav. 2008;94:523–7. doi: 10.1016/j.physbeh.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Oklejewicz M, Van Der Zee EA, Gerkema MP, Daan S. Memory retention in wild-type and TAU mutant syrian hamsters. Behaviour. 2001;138:789–96. [Google Scholar]
- Oster H, Damerow S, Kiessling S, et al. The circadian rhythm of glucocorticoids is regulated by a gating mechanism residing in the adrenal cortical clock. Cell Metab. 2006;4:163–73. doi: 10.1016/j.cmet.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Paolone G, Lee TM, Sarter M. Time to pay attention: Attentional performance time-stamped prefrontal cholinergic activation, diurnality, performance. J Neurosci. 2012;32:12115–28. doi: 10.1523/JNEUROSCI.2271-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezuk P, Mohawk JA, Yoshikawa T, et al. Circadian organization is governed by extra-SCN pacemakers. J Biol Rhythms. 2010;25:432–41. doi: 10.1177/0748730410385204. [DOI] [PubMed] [Google Scholar]
- Pizzo MJ, Crystal JD. Representation of time in time-place learning. Anim Learn Behav. 2002;30:387–93. doi: 10.3758/bf03195963. [DOI] [PubMed] [Google Scholar]
- Pizzo MJ, Crystal JD. Evidence for an alternation strategy in time-place learning. Behav Processes. 2004;67:533–7. doi: 10.1016/j.beproc.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Portaluppi F, Smolensky MH, Touitou Y. Ethics and methods for biological rhythm research on animals and human beings. Chronobiol Int. 2010;27:1911–29. doi: 10.3109/07420528.2010.516381. [DOI] [PubMed] [Google Scholar]
- Ralph MR, Foster RG, Davis FC, Menaker M. Transplanted suprachiasmatic nucleus determines circadian period. Science. 1990;247:975–8. doi: 10.1126/science.2305266. [DOI] [PubMed] [Google Scholar]
- Ralph MR, Ko CH, Antoniadis EA, et al. The significance of circadian phase for performance on a reward-based learning task in hamsters. Behav Brain Res. 2002;136:179–84. doi: 10.1016/s0166-4328(02)00131-6. [DOI] [PubMed] [Google Scholar]
- Ralph MR, Sam K, Rawashdeh OA, et al. Memory for time of day. (time memory) is encoded by a circadian oscillator and is distinct from other context memories. Chronobiol Int. 2013;30:540–7. doi: 10.3109/07420528.2012.754449. [DOI] [PubMed] [Google Scholar]
- Rawashdeh O, Stehle JH. Ageing or NOT, clock genes are important for memory processes: An interesting hypothesis raising many questions. Aging (Albany, NY) 2010;2:259–60. doi: 10.18632/aging.100148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reebs SG. Time-place learning in golden shiners. (Pisces: Cyprinidae). Behav Process. 1996;36:253–62. doi: 10.1016/0376-6357(96)88023-5. [DOI] [PubMed] [Google Scholar]
- Refinetti R. Non-stationary time series and the robustness of circadian rhythms. J Theor Biol. 2004;227:571–81. doi: 10.1016/j.jtbi.2003.11.032. [DOI] [PubMed] [Google Scholar]
- Rijnsdorp A, Daan S, Dijkstra C. Hunting in the kestrel, Falco tinnunculus, and the adaptive significance of daily habits. Oecologia. 1981;50:391–406. doi: 10.1007/BF00344982. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Barsegyan A, Lee S. Adrenal stress hormones, amygdala activation, and memory for emotionally arousing experiences. Prog Brain Res. 2008;167:79–97. doi: 10.1016/S0079-6123(07)67006-X. [DOI] [PubMed] [Google Scholar]
- Ruby NF, Hwang CE, Wessells C, et al. Hippocampal-dependent learning requires a functional circadian system. Proc. Natl Acad Sci USA. 2008;105:15593–8. doi: 10.1073/pnas.0808259105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saksida LM, Wilkie DM. Time-of-day discrimination by pigeons, Columba livia . Anim Learn Behav. 1994;22:143–54. [Google Scholar]
- Shapiro ML. Memory time. Neuron. 2011;71:571–3. doi: 10.1016/j.neuron.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Silver R, Bittman EL. Reproductive mechanisms: Interaction of circadian and interval timing. Ann NY Acad Sci. 1984;423:488–514. doi: 10.1111/j.1749-6632.1984.tb23455.x. [DOI] [PubMed] [Google Scholar]
- Smriga M, Saito H, Nishiyama N. Hippocampal long- and short-term potentiation is modulated by adrenalectomy and corticosterone. Neuroendocrinology. 1996;64:35–41. doi: 10.1159/000127095. [DOI] [PubMed] [Google Scholar]
- Sokolove PG, Bushell WN. The chi square periodogram: Its utility for analysis of circadian rhythms. J Theor Biol. 1978;72:131–60. doi: 10.1016/0022-5193(78)90022-x. [DOI] [PubMed] [Google Scholar]
- Stephan FK, Kovacevic NS. Multiple retention deficit in passive avoidance in rats is eliminated by suprachiasmatic lesions. Behav Biol. 1978;22:456–62. doi: 10.1016/s0091-6773(78)92565-8. [DOI] [PubMed] [Google Scholar]
- Stephan FK, Swann JM, Sisk CL. Anticipation of 24-hr feeding schedules in rats with lesions of the suprachiasmatic nucleus. Behav Neural Biol. 1979a;25:346–63. doi: 10.1016/s0163-1047(79)90415-1. [DOI] [PubMed] [Google Scholar]
- Stephan FK, Swann JM, Sisk CL. Entrainment of circadian rhythms by feeding schedules in rats with suprachiasmatic lesions. Behav Neural Biol. 1979b;25:545–54. doi: 10.1016/s0163-1047(79)90332-7. [DOI] [PubMed] [Google Scholar]
- Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci USA. 1972;69:1583–1586. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan FK. Limits of entrainment to periodic feeding in rats with suprachiasmatic lesions. J Comp Physiol. 1981;143:401–10. [Google Scholar]
- Stephan FK. Entrainment of activity to multiple feeding times in rats with suprachiasmatic lesions. Physiol Behav. 1989;46:489–97. doi: 10.1016/0031-9384(89)90026-7. [DOI] [PubMed] [Google Scholar]
- Stone W, Rudd R, Ragozzino M, Gold P. Glucose attenuation of deficits in memory retrieval in altered light:dark cycles. Psychobiology. 1992;20:47–50. [Google Scholar]
- Storch KF, Weitz CJ. Daily rhythms of food-anticipatory behavioral activity do not require the known circadian clock. Proc Natl Acad Sci USA. 2009;106:6808–13. doi: 10.1073/pnas.0902063106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapp WN, Holloway FA. Phase shifting circadian rhythms produces retrograde amnesia. Science. 1981;211:1056–8. doi: 10.1126/science.7193351. [DOI] [PubMed] [Google Scholar]
- Thorpe CM, Bates ME, Wilkie DM. Rats have trouble associating all three parts of the time-place-event memory code. Behav Process. 2003;63:95–110. doi: 10.1016/s0376-6357(03)00051-2. [DOI] [PubMed] [Google Scholar]
- van der Veen DR, Mulder EG, Oster H, et al. SCN-AVP release of mPer1/mPer2 double-mutant mice in vitro. J Circadian Rhythms. 2008;6:5 (1–10). doi: 10.1186/1740-3391-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Zee EA, Biemans BA, Gerkema MP, Daan S. Habituation to a test apparatus during associative learning is sufficient to enhance muscarinic acetylcholine receptor-immunoreactivity in rat suprachiasmatic nucleus. J Neurosci Res. 2004;78:508–19. doi: 10.1002/jnr.20300. [DOI] [PubMed] [Google Scholar]
- Van der Zee EA, Boersma GJ, Hut RA. The neurobiology of circadian rhythms. Curr Opin Pulm Med. 2009;15:534–9. doi: 10.1097/MCP.0b013e3283319b29. [DOI] [PubMed] [Google Scholar]
- Van der Zee EA, Havekes R, Barf RP, et al. Circadian time-place learning in mice depends on Cry genes. Curr Biol. 2008;18:844–8. doi: 10.1016/j.cub.2008.04.077. [DOI] [PubMed] [Google Scholar]
- Wahl O. Neue Untersuchungen über das Zeitgedächtnis der Bienen. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1932;16:529–89. [Google Scholar]
- Wang HC, Wang YC, Huang AC, et al. Roles of corticosterone in formalin-induced conditioned place aversion in rats. Neurosci Lett. 2009;464:122–6. doi: 10.1016/j.neulet.2009.08.053. [DOI] [PubMed] [Google Scholar]
- Wenger D, Biebach H, Krebs JR. Free-running circadian rhythm of a learned feeding pattern in starlings. Naturwissenschaften. 1991;78:87–9. [Google Scholar]
- Widman DR, Sermania CM, Genismore KE. Evidence for time-place learning in the Morris water maze without food restriction but with increased response cost. Behav Proc. 2004;67:183–93. doi: 10.1016/j.beproc.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Wilkie DM, Carr JAR, Siegenthaler A, et al. Field observations of time-place behaviour in scavenging birds. Behav Proc. 1996;38:77–88. doi: 10.1016/0376-6357(96)00026-5. [DOI] [PubMed] [Google Scholar]
- Yin B, Troger AB. Exploring the 4th dimension: Hippocampus, time, memory revisited. Front Integr Neurosci. 2011;5:36 (1–5). doi: 10.3389/fnint.2011.00036. [DOI] [PMC free article] [PubMed] [Google Scholar]