Abstract
Drug-induced liver injury (DILI) is a major challenge in clinical medicine and drug development. New models are needed for predicting which potential therapeutic compounds will cause DILI in humans, and new markers and mediators of DILI still need to be identified. This review highlights the strengths and weaknesses of using zebrafish as a high-throughput in vivo model for studying DILI. Although the zebrafish liver architecture is different from that of the mammalian liver, the main physiological processes remain similar. Zebrafish metabolize drugs using similar pathways to those in humans; they possess a wide range of cytochrome P450 enzymes that enable metabolic reactions including hydroxylation, conjugation, oxidation, demethylation and de-ethylation. Following exposure to a range of hepatotoxic drugs, the zebrafish liver develops histological patterns of injury comparable to those of mammalian liver, and biomarkers for liver injury can be quantified in the zebrafish circulation. The zebrafish immune system is similar to that of mammals, but the zebrafish inflammatory response to DILI is not yet defined. In order to quantify DILI in zebrafish, a wide variety of methods can be used, including visual assessment, quantification of serum enzymes and experimental serum biomarkers and scoring of histopathology. With further development, the zebrafish may be a model that complements rodents and may have value for the discovery of new disease pathways and translational biomarkers.
Keywords: acetaminophen, drug-induced liver injury, hepatotoxicity, liver toxicity, paracetamol, zebrafish
Introduction
Drug-induced liver injury (DILI) is a major problem in clinical medicine and drug development. The most common drug causing DILI in the UK and the USA is paracetamol (acetaminophen), a commonly used analgesic and antipyretic that is safe when used at therapeutic doses. However, when an accidental or deliberate overdose occurs, a metabolite of the drug is produced in excess, and this can lead to potentially fatal hepatocellular necrosis and acute liver failure. Each year, paracetamol overdose directly results in over 300 deaths in the USA 1 and around 150 in the UK 2. The antidote, N-acetylcysteine, replenishes cellular glutathione 3 and is highly effective at preventing DILI if administered soon after overdose, but its efficacy declines substantially with delayed treatment 4.
Besides challenges with treatment of DILI in clinical medicine, in drug development DILI is a major safety concern and remains one of the main reasons for denial of drug approval, withdrawal of drugs from the market or ‘black box’ warnings by the US Food and Drug Administration (FDA) 5. Drug-induced liver injury due to paracetamol overdose is dose dependent and, to an extent, predictable from the dose ingested and a timed measurement of blood drug concentration. In contrast, idiosyncratic liver toxicity is usually identified in late stages of drug development or after a new drug has already been released to the marketplace, occurring in less than one per 10 000–100 000 subjects who take the medication in therapeutic doses 6. Partly because of its rarity, the pathogenesis of idiosyncratic DILI is incompletely understood, which makes it hard to predict at earlier stages of drug development 7. Therefore, high throughput and improved models are needed for predicting human DILI with potential therapeutic compounds and to identify new markers and mediators of DILI secondary to established hepatotoxic drugs, such as paracetamol.
The zebrafish (Danio rerio) is a promising animal for assessing drug-induced toxicity in a variety of organ systems 8. Well-established zebrafish assays have frequently been used for measurement of cardiac and gastrointestinal function and for assessment of the central nervous system and developmental toxicity 9,10. The zebrafish liver can also be used to study drug toxicity; however, in comparison to other zebrafish organs, the zebrafish model of liver toxicity has been used less frequently.
This review highlights the strengths and weaknesses of zebrafish for studying DILI. The use of this model has the potential to identify new drug targets for the treatment of DILI and play a role in preclinical drug development. Also, the use of zebrafish is in line with the ‘3Rs’ (reduce, refine and replace) approach of animal use for scientific purposes by replacing higher-order animals with lower-order zebrafish (particularly, zebrafish embryos).
Potential advantages of zebrafish as a model for studying DILI
Histopathology and clinical chemistry have been used traditionally to report liver toxicity in established animal models. In order to decrease the cost and time of toxicity studies, alternative test systems have been developed; these include liver slices 11, cultured primary hepatocytes 12 and immortal hepatic cell lines, such as the human hepatoma-derived HepG2 line 13 and the recently derived human hepatocyte HepaRG line 14. The advantage of these ex vivo and in vitro approaches is that they can be used efficiently for high-throughput screening. However, the usefulness of these approaches for toxicological testing of compounds can be questioned based on differences in gene expression between the different systems 15 and the low sensitivity of the cytotoxicity assays, which can be <25% for the detection of hepatotoxic agents 16.
In order to perform liver toxicity testing with a higher degree of sensitivity, in vivo assessment is necessary. This allows study of the dose-dependent toxicity of a drug within the complex physiology of a whole organism. Higher vertebrate organisms (e.g. rodents and pigs) are physiologically similar to humans and have been used for this approach. However, smaller, lower-order vertebrates, such as the zebrafish, have similar molecular and cellular processes that can accurately model human physiology 8. In addition, the zebrafish offers significant advantages in comparison to rodents (Table 1) and other larger animals. The zebrafish embryo is optically transparent and grows outside the uterus. This makes it possible to detect and monitor developmental changes easily from the single-cell stage. For example, the zebrafish embryo has allowed researchers to study embryonic lethal phenotypes; something that was not possible with mammalian models 17. Additionally, an early zebrafish embryo, at 3 days postfertilization, is ∼3.5 mm long. This allows zebrafish embryos to be grown at high stocking densities in multiwell plates. The high fecundity of the zebrafish (each female can lay ∼200 eggs per week) can generate hundreds of embryos for screening, each of which has very rapid development. This reduces the cost of zebrafish husbandry significantly, when compared with larger laboratory animals. Furthermore, the Wellcome Trust's Sanger Institute has sequenced the genome of the zebrafish, and many of these sequences have been annotated (http://vega.sanger.ac.uk/Danio_rerio/Info/Index) 18. Further advantages of the zebrafish have been described elsewhere in the literature 10,19–21.
Table 1.
Comparative advantages of using zebrafish and mice to model drug-induced liver injury
| Advantages of zebrafish | Advantages of mice |
|---|---|
| Optically large and transparent embryos | Characterized inbred strains, including knock-out and knock-in strains |
| Ex utero development | Complement of all mammalian organs and physiological similarity to humans |
| Similar cellular and subcellular processes to humans | Easier to draw blood from mice than fish |
| Rapid development of liver ∼72–96 hours postfertilization | Feasible to perform pharmacokinetic/toxicokinetic studies |
| High fecundity (∼200 eggs per female per week) | Genome duplication of fish results in multiple copies of genes |
| Large numbers of fish can be maintained easily | |
| Embryonic fish can survive up to 7 days without a cardiovascular system | |
| Low overall cost | |
| Easy drug delivery by dissolving in the tank water, with possibility of drug delivery by microinjection | |
| Feasibility of high-throughput screens | |
| High n numbers available per study, allowing improved statistical analysis | |
| Lower-order mammal (in line with ‘3Rs’ principles) |
Zebrafish liver anatomy is different from that of rodents and humans
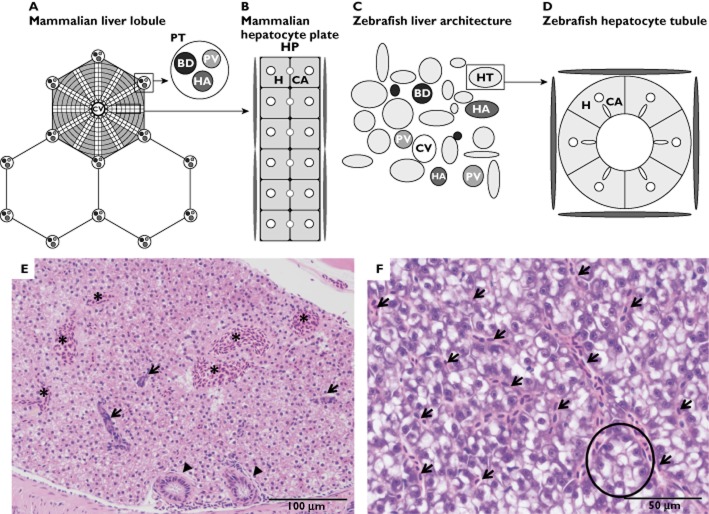
Studies examining the organs of zebrafish, specifically the liver, have revealed multiple similarities with higher vertebrates 22. When liver budding starts at 28 h postfertilization, growth factor and gene expression similar to those of humans and rodents has been reported in zebrafish 23. When hepatic organogenesis is completed at 72 h postfertilization, the liver is perfused with blood and is functional 24. At 120 h postfertilization, the zebrafish is actively seeking food and the yolk sac reserves have become exhausted. By this time, the larval fish already has a fully functional liver. In comparison, in the embryonic mouse the primary liver bud starts to grow around embryonic day 8.5–9 and the liver is mature at embryonic day 18.5, just before birth 25. The tri-lobed liver of the zebrafish is similar to the liver of mammals with regard to biological function; this includes processing of lipids, vitamins, proteins and carbohydrates and the synthesis of serum proteins 22. The main difference between the mammalian and zebrafish liver is the structural organization of the liver tissue. Instead of having the large bile ducts, portal veins and hepatic arteries organized in portal tracts, these are randomly allocated throughout the liver parenchyma in the zebrafish. Hepatocytes in the mammal liver are arranged in plates, whereas in the zebrafish liver they are arranged in tubules. In zebrafish, the bile canaliculi radiate centripetally between hepatocytes to anastomose with a single ductular cell, forming a ductule at the centre of the tubule. These ductules form a network that transports the bile secreted by hepatocytes. Downstream, these ductules merge into intrahepatic bile ducts, converging at the cystic duct, which exits the liver at the hilum to connect with the gallbladder. Subsequently, the gallbladder empties into the intestine through the common bile duct (Figure 1) 23,26. The above-mentioned lack of lobular arrangement impairs morphological differentiation between venules from the portal and hepatic veins, because these vessels are histologically identical.
Figure 1.
Schematic transverse representations of mammalian and zebrafish liver architecture. (A) The mammalian liver lobule, arranged with plates of hepatocytes radiating outward from a central vein (CV). At the corners of each lobule are portal tracts (PT), containing a portal vein (PV), a hepatic artery (HA) and a bile duct (BD). (B) Mammalian bilayered hepatocyte plate. Bicellular canaliculi (CA) are located adjacent to the hepatocytes (H) in the hepatocyte plate (HP). A basal hepatocyte membrane allows transport of oxygen, proteins and different macromolecules to the hepatocytes. Blood enters the liver through the portal vein and hepatic artery, after which it enters the central vein through sinusoid vessels, located between the plates. (C) The zebrafish liver architecture. The portal vein (PV), hepatic artery (HA), bile duct (BD), hepatocyte tubule (HT) and the central vein (CV) are scattered throughout the parenchyma. (D) Zebrafish hepatocytes (H) are arranged in tubules around small bile ducts, which receive bile from the hepatocyte canaliculi (CA). Sinusoids are located at the periphery of these tubules. (E) Histological image of male zebrafish liver (haematoxylin and eosin staining at ×200 magnification). Note the presence of several biliary ducts (arrows), bile ductules (arrowheads) and blood vessels (*), with lack of lobular arrangement. (F) Histological image of female zebrafish liver (haematoxylin and eosin staining at ×400 magnification). This high-power image displays sinusoidal spaces between hepatocytes (arrows) and an instance of the tubular arrangement of hepatocytes (encircled), which is frequently not visible histologically. Note the difference in staining of male and female zebrafish liver
Zebrafish drug metabolism is similar to that of rodents and humans
One of the key physiological functions of the liver is oxidative catalytic transformation, which leads to activation or inactivation of many endogenous and exogenous compounds. This metabolism is mainly performed by the cytochrome P450 (CYP) enzymes, which are predominantly localized in the liver. The metabolic reactions performed by the CYP enzymes include oxidation, reduction and hydrolysis. The CYP enzymes can be divided into two major groups. The first group of enzymes, with generally narrow substrate specificity, are predominantly involved in synthesis, activation or inactivation of endogenous regulatory molecules. The second group predominantly metabolize xenobiotics, but may also metabolize endogenous compounds 27,28.
These reactions are divided in two phases. In phase I, the metabolized compound is oxidized, reduced or hydrolysed. These phase I reactions are predominantly mediated by CYP enzymes. In phase II, conjugation takes place (not CYP enzyme mediated). The rate of these reactions is controlled by expression levels and activity of the specific enzymes 29.
When selecting an animal model for toxicity testing, characterization of the metabolic properties of the selected species is very important. These properties influence DILI, for example, by creating reactive metabolites, and this will determine whether a compound is toxic 30. Therefore, the application of zebrafish as a model of human (hepatic) endogenous and exogenous compound metabolism requires the full range of CYP genes; these have been identified in zebrafish and annotated with regard to their phylogenetic relationships to human CYP enzymes. This essential study was reported by Goldstone and colleagues, who characterised a total of 94 CYP genes in the zebrafish genome 27. Based on homologous amino-acid sequences, they reported that these genes fitted into 18 CYP gene families that are also present in humans and other mammals. The CYP enzyme families 1–4, which predominantly metabolize exogenous compounds, are more diverse in zebrafish than in humans. However, analysis of shared synteny demonstrates an evolutionary relationship between human and zebrafish CYP genes. In the CYP families 5–51, zebrafish have single genes like humans, and there is a high degree of conservation between human and zebrafish sequences 27.
Metabolic experiments demonstrate that drugs are metabolized when exposed to zebrafish embryos by similar reactions to those in humans. An overview of reported metabolic experiments is presented in Table 2. The metabolic degradation of the widely used nonsteroidal anti-inflammatory drug ibuprofen is well studied in different mammals 31,32. The compound is metabolized by different reactions, including oxidation of the parent compound to hydroxyl-ibuprofen and carboxy-ibuprofen, as well as glucuronic acid conjugation of both parent and metabolite compounds 33. In humans, the oxidation of ibuprofen is catalysed by the CYP2C8/9 isoforms 34. When ibuprofen is exposed to zebrafish embryos, hydroxylated ibuprofen can be detected in the zebrafish extracts and water samples, suggesting that zebrafish have an analogous metabolic system to the human CYP2C8/9 35.
Table 2.
Specific metabolic drug reactions reported in zebrafish compared with humans
| Drug metabolism in zebrafish | Similar to human | Human P450 isotype | Ref. | |
|---|---|---|---|---|
| Compound | Reaction observed in zebrafish | |||
| Ibuprofen | Hydroxylation | Yes | CYP2C8/9 | 33 |
| Paracetamol | Hydroxylation | Yes | CYP3A4 | 37 |
| Testosterone | Hydroxylation | Yes | CYP3A4 | 37 |
| Cisapride | Sulfate conjugation | No | CYP3A4 | 38 |
| Verapamil | N-Dealkylation and hydroxylation | Yes | CYP3A4, CYP2C8/9, CYP1A2 | 38 |
| Chlorpromazine | Hydroxylation, oxidation, N-demethylation, glucuronidation and sulfation | Yes | CYP1A2, CYP2D6 | 38 |
| Phenacetin | De-ethylation | Yes | CYP1A2 | 38 |
| Dextromethorphan | Demethylation | Yes | CYP2D6 | 38 |
| Bupropion | Hydroxylation | Yes | CYP2B6 | 38 |
Following exposure to high-dose paracetamol, in humans, rats and mice, the reactive metabolite N-acetyl-p-benzoquinone imine (NAPQI) is formed by phase I metabolism of paracetamol, predominately by CYP3A4 36–38. Recently, Chng and colleagues used a glutathione trapping assay for NAPQI to determine that zebrafish generate the same reactive metabolite as humans. The same authors reported that the zebrafish CYP3A65, orthologue for the human CYP3A4, contributed to the formation of NAPQI, as well as the phase I hydroxylation of testosterone 39.
Alderton and colleagues 40 confirmed that zebrafish embryos are able to perform the metabolic phase I reactions, oxidation, N-demethylation, O-demethylation and N-dealkylation, as well as the metabolic phase II metabolic reactions, sulfation and glucuronidation. The metabolites of three compounds, namely cisapride, verapamil and chlorpromazine, were profiled. With cisapride, the mammalian phase I reactions (piperidine N-delakylation and fluorophenyl ring oxidation) and phase II reactions (glucuronidation, resulting in glucuronide conjugates) were not observed in zebrafish 41. However, following exposure of zebrafish to verapamil, a number of metabolites were formed by N-dealkylation and hydroxylation; these reactions are also present in mammals 42. Three major metabolites of chlorpromazine, which are excreted in human urine, were also excreted by zebrafish; these metabolites were formed by hydroxylation, oxidation, N-demethylation, glucuronidation and sulfation. Alderton and colleagues also reported that zebrafish embryos were able to de-ethylate phenacetin, demethylate dextromethorphan and hydroxylate bupropion 40.
The nuclear receptor, pregnane X receptor (PXR), is involved in the transcriptional regulation of cytochrome P4503A (CYP3A) and the multidrug resistance 1 transporter (MDR1) 43,44. Studies have confirmed that CYP enzymes can be induced and inhibited in zebrafish as reported in mammals. Bresolin and colleagues 45 studied the in vivo expression of PXR, CYP3A and MDR1 genes in the liver of zebrafish treated with the synthetic steroid pregnenolone 16α-carboninitrile, a potent PXR agonist 46. The liver of the fish treated with pregnenolone 16α-carboninitrile had a 1.9-fold increase in PXR, followed by a 1.8-fold increase of CYP3A and 1.6-fold increase in MDR1. This suggests that the regulation of PXR, CYP3A and MDR1 is conserved in zebrafish and similar to mammals 45.
Tseng and colleagues 47 studied the effect of different drugs on CYP3A expression in zebrafish. They found that CYP3A65 expression was upregulated in the embryo (84 h postfertilization) intestine by rifampicin and dexamethasone. In addition to the PXR pathway, the aryl hydrocarbon receptor (AHR2) has a role in the pathway that regulates gene expression and is activated by endogenous and exogenous compounds, such as drugs and xenobiotics 48. The AHR2 is present in different mammals, such as humans, mice and rats, and regulates expression levels of enzymes involved in phase I metabolism, including CYP1A2, CYP1B1 and aldehyde dehydrogenase 3A1 (ALDH3A1), as well as phase II metabolism, including NAD(P)H dehydrogenase quinone 1 (NQO1), UDP glucuronosyltransferase 1A2 (UGT1A2) and glutathione S-transferase α1 (STA1) 49. Expression of CYP3A65 was increased by exposing fish to 2,3,7,8-tetrachloro-dibenzo-p-dioxin, an AHR2 ligand 50, during early embryonic stages, and inhibition of AHR2 translation by antisense morpholino oligonucleotides inhibited both normal and 2,3,7,8-tetrachloro-dibenzo-p-dioxin-stimulated CYP3A65 transcription in embryonic intestine. These data suggest that AHR2 regulates CYP3A65 expression in zebrafish 47.
In summary, the zebrafish liver contains enzymes that metabolize a variety of endogenous and exogenous compounds in a similar manner to human liver. Additionally, these enzymes are subject to similar regulation mechanisms to those reported in humans. These findings support the potential of the zebrafish as an animal model for DILI.
The zebrafish immune system is similar to that of rodents and humans
Hepatic inflammation is commonly reported in various liver diseases, including DILI. Hepatotoxic drugs can have a direct effect on liver cells to release damage-associated molecular patterns (DAMPS) that stimulate immune cell secretion of chemokines and cytokines. Various immune cells, such as lymphocytes, neutrophils and macrophages, can subsequently infiltrate the liver. This complex immune response has been widely described by several authors 51–53. Additionally, specific genetic backgrounds can be a risk factor for idiosyncratic DILI in humans 54,55. For example, a variety of leukocyte antigen (HLA) haplotypes are associated with immunological drug hypersensitivity (e.g. amoxicillin/clavulanate and abacavir) 56–59.
Many similarities exist between the zebrafish and the mammalian immune systems. Different studies of haematopoiesis in zebrafish have demonstrated that most, if not all, cell types of the human immune system have zebrafish counterparts, although the sites of origin differ 60. There is a variation in the repertoire of chemokine receptors in different species, regardless of the specific evolutionary position. Despite this, the expression and function of orthologous chemokine receptors in lower and higher vertebrates are highly similar 61. While the zebrafish metabolizes drugs using similar pathways to humans, whether a similar immune response takes place with DILI in zebrafish is yet to be confirmed.
A range of drugs induce liver toxicity in zebrafish
Different methods have been used to assess liver toxicity in zebrafish, for example, visual assessment of gross and microscopic morphological changes, serum enzyme and biomarker tests, hepatic excretory tests and assessment of alterations in chemical constituents of the liver.
Gross/subgross visual phenotypic assessment
The ability to perform assays for liver toxicity with visually assessable phenotypic end-points enables the transparent larval zebrafish to be used in high-throughput screening.
A comparative toxic screen of 50 different compounds classified to be hepatotoxic by the US FDA, and non-toxic controls, was performed in zebrafish embryos. The compounds were screened in a researcher-blinded fashion for evaluation of three specific phenotypic end-points of liver toxicity, i.e. change in liver size, liver morphological abnormality and yolk-sac retention. A sensitivity for hepatotoxic drugs of 86% and specificity for nonhepatotoxic drugs of 77% was reported, which resulted in an overall correlation of 84% with mammalian in vivo data 62. However, when four compounds were excluded from the analysis because of low uptake into the embryo from the tank water, an increased sensitivity, specificity and overall predictability of 97, 77 and 91%, respectively, was reported 63.
He and colleagues 64 exposed zebrafish embryos at 120 h postfertilization to six known mammalian hepatotoxic drugs (acetaminophen, aspirin, tetracycline hydrochloride, sodium valproate, cyclophosphamide and erythromycin) and two nontoxic compounds (sucrose and biotin), after which three phenotypic visual end-points of liver toxicity were assessed quantitatively. These end-points were liver degeneration score, changes in liver size and shape and yolk-sac retention. These end-points were easily measured under a light microscope without the need for dissection. All six hepatotoxic compounds induced liver degeneration, reduced liver size and led to yolk-sac retention, which suggested that this assay could be predictive for liver toxicity. Zhang and colleagues 65 have developed a transgenic zebrafish line (LiPan) that expresses a liver-specific fluorescent protein (DsRed) under the fabp10a promoter. They reported that the LiPan line could identify hepatotoxic drugs by detecting changes in both liver red fluorescence and liver size in a dosage-dependent fashion. This was demonstrated by exposing the LiPan line to the hepatotoxic drugs paracetamol, aspirin, isoniazid and phenylbutazone.
Liver histopathology
Specific changes in zebrafish liver histology have been reported. North and colleagues reported hepatic necrosis after zebrafish were treated with paracetamol 66. As described for other mammals, exposure of zebrafish to hexachlorocyclohexane results in specific histological changes, such as hepatic macrovesicular triglyceride droplets, glycogen depletion and the presence of club-shaped mitochondria 67. Exposure of zebrafish to thioacetamide induces steatohepatitis, which is accompanied by the accumulation of fatty droplets and apoptosis 68. Zebrafish exposed to ethanol display histological changes such as steatosis, as seen in alcoholic liver disease in humans 69. In conclusion, both embryonic and adult zebrafish are amenable to study of the histological changes that accompany different liver pathologies, such as steatosis, apoptosis and necrosis.
Circulating biomarkers
Whilst zebrafish embryos offer a range of advantages that facilitate high-throughput screening, adult zebrafish are needed if circulating biomarkers are to be measured.
Murtha and colleagues determined multiple serum biochemical values in zebrafish (±1 year old), such as total bilirubin concentration (mean ± SD, 0.38 ± 0.1 mg dl−1; range, 0.2–0.6 mg dl−1) and serum alanine transaminase (ALT) activity (mean ± SD, 376 ± 25.3 U l−1; range, 343–410 U l−1) 70. However, in our laboratory we have reported lower ALT activity (range, 12–137 U l−1) in serum from zebrafish (5–24 months old). In a paracetamol-induced liver toxicity model in adult zebrafish, North and colleagues demonstrated that ALT activity increased in zebrafish in a dose- and time-dependent fashion 66. Injury was reduced by acetylcysteine treatment of paracetamol-exposed zebrafish, as is the case in humans 4. We have observed similar effects of paracetamol on zebrafish in our laboratory. In the same model, we reported an increase in the circulating concentration of microRNA-122, a new experimental biomarker for liver toxicity in humans 71, in fish with liver injury 72. Cox and colleagues reported that after paracetamol exposure, inhibition of the enzymic regulator S-nitrosoglutathione reductase (GSNOR) minimized liver toxicity in zebrafish. A GSNOR-specific inhibitor improved survival and histology and lowered ALT activity through the cytoprotective Nrf2 pathway. Paracetamol toxicity studies in GSNOR-deficient mice confirmed conservation of the hepatoprotective properties of S-nitrosothiol signalling across vertebrates 73. This supports use of the zebrafish as a translational model of human paracetamol toxicity and biomarker research.
Challenges in using zebrafish as a new model for DILI
Although a substantial amount of research demonstrates the potential of zebrafish as a model of liver toxicity, there are a number of challenges.
Zebrafish are often exposed to a drug by dissolving the drug in the water, which enables easy and fast drug administration. This is an advantage of the model that allows for high-throughput phenotypic screening, especially if transgenic lines are used 74. The problem with this method of drug administration is that, although the concentration of drug in water is known, the amount taken up by the fish is imprecise and variable, which limits the study of toxicokinetics. Berghmans and colleagues 75 studied the uptake of nine compounds in zebrafish embryos by dissolving the compounds in the water and found a large variability in the bioavailability of the different compounds. This was because the physiochemical properties of different compounds determine the absorption of the compounds into the fish through the gills and intestine, rather than simply their aqueous concentration. If required, drugs can be injected into the yolk sac of embryonic fish, and this method can therefore quantify the administered dose at the expense of being time consuming 76.
The relationship between the lipophilicity of the drug and the amount of compound penetrating the zebrafish has been determined 75; however, no single physiochemical property can accurately predict the uptake of different types of compounds 77. Therefore, bioanalysis should be performed to correlate the amount of drug in the fish (the real body burden) and the observed toxic effects 78. For instance, sodium valproate, a potentially hepatotoxic drug in humans, did not cause toxic effects in zebrafish embryos, possibly due to poor uptake of this drug. In contrast, valproic acid did cause liver toxicity in zebrafish embryos with higher blood concentrations, indicating increased uptake 62. To overcome the possible problem of absorption, the amount of drug taken up by the fish can be determined by using radio-labelled compound and liquid scintillation counting or radio high-performance liquid chromatography. Other methods that determine the uptake of drug into fish include extracting the embryos with acetonitrile and then using liquid chromatography–tandem mass spectrometry analysis 79, and determining the uptake of compounds by drawing blood from adult zebrafish and measuring the concentration of the compound in blood plasma. However, because of the low blood yields obtained from zebrafish, typically 3–20 μl of whole blood 80–84, 10–20 fish may have to be pooled. Zang and colleagues 84 described a recovery method that allows for serial blood sampling from adult zebrafish, but it takes 1–2 weeks for fish to recover normal haemoglobin values after taking a small blood sample of 2 μl. This delayed recovery may limit the application to toxicity studies.
Goldstone and colleagues 27 demonstrated that 66 of the 88 studied CYP genes in zebrafish embryos have a differential level of expression during development between 3 and 48 h postfertilization. This stresses the importance of age on toxicity when zebrafish embryos are used. In liver toxicity studies, embryos must be >3 days postfertilization 27. Circadian rhythms will influence an organism's susceptibility and responses to xenobiotic exposure. It is established that ATP binding cassette (ABC) transporters have a significant impact on bio-availability, metabolism and excretion of drugs. The gene expression of some transporters, including P-glycoprotein, could be under circadian transcriptional regulation in zebrafish, as reported in mice 85. Therefore, age-related and circadian-related gene expression profiles will impact on the relative higher amount of toxic metabolite, which might influence susceptibility. It is for this reason that standardization of protocols when using embryonic stages is an important consideration in high-throughput screens.
Conclusion
Early identification of hepatotoxic compounds would accelerate the process of drug discovery and development and reduce the enormous costs. The zebrafish appears to be a model that may complement established models. However, before the model can be applied on wider scale more validation is needed to confirm the translatability of the model to humans. This may include testing established human hepatotoxic and nonhepatotoxic compounds, comparing dose responses between fish and humans and developing translational biomarkers that bridge between fish, rodents and humans. Furthermore, the immunological response observed with DILI in humans has to be studied in zebrafish to confirm mechanistic similarity. Ultimately, use of the zebrafish as a model for DILI is promising and may enable better decision making in the early stages of drug discovery, before a compound is tested in higher mammals.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
References
- Bronstein AC, Spyker DA, Cantilena LR, Jr, Green J, Rumack BH, Heard SE. 2006 annual report of the American Association of Poison Control Centers' National Poison Data System (NPDS) Clin Toxicol (Phila) 2007;45:815–917. doi: 10.1080/15563650701754763. [DOI] [PubMed] [Google Scholar]
- Hawton K, Bergen H, Simkin S, Dodd S, Pocock P, Bernal W, Gunnell D, Kapur N. Long term effect of reduced pack sizes of paracetamol on poisoning deaths and liver transplant activity in England and Wales: interrupted time series analyses. BMJ (clinical research edition) 2013;346:f403. doi: 10.1136/bmj.f403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauterburg BH, Corcoran GB, Mitchell JR. Mechanism of action of N-acetylcysteine in the protection against the hepatotoxicity of acetaminophen in rats in vivo. J Clin Invest. 1983;71:980–991. doi: 10.1172/JCI110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumack BH, Bateman DN. Acetaminophen and acetylcysteine dose and duration: past, present and future. Clin Toxicol (Phila) 2012;50:91–98. doi: 10.3109/15563650.2012.659252. [DOI] [PubMed] [Google Scholar]
- Temple RJ. 2001. Hepatotoxicity through the years: impact on the FDA Available at http://www.fda.gov/downloads/Drugs/ScienceResearch/ResearchAreas/ucm122149.pdf (last accessed March 2014)
- Farrell GC, Liddle C. Drugs and the liver updated, 2002. Semin Liver Dis. 2002;22:109–113. [PubMed] [Google Scholar]
- McDonnell ME, Braverman LE. Drug-related hepatotoxicity. N Engl J Med. 2006;354:2191–2193. doi: 10.1056/NEJMc060733. author reply 91-3. [DOI] [PubMed] [Google Scholar]
- Lieschke GJ, Currie PD. Animal models of human disease: zebrafish swim into view. Nat Rev Genet. 2007;8:353–367. doi: 10.1038/nrg2091. [DOI] [PubMed] [Google Scholar]
- McGrath P, Li CQ. Zebrafish: a predictive model for assessing drug-induced toxicity. Drug Discov Today. 2008;13:394–401. doi: 10.1016/j.drudis.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Barros TP, Alderton WK, Reynolds HM, Roach AG, Berghmans S. Zebrafish: an emerging technology for in vivo pharmacological assessment to identify potential safety liabilities in early drug discovery. Br J Pharmacol. 2008;154:1400–1413. doi: 10.1038/bjp.2008.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerche-Langrand C, Toutain HJ. Precision-cut liver slices: characteristics and use for in vitro pharmaco-toxicology. Toxicology. 2000;153:221–253. doi: 10.1016/s0300-483x(00)00316-4. [DOI] [PubMed] [Google Scholar]
- LeCluyse EL. Human hepatocyte culture systems for the in vitro evaluation of cytochrome P450 expression and regulation. Eur J Pharm Sci. 2001;13:343–368. doi: 10.1016/s0928-0987(01)00135-x. [DOI] [PubMed] [Google Scholar]
- Schoonen WG, Westerink WM, de Roos JA, Debiton E. Cytotoxic effects of 100 reference compounds on Hep G2 and HeLa cells and of 60 compounds on ECC-1 and CHO cells. I mechanistic assays on ROS, glutathione depletion and calcein uptake. Toxicol in Vitro. 2005;19:505–516. doi: 10.1016/j.tiv.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Aninat C, Piton A, Glaise D, Le Charpentier T, Langouet S, Morel F, Guguen-Guillouzo C, Guillouzo A. Expression of cytochromes P450, conjugating enzymes and nuclear receptors in human hepatoma HepaRG cells. Drug Metab Dispos. 2006;34:75–83. doi: 10.1124/dmd.105.006759. [DOI] [PubMed] [Google Scholar]
- Boess F, Kamber M, Romer S, Gasser R, Muller D, Albertini S, Suter L. Gene expression in two hepatic cell lines, cultured primary hepatocytes, and liver slices compared to the in vivo liver gene expression in rats: possible implications for toxicogenomics use of in vitro systems. Toxicol Sci. 2003;73:386–402. doi: 10.1093/toxsci/kfg064. [DOI] [PubMed] [Google Scholar]
- O'Brien PJ, Irwin W, Diaz D, Howard-Cofield E, Krejsa CM, Slaughter MR, Gao B, Kaludercic N, Angeline A, Bernardi P, Brain P, Hougham C. High concordance of drug-induced human hepatotoxicity with in vitro cytotoxicity measured in a novel cell-based model using high content screening. Arch Toxicol. 2006;80:580–604. doi: 10.1007/s00204-006-0091-3. [DOI] [PubMed] [Google Scholar]
- Chen JN, Haffter P, Odenthal J, Vogelsang E, Brand M, van Eeden FJ, Furutani-Seiki M, Granato M, Hammerschmidt M, Heisenberg CP, Jiang YJ, Kane DA, Kelsh RN, Mullins MC, Nusslein-Volhard C. Mutations affecting the cardiovascular system and other internal organs in zebrafish. Development (Cambridge, England) 1996;123:293–302. doi: 10.1242/dev.123.1.293. [DOI] [PubMed] [Google Scholar]
- Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, Collins JE, Humphray S, McLaren K, Matthews L, McLaren S, Sealy I, Caccamo M, Churcher C, Scott C, Barrett JC, Koch R, Rauch GJ, White S, Chow W, Kilian B, Quintais LT, Guerra-Assuncao JA, Zhou Y, Gu Y, Yen J, Vogel JH, Eyre T, Redmond S, Banerjee R, Chi J, Fu B, Langley E, Maguire SF, Laird GK, Lloyd D, Kenyon E, Donaldson S, Sehra H, Almeida-King J, Loveland J, Trevanion S, Jones M, Quail M, Willey D, Hunt A, Burton J, Sims S, McLay K, Plumb B, Davis J, Clee C, Oliver K, Clark R, Riddle C, Elliot D, Threadgold G, Harden G, Ware D, Mortimore B, Kerry G, Heath P, Phillimore B, Tracey A, Corby N, Dunn M, Johnson C, Wood J, Clark S, Pelan S, Griffiths G, Smith M, Glithero R, Howden P, Barker N, Stevens C, Harley J, Holt K, Panagiotidis G, Lovell J, Beasley H, Henderson C, Gordon D, Auger K, Wright D, Collins J, Raisen C, Dyer L, Leung K, Robertson L, Ambridge K, Leongamornlert D, McGuire S, Gilderthorp R, Griffiths C, Manthravadi D, Nichol S, Barker G, Whitehead S, Kay M, Brown J, Murnane C, Gray E, Humphries M, Sycamore N, Barker D, Saunders D, Wallis J, Babbage A, Hammond S, Mashreghi-Mohammadi M, Barr L, Martin S, Wray P, Ellington A, Matthews N, Ellwood M, Woodmansey R, Clark G, Cooper J, Tromans A, Grafham D, Skuce C, Pandian R, Andrews R, Harrison E, Kimberley A, Garnett J, Fosker N, Hall R, Garner P, Kelly D, Bird C, Palmer S, Gehring I, Berger A, Dooley CM, Ersan-Urun Z, Eser C, Geiger H, Geisler M, Karotki L, Kirn A, Konantz J, Konantz M, Oberlander M, Rudolph-Geiger S, Teucke M, Osoegawa K, Zhu B, Rapp A, Widaa S, Langford C, Yang F, Carter NP, Harrow J, Ning Z, Herrero J, Searle SM, Enright A, Geisler R, Plasterk RH, Lee C, Westerfield M, de Jong PJ, Zon LI, Postlethwait JH, Nusslein-Volhard C, Hubbard TJ, Roest Crollius H, Rogers J, Stemple DL, Begum S, Lloyd C, Lanz C, Raddatz G, Schuster SC. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496:498–503. doi: 10.1038/nature12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs JP. The zebrafish: a new model organism for integrative physiology. Am J Physiol Regul Integr Comp Physiol. 2002;282:R3–9. doi: 10.1152/ajpregu.00589.2001. [DOI] [PubMed] [Google Scholar]
- Santoriello C, Zon LI. Hooked! Modeling human disease in zebrafish. J Clin Invest. 2012;122:2337–2343. doi: 10.1172/JCI60434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth A, Stemple DL, Barroso I. The emerging use of zebrafish to model metabolic disease. Dis Model Mech. 2013;6:1080–1088. doi: 10.1242/dmm.011346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menke AL, Spitsbergen JM, Wolterbeek AP, Woutersen RA. Normal anatomy and histology of the adult zebrafish. Toxicol Pathol. 2011;39:759–775. doi: 10.1177/0192623311409597. [DOI] [PubMed] [Google Scholar]
- Tao T, Peng J. Liver development in zebrafish (Danio rerio) J Genet Genomics. 2009;36:325–334. doi: 10.1016/S1673-8527(08)60121-6. [DOI] [PubMed] [Google Scholar]
- Isogai S, Horiguchi M, Weinstein BM. The vascular anatomy of the developing zebrafish: an atlas of embryonic and early larval development. Dev Biol. 2001;230:278–301. doi: 10.1006/dbio.2000.9995. [DOI] [PubMed] [Google Scholar]
- Zhao R, Duncan SA. Embryonic development of the liver. Hepatology (Baltimore, Md) 2005;41:956–967. doi: 10.1002/hep.20691. [DOI] [PubMed] [Google Scholar]
- Lorent K, Yeo SY, Oda T, Chandrasekharappa S, Chitnis A, Matthews RP, Pack M. Inhibition of Jagged-mediated Notch signaling disrupts zebrafish biliary development and generates multi-organ defects compatible with an Alagille syndrome phenocopy. Development (Cambridge, England) 2004;131:5753–5766. doi: 10.1242/dev.01411. [DOI] [PubMed] [Google Scholar]
- Goldstone JV, McArthur AG, Kubota A, Zanette J, Parente T, Jonsson ME, Nelson DR, Stegeman JJ. Identification and developmental expression of the full complement of Cytochrome P450 genes in Zebrafish. BMC Genomics. 2010;11:643. doi: 10.1186/1471-2164-11-643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebert DW, Dalton TP. The role of cytochrome P450 enzymes in endogenous signalling pathways and environmental carcinogenesis. Nat Rev Cancer. 2006;6:947–960. doi: 10.1038/nrc2015. [DOI] [PubMed] [Google Scholar]
- Williams RT. The metabolism of certain drugs and food chemicals in man. Ann N Y Acad Sci. 1971;179:141–154. doi: 10.1111/j.1749-6632.1971.tb46896.x. [DOI] [PubMed] [Google Scholar]
- Park BK, Boobis A, Clarke S, Goldring CE, Jones D, Kenna JG, Lambert C, Laverty HG, Naisbitt DJ, Nelson S, Nicoll-Griffith DA, Obach RS, Routledge P, Smith DA, Tweedie DJ, Vermeulen N, Williams DP, Wilson ID, Baillie TA. Managing the challenge of chemically reactive metabolites in drug development. Nat Rev Drug Discov. 2011;10:292–306. doi: 10.1038/nrd3408. [DOI] [PubMed] [Google Scholar]
- Adams SS, Bough RG, Cliffe EE, Lessel B, Mills RF. Absorption, distribution and toxicity of ibuprofen. Toxicol Appl Pharmacol. 1969;15:310–330. doi: 10.1016/0041-008x(69)90032-5. [DOI] [PubMed] [Google Scholar]
- Smith HS, Voss B. Pharmacokinetics of intravenous ibuprofen: implications of time of infusion in the treatment of pain and fever. Drugs. 2012;72:327–337. doi: 10.2165/11599230-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Kepp DR, Sidelmann UG, Tjornelund J, Hansen SH. Simultaneous quantitative determination of the major phase I and II metabolites of ibuprofen in biological fluids by high-performance liquid chromatography on dynamically modified silica. J Chromatogr B Biomed Sci Appl. 1997;696:235–241. doi: 10.1016/s0378-4347(97)00239-9. [DOI] [PubMed] [Google Scholar]
- Brown CM, Reisfeld B, Mayeno AN. Cytochromes P450: a structure-based summary of biotransformations using representative substrates. Drug Metab Rev. 2008;40:1–100. doi: 10.1080/03602530802309742. [DOI] [PubMed] [Google Scholar]
- Jones HS, Trollope HT, Hutchinson TH, Panter GH, Chipman JK. Metabolism of ibuprofen in zebrafish larvae. Xenobiotica. 2012;42:1069–1075. doi: 10.3109/00498254.2012.684410. [DOI] [PubMed] [Google Scholar]
- Dahlin DC, Miwa GT, Lu AY, Nelson SD. N-acetyl-p-benzoquinone imine: a cytochrome P-450-mediated oxidation product of acetaminophen. Proc Natl Acad Sci U S A. 1984;81:1327–1331. doi: 10.1073/pnas.81.5.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine JE, Auriola S, Pasanen M, Juvonen RO. Acetaminophen bioactivation by human cytochrome P450 enzymes and animal microsomes. Xenobiotica. 2009;39:11–21. doi: 10.1080/00498250802512830. [DOI] [PubMed] [Google Scholar]
- Jemnitz K, Veres Z, Monostory K, Kobori L, Vereczkey L. Interspecies differences in acetaminophen sensitivity of human, rat, and mouse primary hepatocytes. Toxicol in Vitro. 2008;22:961–967. doi: 10.1016/j.tiv.2008.02.001. [DOI] [PubMed] [Google Scholar]
- Chng HT, Ho HK, Yap CW, Lam SH, Chan EC. An investigation of the bioactivation potential and metabolism profile of Zebrafish versus human. J Biomol Screen. 2012;17:974–986. doi: 10.1177/1087057112447305. [DOI] [PubMed] [Google Scholar]
- Alderton W, Berghmans S, Butler P, Chassaing H, Fleming A, Golder Z, Richards F, Gardner I. Accumulation and metabolism of drugs and CYP probe substrates in zebrafish larvae. Xenobiotica. 2010;40:547–557. doi: 10.3109/00498254.2010.493960. [DOI] [PubMed] [Google Scholar]
- Meuldermans W, Van Peer A, Hendrickx J, Lauwers W, Swysen E, Bockx M, Woestenborghs R, Heykants J. Excretion and biotransformation of cisapride in dogs and humans after oral administration. Drug Metab Dispos. 1988;16:403–409. [PubMed] [Google Scholar]
- Eichelbaum M, Ende M, Remberg G, Schomerus M, Dengler HJ. The metabolism of DL-[14C]verapamil in man. Drug Metab Dispos. 1979;7:145–148. [PubMed] [Google Scholar]
- Mani S, Dou W, Redinbo MR. PXR antagonists and implication in drug metabolism. Drug Metab Rev. 2013;45:60–72. doi: 10.3109/03602532.2012.746363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Lu J, Cheng J, Wang L, Matsubara T, Csanaky IL, Klaassen CD, Gonzalez FJ, Ma X. Human PXR modulates hepatotoxicity associated with rifampicin and isoniazid co-therapy. Nat Med. 2013;19:418–420. doi: 10.1038/nm.3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresolin T, Rebelo M, Celso Dias Bainy A. Expression of PXR, CYP3A and MDR1 genes in liver of zebrafish. Comp Biochem Physiol C Toxicol Pharmacol. 2005;140:403–407. doi: 10.1016/j.cca.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Moore LB, Maglich JM, McKee DD, Wisely B, Willson TM, Kliewer SA, Lambert MH, Moore JT. Pregnane X receptor (PXR), constitutive androstane receptor (CAR), and benzoate X receptor (BXR) define three pharmacologically distinct classes of nuclear receptors. Mol Endocrinol. 2002;16:977–986. doi: 10.1210/mend.16.5.0828. [DOI] [PubMed] [Google Scholar]
- Tseng HP, Hseu TH, Buhler DR, Wang WD, Hu CH. Constitutive and xenobiotics-induced expression of a novel CYP3A gene from zebrafish larva. Toxicol Appl Pharmacol. 2005;205:247–258. doi: 10.1016/j.taap.2004.10.019. [DOI] [PubMed] [Google Scholar]
- Xu C, Li CY, Kong AN. Induction of phase I, II and III drug metabolism/transport by xenobiotics. Arch Pharm Res. 2005;28:249–268. doi: 10.1007/BF02977789. [DOI] [PubMed] [Google Scholar]
- Rowlands JC, Gustafsson JA. Aryl hydrocarbon receptor-mediated signal transduction. Crit Rev Toxicol. 1997;27:109–134. doi: 10.3109/10408449709021615. [DOI] [PubMed] [Google Scholar]
- Poellinger L, Gottlicher M, Gustafsson JA. The dioxin and peroxisome proliferator-activated receptors: nuclear receptors in search of endogenous ligands. Trends Pharmacol Sci. 1992;13:241–245. doi: 10.1016/0165-6147(92)90076-i. [DOI] [PubMed] [Google Scholar]
- Tian Z, Chen Y, Gao B. Natural killer cells in liver disease. Hepatology (Baltimore, Md) 2013;57:1654–1662. doi: 10.1002/hep.26115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeschke H, Williams CD, Ramachandran A, Bajt ML. Acetaminophen hepatotoxicity and repair: the role of sterile inflammation and innate immunity. Liver Int. 2012;32:8–20. doi: 10.1111/j.1478-3231.2011.02501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju C, Reilly T. Role of immune reactions in drug-induced liver injury (DILI) Drug Metab Rev. 2012;44:107–115. doi: 10.3109/03602532.2011.645579. [DOI] [PubMed] [Google Scholar]
- Wilke RA, Lin DW, Roden DM, Watkins PB, Flockhart D, Zineh I, Giacomini KM, Krauss RM. Identifying genetic risk factors for serious adverse drug reactions: current progress and challenges. Nat Rev Drug Discov. 2007;6:904–916. doi: 10.1038/nrd2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uetrecht J. Idiosyncratic drug reactions: current understanding. Annu Rev Pharmacol Toxicol. 2007;47:513–539. doi: 10.1146/annurev.pharmtox.47.120505.105150. [DOI] [PubMed] [Google Scholar]
- Daly AK, Donaldson PT, Bhatnagar P, Shen Y, Pe'er I, Floratos A, Daly MJ, Goldstein DB, John S, Nelson MR, Graham J, Park BK, Dillon JF, Bernal W, Cordell HJ, Pirmohamed M, Aithal GP, Day CP. HLA-B*5701 genotype is a major determinant of drug-induced liver injury due to flucloxacillin. Nat Genet. 2009;41:816–819. doi: 10.1038/ng.379. [DOI] [PubMed] [Google Scholar]
- Donaldson PT, Daly AK, Henderson J, Graham J, Pirmohamed M, Bernal W, Day CP, Aithal GP. Human leucocyte antigen class II genotype in susceptibility and resistance to co-amoxiclav-induced liver injury. J Hepatol. 2010;53:1049–1053. doi: 10.1016/j.jhep.2010.05.033. [DOI] [PubMed] [Google Scholar]
- Martin AM, Nolan D, Gaudieri S, Almeida CA, Nolan R, James I, Carvalho F, Phillips E, Christiansen FT, Purcell AW, McCluskey J, Mallal S. Predisposition to abacavir hypersensitivity conferred by HLA-B*5701 and a haplotypic Hsp70-Hom variant. Proc Natl Acad Sci U S A. 2004;101:4180–4185. doi: 10.1073/pnas.0307067101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallal S, Nolan D, Witt C, Masel G, Martin AM, Moore C, Sayer D, Castley A, Mamotte C, Maxwell D, James I, Christiansen FT. Association between presence of HLA-B*5701, HLA-DR7, and HLA-DQ3 and hypersensitivity to HIV-1 reverse-transcriptase inhibitor abacavir. Lancet. 2002;359:727–732. doi: 10.1016/s0140-6736(02)07873-x. [DOI] [PubMed] [Google Scholar]
- Berman J, Hsu K, Look AT. Zebrafish as a model organism for blood diseases. Br J Haematol. 2003;123:568–576. doi: 10.1046/j.1365-2141.2003.04682.x. [DOI] [PubMed] [Google Scholar]
- Bajoghli B. Evolution and function of chemokine receptors in the immune system of lower vertebrates. Eur J Immunol. 2013;43:1686–1692. doi: 10.1002/eji.201343557. [DOI] [PubMed] [Google Scholar]
- Jones M, Ball JS, Dodd A, Hill AJ. Comparison between zebrafish and Hep G2 assays for the predictive identification of hepatotoxins. Toxicology. 2009;262:13–14. [Google Scholar]
- Hill A, Mesens N, Steemans M, Xu JJ, Aleo MD. Comparisons between in vitro whole cell imaging and in vivo zebrafish-based approaches for identifying potential human hepatotoxicants earlier in pharmaceutical development. Drug Metab Rev. 2012;44:127–140. doi: 10.3109/03602532.2011.645578. [DOI] [PubMed] [Google Scholar]
- He JH, Guo SY, Zhu F, Zhu JJ, Chen YX, Huang CJ, Gao JM, Dong QX, Xuan YX, Li CQ. A zebrafish phenotypic assay for assessing drug-induced hepatotoxicity. J Pharmacol Toxicol Methods. 2013;67:25–32. doi: 10.1016/j.vascn.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Zhang X, Li C, Gong Z. Development of a convenient in vivo hepatotoxin assay using a transgenic zebrafish line with liver-specific DsRed Expression. PloS ONE. 2014;9:e91874. doi: 10.1371/journal.pone.0091874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North TE, Babu IR, Vedder LM, Lord AM, Wishnok JS, Tannenbaum SR, Zon LI, Goessling W. PGE2-regulated wnt signaling and N-acetylcysteine are synergistically hepatoprotective in zebrafish acetaminophen injury. Proc Natl Acad Sci U S A. 2010;107:17315–17320. doi: 10.1073/pnas.1008209107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunbeck T, Gorge G, Storch V, Nagel R. Hepatic steatosis in zebra fish (Brachydanio rerio) induced by long-term exposure to gamma-hexachlorocyclohexane. Ecotoxicol Environ Saf. 1990;19:355–374. doi: 10.1016/0147-6513(90)90036-5. [DOI] [PubMed] [Google Scholar]
- Amali AA, Rekha RD, Lin CJ, Wang WL, Gong HY, Her GM, Wu JL. Thioacetamide induced liver damage in zebrafish embryo as a disease model for steatohepatitis. J Biomed Sci. 2006;13:225–232. doi: 10.1007/s11373-005-9055-5. [DOI] [PubMed] [Google Scholar]
- Passeri MJ, Cinaroglu A, Gao C, Sadler KC. Hepatic steatosis in response to acute alcohol exposure in zebrafish requires sterol regulatory element binding protein activation. Hepatology (Baltimore, Md) 2009;49:443–452. doi: 10.1002/hep.22667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murtha JM, Qi W, Keller ET. Hematologic and serum biochemical values for zebrafish (Danio rerio) Comp Med. 2003;53:37–41. [PubMed] [Google Scholar]
- Starkey Lewis PJ, Dear J, Platt V, Simpson KJ, Craig DG, Antoine DJ, French NS, Dhaun N, Webb DJ, Costello EM, Neoptolemos JP, Moggs J, Goldring CE, Park BK. Circulating microRNAs as potential markers of human drug-induced liver injury. Hepatology (Baltimore, Md) 2011;54:1767–1776. doi: 10.1002/hep.24538. [DOI] [PubMed] [Google Scholar]
- Vliegenthart AD, Starkey Lewis P, Tucker CS, Del Pozo J, Rider S, Antoine DJ, Dubost V, Westphal M, Moulin P, Bailey MA, Moggs JG, Goldring CE, Park BK, Dear JW. Retro-orbital blood acquisition facilitates circulating microRNA measurement in zebrafish with paracetamol hepatotoxicity. Zebrafish. 2014;11:219–226. doi: 10.1089/zeb.2013.0912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox AG, Saunders DC, Kelsey PB, Jr, Conway AA, Tesmenitsky Y, Marchini JF, Brown KK, Stamler JS, Colagiovanni DB, Rosenthal GJ, Croce KJ, North TE, Goessling W. S-nitrosothiol signaling regulates liver development and improves outcome following toxic liver injury. Cell Reports. 2014;6:56–69. doi: 10.1016/j.celrep.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns CG, Milan DJ, Grande EJ, Rottbauer W, MacRae CA, Fishman MC. High-throughput assay for small molecules that modulate zebrafish embryonic heart rate. Nat Chem Biol. 2005;1:263–264. doi: 10.1038/nchembio732. [DOI] [PubMed] [Google Scholar]
- Berghmans S, Butler P, Goldsmith P, Waldron G, Gardner I, Golder Z, Richards FM, Kimber G, Roach A, Alderton W, Fleming A. Zebrafish based assays for the assessment of cardiac, visual and gut function–potential safety screens for early drug discovery. J Pharmacol Toxicol Methods. 2008;58:59–68. doi: 10.1016/j.vascn.2008.05.130. [DOI] [PubMed] [Google Scholar]
- Langheinrich U. Zebrafish: a new model on the pharmaceutical catwalk. Bioessays. 2003;25:904–912. doi: 10.1002/bies.10326. [DOI] [PubMed] [Google Scholar]
- Doshna C, Benbow J, Depasquale M, Okerberg C, Turnquist S, Stedman D, Chapin R, Sivaraman L, Waldron G, Navetta K, Brady J, Banker M, Casimiro-Garcia A, Hill A, Jones M, Ball J, Aleo M. Multi-phase analysis of uptake and toxicity in Zebrafish: relationship to compound physical-chemical properties. Toxicol Sci. 2009;108:78. [Google Scholar]
- Van den Bulck K, Hill A, Mesens N, Diekman H, De Schaepdrijver L, Lammens L. Zebrafish developmental toxicity assay: a fishy solution to reproductive toxicity screening, or just a red herring? Reprod Toxicol. 2011;32:213–219. doi: 10.1016/j.reprotox.2011.06.119. [DOI] [PubMed] [Google Scholar]
- Diekmann H, Hill A. ADMETox in zebrafish. Drug Discov Today Dis Models. 2013;10:e31–e35. [Google Scholar]
- Babaei F, Ramalingam R, Tavendale A, Liang Y, Yan LS, Ajuh P, Cheng SH, Lam YW. Novel blood collection method allows plasma proteome analysis from single zebrafish. J Proteome Res. 2013;12:1580–1590. doi: 10.1021/pr3009226. [DOI] [PubMed] [Google Scholar]
- Jagadeeswaran P, Sheehan JP, Craig FE, Troyer D. Identification and characterization of zebrafish thrombocytes. Br J Haematol. 1999;107:731–738. doi: 10.1046/j.1365-2141.1999.01763.x. [DOI] [PubMed] [Google Scholar]
- Eames SC, Philipson LH, Prince VE, Kinkel MD. Blood sugar measurement in zebrafish reveals dynamics of glucose homeostasis. Zebrafish. 2010;7:205–213. doi: 10.1089/zeb.2009.0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedroso GL, Hammes TO, Escobar TD, Fracasso LB, Forgiarini LF, da Silveira TR. Blood collection for biochemical analysis in adult zebrafish. J Vis Exp. 2012;63:e3865. doi: 10.3791/3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zang L, Shimada Y, Nishimura Y, Tanaka T, Nishimura N. A novel, reliable method for repeated blood collection from aquarium fish. Zebrafish. 2013;10:425–432. doi: 10.1089/zeb.2012.0862. [DOI] [PubMed] [Google Scholar]
- Ando H, Yanagihara H, Sugimoto K, Hayashi Y, Tsuruoka S, Takamura T, Kaneko S, Fujimura A. Daily rhythms of P-glycoprotein expression in mice. Chronobiol Int. 2005;22:655–665. doi: 10.1080/07420520500180231. [DOI] [PubMed] [Google Scholar]