Abstract
OBJECTIVE
This multicenter non-inferiority study evaluated the safety of infant formulas enriched with bovine milk fat globule membrane (MFGM) fractions.
METHODS
Healthy, full-term infants (n = 119) age ≤14 days were randomized to standard infant formula (control), standard formula enriched with a lipid-rich MFGM fraction (MFGM-L), or standard formula enriched with a protein-rich MFGM fraction (MFGM-P). Primary outcome was mean weight gain per day from enrollment to age 4 months (non-inferiority margin: −3.0 g/day). Secondary (length, head circumference, tolerability, morbidity, adverse events) and exploratory (phospholipids, metabolic markers, immune markers) outcomes were also evaluated.
RESULTS
Weight gain was non-inferior in the MFGM-L and MFGM-P groups compared with the control group. Among secondary and exploratory outcomes, few between-group differences were observed. Formula tolerance rates were high (>94%) in all groups. Adverse event and morbidity rates were similar across groups except for a higher rate of eczema in the MFGM-P group (13.9% vs control [3.5%], MFGM-L [1.4%]).
CONCLUSION
Both MFGM-enriched formulas met the primary safety endpoint of non-inferiority in weight gain and were generally well tolerated, although a higher rate of eczema was observed in the MFGM-P group.
Keywords: infant feeding, milk fat globule membrane, growth, safety, formula tolerance
Introduction
Human milk is widely recommended as the optimal source of nutrition for infants.1,2 While the composition of milk changes over the course of lactation,3 fat provides approximately half of the energy of human milk. Fats in milk are secreted in highly specialized fat globules, which consist of a central core of lipid surrounded first by a monolayer of polar lipid, and then by a lipid bilayer with a glycosylated surface. This tri-layer membrane, known as the milk fat globule membrane (MFGM), acts to stabilize the globule as an emulsion and protects it from coalescence and degradation.4 Increasing evidence suggests that the components of the MFGM provide important nutritional and immunological benefits in the developing infant.5 A clinical study in infants fed a low-energy, low-protein formula supplemented with bovine MFGM from ages 2 to 6 months had higher cognitive development scores at 1 year of age, compared with infants fed a standard infant formula.6 A study in Peru in older infants, 6 to 11 months of age, fed an MFGM-enriched complementary food reported beneficial effects in reducing diarrhea morbidity.7
The MFGM in human and bovine milks is comprised primarily of membrane-specific proteins and polar lipids.4,5 While reports of the composition of the MFGM are highly variable, approximately 25% to 70% of the MFGM consists of a diverse group of proteins and accounts for 1% to 2% of the total protein in milk.4,8–11 The majority of the proteins comprising the MFGM are glycoproteins and include mucin, lactadherin, and lactoferrin.12,13 The lipid fraction of the MFGM consists mainly of glycerophospholipids and sphingolipids, including sphingomyelin (SM) and glycosphingolipids.5 Total phospholipid levels are comparable in human and bovine MFGM, although differences in specific phospholipid classes have been identified. For example, compared with bovine milk, human milk has higher levels of SM, plasmalogens, and gangliosides.5,14 Differences in the fatty acid composition of MFGM phospholipids have also been documented.15,16
Several individual components of the MFGM have been shown to play important roles in growth, development of the central nervous system,4,17 immune function, and digestion,4 and are found in lower concentrations in infant formulas than in human milk.6,18–20 Given the potential role of MFGM protein and lipid fractions in infant development, and despite some differences in the composition of human and bovine MFGM, it is plausible that addition of bovine MFGM fractions to infant formula would confer added advantage to the overall bioactivity of the formula and possibly narrow the nutritional gap between conventional infant formulas and human milk. The objective of this pilot study was to evaluate the safety of standard infant formula supplemented with either a lipid-rich or protein-rich bovine MFGM fraction fed to healthy infants starting at approximately 2 weeks of age and continuing through age 4 months.
Materials and Methods
Study design
This was a multicenter, randomized, double-blind, parallel group, reference-controlled pilot study conducted to evaluate the safety of MFGM-enriched infant formulas. The study was conducted between November 25, 2009, and December 15, 2010, at three hospitals in France and one obstetric, gynecologic, and neonatology clinic in Italy. It was conducted in accordance with the guidelines of the Declaration of Helsinki, the World Medical Association General Assembly, and the International Conference on Harmonization Guidelines on Good Clinical Practice after acceptance by local ethical review boards. Written informed consent was obtained from the infants’ parents or legal guardians at the time of enrollment.
Population
The study population consisted of healthy full-term infants aged 14 days or younger at the time of enrollment whose mothers had chosen not to breastfeed beyond 14 days of the child’s life. Eligible infants had a gestational age of at least 37 weeks and a weight between 2500 g and 4500 g at birth. Only infants from singleton pregnancies were included. Exclusion criteria included any significant prenatal, postnatal, or congenital illness or malformation that could potentially affect normal growth; ongoing antibiotic therapy; re-hospitalization for more than 2 days in the first 14 days of life (except those re-hospitalized due to simple non-cholestatic icterus); assessment that parents were likely to be noncompliant; and infant participation in another clinical trial.
Study formulas
Eligible infants were randomized to one of the three feeding groups: control–standard infant formula (NAN 1, Nestlé Nutrition, Nestec Ltd., Vevey, Switzerland); MFGM-L–standard formula enriched with a bovine-derived, lipid-rich MFGM fraction (Fonterra Co-operative Group Limited, Auckland, New Zealand); or MFGM-P–standard formula enriched with a bovine-derived, protein-rich MFGM fraction (Lacprodan® MFGM-10, Arla Foods Ingredients Group P/S, Viby J, Denmark). The study statistician produced a stratified randomization list based on two stratification factors: site and gender. The investigator was provided with a set of sealed envelopes containing the identity codes and types of formula. Envelopes remained sealed unless there was an urgent need to know a subject’s assigned formula.
All formulas were given to infants starting at the age of 14 days (day 14 ± 3) and were continued for an additional 14 weeks, until the age of 4 months (day 112). The MFGM-enriched investigational formulas contained different MFGM fractions that provided different levels of phospholipids (Table 1) while maintaining an overall nutrient profile that was the same as that provided by the control formula (Table 2). Formulas were manufactured, packaged in identical cans, and coded by the study sponsor. The investigator, study staff, and parents were blinded to the identity of the formulas. Preparation instructions were provided on formula labels. Parents were advised to feed their infants as they deemed appropriate, based on the infant’s appetite, age, and weight. Parents were asked to avoid introducing complementary food during the first 4 months of the study, unless otherwise advised by the investigator or the infant’s physician. After 4 months, the formula was switched to a commercial follow-up formula for an additional 8 months at the three sites in France and 2 months at the site in Italy, in accordance with local ethical committee requirements for a safety follow-up period.
Table 1.
Phospholipid content of reconstituted study formulas and human milk.
Table 2.
Nutrient analysis (per 100 kcal) of control and MFGM-enriched formulas.
| NUTRIENT | CONTROL | MFGM-L | MFGM-P |
|---|---|---|---|
| Per 100 kcal | |||
| Protein, g | 1.9 | 2.0 | 1.9 |
| Fat, g | 5.3 | 5.5 | 5.5 |
| Iron, mg | 1.1 | 1.1 | 1.1 |
| Calcium, mg | 56.8 | 60.3 | 59.7 |
| Iodine, μg | 23.1 | 21.2 | 21.0 |
| Zinc, mg | 0.7 | 0.7 | 0.7 |
| Vitamin A, μg RE | 141.4 | 142.8 | 142.8 |
| Vitamin D, μg | 1.7 | 1.7 | 1.7 |
| Vitamin C, mg | 22.0 | 21.6 | 20.6 |
| Folic acid, μg | 11.4 | 14.3 | 14.5 |
| Vitamin B12, μg | 0.4 | 0.4 | 0.6 |
Observation period
Baseline demographic characteristics and anthropometric measurements were collected at the screening visit (between ages 0 and 13 days). Additional clinic visits were scheduled at ages 14 (±3), 56 (±5), 84 (±7), and 112 (±7) days to dispense formula, monitor the infant’s general health and potential adverse events (AEs), and perform data collection procedures. Three additional follow-up visits scheduled at 6, 9, and 12 months of age were offered to the parents of infants at the three sites in France.
Prior to each visit, parents were asked to record the feeding history of their infants for 3 consecutive days before each visit on a 3-day diary card. Feeding history included the number of times the subject was fed, the volume of milk ingested in each feed, and the volume of milk that remained after each feed. Details regarding the infant’s medications (including antibiotic use), treatments, and supplementary food intake were also recorded by parents on these diary cards, as well as episodes of spitting up, vomiting, crying, fussing, or colic. Additional data collection activities at the clinic visits included the collection of stool samples at days 14, 56, and 112, and a blood sample at day 112. Samples were stored at −80 °C (or if not possible at −20 °C) until laboratory assessments were performed.
Safety outcomes
The primary goal of this study was to evaluate the safety of the investigational formulas. The primary safety outcome was mean weight gain (g/day) from baseline (age 0–13 days) to age 112 days (∼age 4 months), with a non-inferiority margin of −3.0 g/day.21 Secondary safety outcomes included changes in recumbent length and head circumference, formula tolerability, morbidity, and AEs.
Anthropometric measurements
Infants were weighed without clothing on calibrated electronic weight scales. Recumbent length was measured at each visit in centimeters (±1 mm) using a standardized length board. Head circumference (±1 mm) was measured approximately 2.5 cm above the eyebrows using a standard nonelastic-coated measuring tape.22
Formula tolerance and compliance
Formula tolerance (yes/no) was determined by the investigator at each visit based on parents’ reports of spitting up, vomiting, crying, fussing, or colic in the 3-day diary. At the initial visit, the investigator explained each symptom to the parents and how to record these in the diary. Formula compliance was based on the feeding history reported in the 3-day diaries. Noncompliance was defined as two or more visits with nonexclusive feeding (ie, having fed >1 bottle per week of another standard formula, not providing study formula for >3 consecutive days, or giving complementary food before age 4 months).
Morbidity and adverse events
Predefined morbidity concerns monitored in the trial included respiratory symptoms (defined as runny nose and/or chronic cough [based on investigator’s clinical judgment; generally >2 weeks duration] suggestive of allergy), fever, eczema, ear infection, colic, constipation, and diarrhea (defined as three or more loose or watery stools in 24 hours). AEs, defined as any untoward medical occurrence in the form of signs, symptoms, or diseases that emerged or worsened during the study, regardless of causal relationship, were assessed based on parental reports, daily records, and physician examination. Morbidity episodes that met the criteria of an AE were recorded as such.
Exploratory outcomes
In light of emerging evidence suggesting that components of the MFGM may provide nutritional and immunological benefits in the developing infant, a number of exploratory metabolic and immune outcomes were evaluated in this study. These included plasma and red blood cell (RBC) membrane phospholipid levels, metabolic markers, fecal immune markers, and immune responses to polio and Haemophilus influenzae type B (HiB) vaccines. Blood samples collected at the end of the feeding intervention (day-112 visit) were used to measure: (1) phospholipid (ie, SM, phosphatidylcholine [PC], phosphatidylethanolamine [PE], and phosphatidylinositol phosphate [PIP]) levels in the plasma and RBC membrane; (2) plasma cardiolipin and cholesterol ester levels; (3) metabolic markers including insulin growth factor 1 (IGF-1), IGF binding protein-3 (IGF BP3), leptin, and C-peptide; and (4) vaccine-specific immune response to polio virus type 1, 2, and 3 and HiB (plasma anti-polio immunoglobulin G [IgG] and anti-HiB IgG). Stool samples collected at the days 14, 56, and 112 visits were used to measure fecal markers of immune function including total immunoglobulin A (IgA), alpha-1-antitrypsin, and calprotectin.
Lipids were analyzed as described previously23 using the extraction method of Folch et al.24 followed by separation of lipid classes by thin layer chromatography and transesterification using the technique of Morrison and Smith.25 Enzyme immunoassay was used to measure anti-HIB IgG (VaccZyme™ Hib IgG, The Binding Site Group Ltd, Birmingham, UK), leptin (Leptin ELISA kit, Mediagnost, Reutlingen, Germany), IGF-BP3 (IGF-BP3 ELISA kit, Mediagnost, Reutlingen, Germany), total IgA (sIgA ELISA Kit, Immundiagnostik AG, Bensheim, Germany), calprotec-tin (Calprest, Eurospital, S.p.A., Trieste, Italy), alpha-1-antitrypsin (α1-Antitrypsin ELISA kit; Immundiagnostik AG, Bensheim, Germany). Chemiluminescent immunoassay was used to measure C-peptide (LIAISON® C-Peptid; DiaSorin S.p.A., Saluggia, Italy) and IGF-1 (LIAISON® IGF-1 DiaSorin S.p.A., Saluggia, Italy); a neutralization assay (Biomnis, Lyon, France) was used for anti-polio IgG.
Statistical methods
The sample size required to evaluate non-inferiority of the primary outcome (weight gain, g/day) with 80% power at α = 0.05 (two sided), assuming a non-inferiority margin of −3.0 g/day and standard deviation (SD) for weight gain of 6.1 g/day, was calculated as 52 infants per group. An enrollment target of 70 infants per group was set to allow for anticipated dropouts. Statistical analyses were performed using SAS® Version 9.2 for Windows in accordance with both intent-to-treat (ITT) and per protocol (PP) approaches. The ITT analyses included all randomized subjects with post-baseline data available. The PP analyses included all randomized subjects who completed the 4-month investigational feeding period (through at least age 112 days) without a major protocol violation (ie, failure to meet enrollment criteria, failure to attend ≥1 study visit within the defined window visit, deviation in vaccination timing, and parent under minimum age requirement at consent signing) and who were considered compliant with study feedings (ie, received assigned formula exclusively for at least two of the three visits after study feedings were initiated). No substantive differences in results were identified between the ITT and PP analyses; therefore, only ITT results are reported here.
Non-inferiority in weight gain from baseline to age 112 days was compared between groups using analysis of variance (ANOVA) with gender and formula as fixed factors. Pairwise comparisons between each MFGM-enriched formula group and the control group were deduced from the ANOVA model. Non-inferiority was determined based on the predefined margin of a clinically relevant difference of −3.0 g/day. Non-inferiority was met if the lower bound of the two-sided 95% confidence interval (CI) was greater than −3.0 g/day. Analysis of covariance with formula as the factor and baseline value as the covariate was used to compare group differences in length and head circumference gains, and z-scores (WHO) for weight, length, and head circumference. In addition, anthropometric parameters were analyzed in male and female subpopulations given established differences in growth rates between the sexes. Pairwise comparisons (Fisher’s exact test or Kruskal–Wallis test) were performed to evaluate differences in morbidity, tolerance, and exploratory outcomes between each MFGM formula group and the control group. Post hoc significance tests across the three feeding groups were additionally performed for the most common AEs and morbidities. These additional analyses were done to account for the potential bias of non-Gaussian data (unequal variance) as a result of having unequal group sizes.
Results
Study population
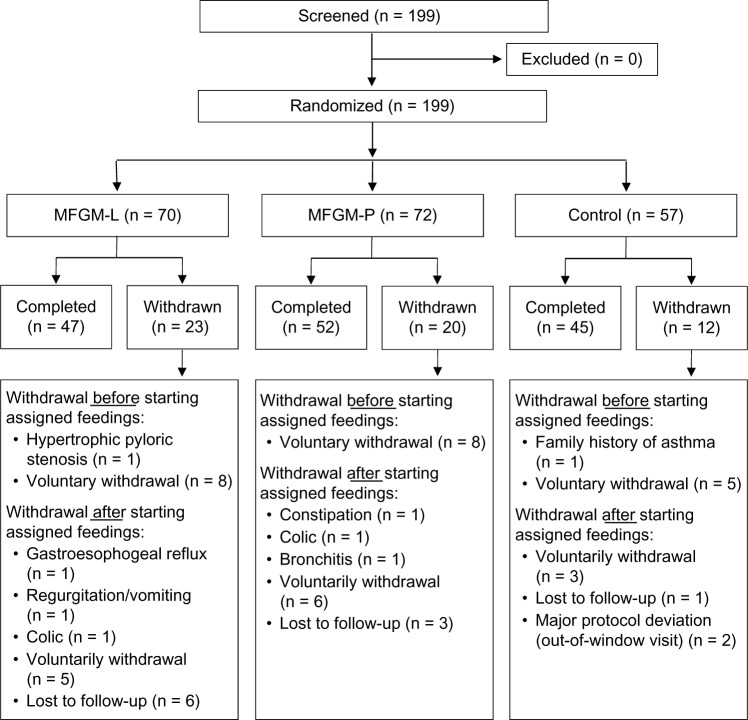
A total of 199 infants were screened and randomized (Fig. 1). Although subjects were intended to be randomized in an equal ratio across groups, the number randomized to the control formula was lower (n = 57) than the number randomized to MFGM-L (n = 70) or MFGM-P (n = 72) groups due to a supply shortage of the control formula. Baseline characteristics and demographics (Table 3) were similar among groups. A total of 46 (80.7%), 50 (71.4%), and 53 (73.6%) infants in the control, MFGM-L, and MFGM-P groups, respectively, were compliant with assigned feedings (P = 0.48).
Figure 1.
Subject disposition.
Table 3.
Demographic and baseline characteristics of the ITT population.
| CONTROL (n = 57) | MFGM-L (n = 70) | MFGM-P (n = 72) | |
|---|---|---|---|
| Male, n (%) | 31 (54.4) | 39 (55.7) | 40 (55.6) |
| Characteristics at birth | |||
| Gestational age (weeks) | 39.3 ± 1.1 | 39.4 ± 1.1 | 39.3 ± 1.3 |
| Weight at birth (g) | 3268.9 ± 374.1 | 3258.5 ± 354.9 | 3328.8 ± 436.8 |
| Length at birth (cm) | 48.9 ± 1.8 | 49.1 ± 1.7 | 49.3 ± 2.0 |
| Head circumference at birth (cm) | 34.1 ± 1.3 | 34.3 ± 1.2 | 34.5 ± 1.3 |
| Characteristics at baseline | |||
| Age (d) | 13.7 ± 1.8 | 13.6 ± 2.0 | 13.9 ± 1.8 |
| Weight (g) | 3494.2 ± 373.8 | 3498.8 ± 344.9 | 3498.0 ± 450.7 |
| Length (cm) | 50.7 ± 1.7 | 50.7 ± 1.6 | 50.9 ± 2.0 |
| Head circumference (cm) | 35.8 ± 1.3 | 35.8 ± 1.5 | 35.9 ± 1.3 |
| Parental characteristics | |||
| Maternal age (y) | 27.8 ± 6.4 | 28.6 ± 5.7 | 29.5 ± 6.3 |
| Paternal age (y) | 30.5 ± 7.9 | 32.1 ± 7.7 | 33.0 ± 7.7 |
| Maternal smoking status | |||
| Nonsmoker, n (%) | 27 (47.4) | 43 (61.4) | 43 (59.7) |
| Former smoker, n (%) | 11 (19.3) | 10 (14.3) | 14 (19.4) |
| Current smoker, n (%) | 19 (33.3) | 17 (24.3) | 15 (20.8) |
| Alcohol use during pregnancy, n (%) | 2 (3.5) | 3 (4.3) | 1 (1.4) |
Note: Data are means ± standard deviation or number (%).
Weight gain and growth
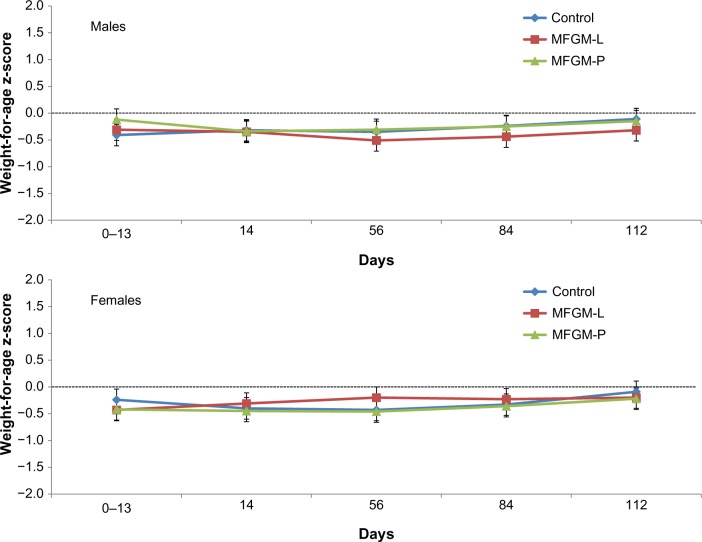
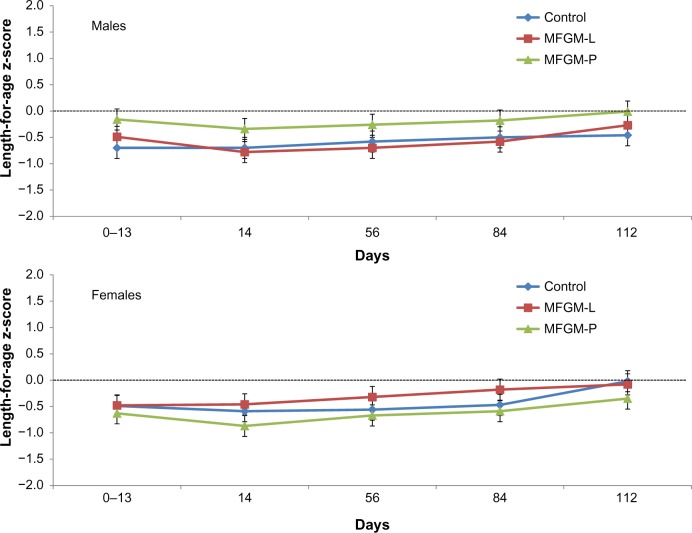
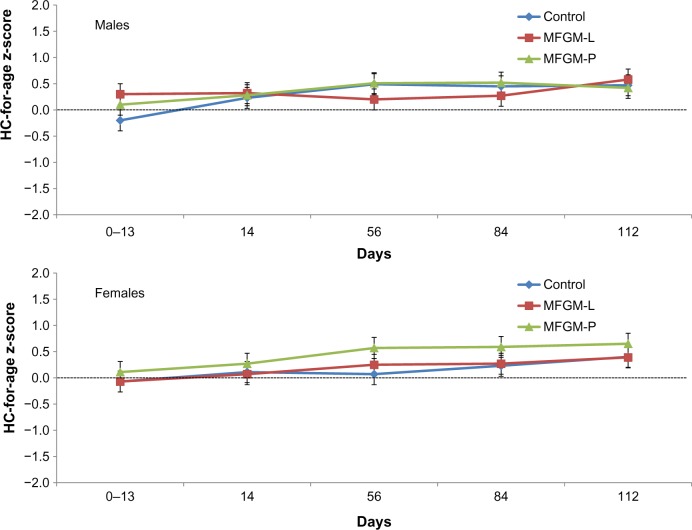
In both MFGM groups, the lower bound of the 95% CI for difference in weight gain vs control was greater than the non-inferiority margin of −3.0 g/day. The difference in mean weight gain between the MFGM-L group and the control group was −0.8 g/day (95% CI, −2.81 to 1.22). The difference between the MFGM-P group and the control group was −0.65 g/day (95% CI, −2.66 to 1.36). Weight z-scores were within −1.0 to +1.0 throughout the study in all three groups, indicating normal growth, with no significant differences observed between groups (Fig. 2). Similarly, there were no significant differences between the MFGM-enriched and control formula groups in length or head circumference gains over the course of the study, and length (Fig. 3) and head circumference (Fig. 4) z-scores were within −1.0 to +1.0 throughout the study.
Figure 2.
Weight-for-age z-scores (WHO) in males and females in the ITT population.
Figure 3.
Length-for-age z-scores (WHO) in males and females in the ITT population.
Figure 4.
Head circumference for age z-scores (WHO) in males and females in the ITT population. HC = head circumference.
Formula tolerance
Formula tolerability was high (94.2%–100% at each visit) and no significant differences were observed between the MFGM-enriched and control formulas.
Morbidity
Predefined morbidity concerns monitored in the trial included respiratory symptoms (runny nose and/or chronic cough), fever, eczema, ear infection, colic, constipation, and diarrhea. Rates of specific morbidities did not differ significantly between the MFGM formula and control groups, with the exception of respiratory symptoms reported by parents in the MFGM-P group (P = 0.01) at the day-56 visit. Compared with the control group, the MFGM-P group had a higher rate of mild respiratory symptoms (12.7% vs 2.1%), but a lower rate of moderate/severe symptoms (0.0% vs 8.4%). No between-group differences were observed in respiratory symptoms at the other clinic visits. The rates of eczema cases recorded as a morbidity did not differ in pairwise comparisons between each MFGM formula group and the control group (eg, lowest pairwise P = 0.12 for MFGM-P vs control at day 112). However, post hoc significance testing done across all three formula groups (to account for differences in group sizes) showed a significantly higher rate of eczema in the MFGM-P group compared with control (P = 0.01).
Adverse events
Treatment-emergent AEs (TEAEs) occurred in 45 (78.9%) infants (120 events) in the control group, 50 (69.4%) infants (138 events) in the MFGM-P group, and 51 (72.9%) infants (140 events) in the MFGM-L group. Rates of the most commonly reported TEAEs (Table 4) were generally balanced across groups. Post hoc significance testing across the three feeding groups revealed that the rate of eczema in the MFGM-P group (13.9%) was significantly higher than the rates reported in the other groups (3.5% in control; 1.4% in MFGM-L; P = 0.001). This finding was consistent with the post hoc morbidity results for eczema (reported above). Eleven infants experienced serious TEAEs (3 [5.3%] in control, 5 [7.1%] in MFGM-L, and 3 [4.2%] in MFGM-P), although only one (bronchitis in the MFGM-P group) was considered by the investigator to be related to feeding (ie, formula contributed to vomiting).
Table 4.
Treatment-emergent adverse events occurring in ≥5% of infants in the ITT population.
| CONTROL (n = 57) | MFGM-L (n = 70) | MFGM-P (n = 72) | |
|---|---|---|---|
| n (%) | |||
| Conjunctivitis | 4 (7.0) | 3 (4.3) | 6 (8.3) |
| Constipation | 2 (3.5) | 8 (11.4) | 7 (9.7) |
| Diarrhea | 4 (7.0) | 4 (5.7) | 7 (9.7) |
| Infantile colic | 5 (8.8) | 5 (7.1) | 5 (6.9) |
| Regurgitation | 8 (14.0) | 7 (10.0) | 5 (6.9) |
| Vomiting | 4 (7.0) | 2 (2.9) | 3 (4.2) |
| Pyrexia | 4 (7.0) | 5 (7.1) | 6 (8.3) |
| Bronchiolitis | 11 (19.3) | 9 (12.9) | 7 (9.7) |
| Bronchitis | 3 (5.3) | 4 (5.7) | 8 (11.1) |
| Ear infection | 10 (17.5) | 3 (4.3) | 6 (8.3) |
| Gastroenteritis | 5 (8.8) | 8 (11.4) | 5 (6.9) |
| Nasopharyngitis | 15 (26.3) | 18 (25.7) | 15 (20.8) |
| Oral candidiasis | 5 (8.8) | 5 (7.1) | 1 (1.4) |
| Rhinitis | 2 (3.5) | 5 (7.1) | 5 (6.9) |
| Eczema* | 2 (3.5) | 1 (1.4) | 10 (13.9) |
Note:
P = 0.001 for post hoc global comparison across all groups.
Exploratory endpoints
Blood samples drawn on the day-112 visit revealed no significant differences in either plasma or RBC membrane levels of phospholipids (PC, PE, SM, and PIP) between infants fed the MFGM-enriched formulas and those fed control formula (Table 5). Similarly, mean plasma cardiolipin and cholesterol ester values were not significantly different between the MFGM and control formula groups. Exploratory analysis of metabolic markers using the day-112 blood samples revealed no differences in IGF-1, leptin, and IGF BP3 levels in the MFGM groups compared with the control group. However, mean ± SD blood C-peptide levels were significantly lower (P = 0.03) in the MFGM-L (2.0 ± 0.9 ng/mL) and MFGM-P groups (1.8 ± 1.0 ng/mL) compared with the control formula group (2.4 ± 0.9 ng/mL).
Table 5.
Concentration of MFGM phospholipids in plasma and RBC membranes.
| CONTROL | MFGM-L | MFGM-P | P-VALUE* | |
|---|---|---|---|---|
| Mean (SD) | ||||
| Phosphatidylcholine (PC) | ||||
| RBC, n | 36 | 43 | 41 | |
| RBC, mg/g | 0.69 (0.14) | 0.65 (0.14) | 0.66 (0.43) | 0.60 |
| Plasma, n | 35 | 42 | 41 | |
| Plasma, mg/mL | 0.87 (0.34) | 0.85 (0.35) | 0.87 (0.28) | 0.76 |
| Phosphatidylethanolamine (PE) | ||||
| RBC, n | 36 | 43 | 41 | |
| RBC, mg/g | 0.55 (0.16) | 0.49 (0.16) | 0.56 (0.13) | 0.10 |
| Plasma, n | 22 | 29 | 31 | |
| Plasma, mg/mL | 0.04 (0.02) | 0.04 (0.02) | 0.04 (0.02) | 0.94 |
| Sphingomyelin (SM) | ||||
| RBC, n | 36 | 43 | 41 | |
| RBC, mg/g | 0.57 (0.12) | 0.56 (0.12) | 0.56 (0.12) | 0.94 |
| Plasma, n | 35 | 42 | 41 | |
| Plasma, mg/mL | 0.14 (0.06) | 0.14 (0.06) | 0.15 (0.04) | 0.70 |
| Phosphatidylinositol phosphate (PIP) | ||||
| RBC, n | 36 | 43 | 41 | |
| RBC, mg/g | 0.43 (0.16) | 0.45 (0.15) | 0.47 (0.18) | 0.53 |
| Plasma, n | 35 | 42 | 41 | |
| Plasma, mg/mL | 0.18 (0.06) | 0.18 (0.07) | 0.18 (0.06) | 0.93 |
Note:
Comparison of the 3 groups globally was performed using the Kruskal–Wallis test.
Due to differences in vaccination schedules, immune responses to polio and HiB vaccines were evaluated separately in infants from France and those from Italy. With one exception, no between-group differences were observed in polio virus type 1, 2, and 3, and HiB antibodies at day 112 in either country. The exception was a lower mean polio virus type 1 IgG level in the MFGM-P group in Italy (P = 0.04). It is notable, however, that IgG levels against polio virus type 1 were below the lower limit of quantification (LLOQ) in the MFGM-P group in Italy. Consequently, mean polio virus type 1 IgG level (kIU/L) in this group was recorded as LLOQ/2 (0.25 ± 0.0) in accordance with a priori statistical analysis rules. By comparison, polio virus type 1 IgG levels in the MFGM-L and the control groups were 1.92 ± 2.22 and 0.93 ± 1.13, respectively. Immune function was also evaluated by assessing the fecal immune markers alpha-1-antitrypsin, secretory IgA, and calprotectin using stool samples collected during clinic visits on days 14, 56, and 112. No significant differences between groups in levels of these fecal immune markers were observed at any time point.
Discussion
The primary objective of the study was to characterize the safety of two bovine MFGM-enriched investigational infant formulas in healthy term infants based on the primary safety endpoint of non-inferiority in weight gain in infants assigned to control formula. Both MFGM-enriched formulas demonstrated non-inferiority in weight gain, as the lower bound of the 95% CIs for difference in weight gain compared with control was greater than −3.0 g/day. Assessments of length and head circumference also showed no differences between groups. Both MFGM-enriched investigational formulas were well tolerated, with no differences seen in parents’ reports of vomiting, spitting up, fussing, crying, and colic. Overall, AE reports did not reveal any major safety concerns. The study found evidence of a higher rate of eczema in the group receiving formula enriched with MFGM-P, although this difference was statistically significant only when the three study groups were compared in post hoc analyses performed to account for potential bias arising from having fewer infants in the control formula group compared with the MFGM-enriched formula groups. Caution is therefore warranted in extrapolating this finding. Notably, a higher risk of eczema has not been reported in a separate clinical trial that evaluated the MFGM-P fraction added to formula for infants between less than 2 months and 6 months of age.6
As a general rule, infant formulas lack MFGM because this fraction is lost during dairy processing. However, data supporting the potential benefits of MFGM enrichment are accumulating, suggesting that MFGM enrichment may help narrow the gap between breast milk and infant formulas. In a recent study, Timby et al randomized 160 infants less than 2 months of age to a low-energy, low-protein experimental infant formula supplemented with bovine MFGM or a standard formula until 6 months of age.6 A breastfed reference group of infants was also evaluated. The investigational formula provided 60 kcal and 1.20 g of protein/100 mL. Infants fed the lower energy formulation upregulated their ingested volume of formula over the course of the study. Rates of linear growth, weight gain, and changes in body mass index, percentage body fat, and head circumference were similar between groups, which is consistent with the anthropometric results in the current study. However, by 12 months, cognitive test scores among the infants assigned to the lower energy, lower protein MFGM-supplemented formula were significantly higher than among the infants assigned to the standard formula and comparable to those among the breastfed infants. These results are promising, although replication in future studies will be important, as well as determining whether effects on cognitive function are due to reductions in the concentration of energy or protein or the added MFGM components, or a combination of these factors.
Evidence also suggests that MFGM may exhibit antibacterial and antiviral activity.12,26–28 Preclinical studies have demonstrated that several proteins in the MFGM inhibit the activity of various pathogens, including Escherichia coli and rotaviruses, and enterotoxins.12,26–28 Moreover, an infant formula supplemented with sphingolipids (gangliosides) was shown to reduce fecal E. coli counts and increase levels of beneficial bifidobacteria.12,29 A randomized, double-blind, controlled study of the impact of an MFGM-enriched complementary food on diarrhea, anemia, and micronutrient status in Peruvian infants aged 6 to 11 months found that infants receiving the MFGM-enriched food experienced a reduced prevalence of diarrhea (3.84% vs 4.37% with controls; P < 0.05) as well as a 46% reduction in the likelihood of bloody diarrhea.7 No differences in anemia or micronutrient status were observed.7 The beneficial effects of MFGM supplementation on diarrhea observed by Zavaleta et al may have resulted from modulation of gut microbiota or positive changes in the developing infant immune system in this population of older infants.7 In contrast, our study showed no significant differences in immune function or the occurrence of diarrhea among subjects assigned to MFGM-enriched and control formulas. Such an effect may not have been detectable in the current study population, because the rates of diarrhea in the population were low or because a larger sample size was needed to adequately evaluate this outcome.
Although a lower immune response to the Polio virus type 1 was observed in the MFGM-P group, responses to other polio virus types did not differ among the groups, suggesting that the observed difference may be an artifact. Also this finding was observed only in the MFGM-P group from Italy, but not from France, and thus might be due to differences in the schedules of infant vaccination between the two countries; subjects in France had received booster doses, while those in Italy were vaccinated only once during the first period of the study. As the measurements of the immune response to the Polio virus type 1 at this site were below the LLQ, these findings must be interpreted with caution. This study also showed that C-peptide levels, which have been shown to be lower in breastfed infants than in formula-fed infants,30 were significantly lower in the groups fed MFGM-enriched infant formulas compared with the group fed control formula. C-peptide is a byproduct of insulin production, facilitating the efficient assembly, folding, and processing of insulin. Consequently, C-peptide levels are used as an indicator of insulin production. The clinical significance of this finding, if any, is not known at this time.
Overall, care must be taken in interpreting the exploratory endpoints in this study. Although the study was adequately powered to evaluate non-inferiority of the MFGM-enriched formulas with respect to weight gain, limitations of the study for investigating other outcomes include its short duration and relatively small sample size, as well as the unequal allocation of subjects among groups, which may have introduced some degree of bias between the control and the experimental groups. Despite these limitations, the safety profile of MFGM-enriched infant formulas that emerges from this study combined with the potential cognitive benefits observed by others suggests the importance of continued investigation of these fractions.6 Future studies should be planned with longer follow-up times, preferably a year or longer, and include markers of cognitive function.
Conclusion
Both MFGM-enriched formulas met the primary safety endpoint of non-inferiority in weight gain compared to infants assigned to the control formula. In general, the formulas were well tolerated. Our findings provide preliminary support for further clinical evaluation of MFGM-enriched infant formulas.
Acknowledgments
The authors acknowledge Nicole Cooper for providing medical writing services.
Footnotes
Author Contributions
Conceived and designed the experiments: CB, PS, SP. Recruited study participants and supervised data collection: CB, GP, ES, BG. Conducted lipid analyses: CV. All authors jointly contributed to analysis and interpretation of data. Jointly developed the structure and arguments for the paper: CB, PS, SP. Led the development of the first draft of the manuscript: CB, PS, with assistance from medical writer (NC). All authors reviewed manuscript drafts and approved the final version.
ACADEMIC EDITOR: Praveen Kumar, Associate Editor
FUNDING: This study was funded by Nestlé Nutrition. Medical writing assistance was also funded by Nestlé Nutrition. The authors confirm that the funder had no influence over the study design, content of the article, or selection of this journal.
COMPETING INTERESTS: PS and SP are employees of Nestlé. CB discloses grants and personal fees from Nestlé Switzerland. Other authors disclose no competing interests.
Paper subject to independent expert blind peer review by minimum of two reviewers. All editorial decisions made by independent academic editor. Prior to publication all authors have given signed confirmation of agreement to article publication and compliance with all applicable ethical and legal requirements, including the accuracy of author and contributor information, disclosure of competing interests and funding sources, compliance with ethical requirements relating to human and animal study participants, and compliance with any copyright requirements of third parties.ClinicalTrials.gov Identifier: NCT02111837
REFERENCES
- 1.World Health Organization . Global Strategy for Infant and Young Child Feeding. Geneva, Switzerland: World Health Organization; 2003. pp. 7–10. [Google Scholar]
- 2.American Academy of Pediatrics Policy statement: breastfeeding and the use of human milk. Pediatrics. 2012;129:e827–41. doi: 10.1542/peds.2011-3552. [DOI] [PubMed] [Google Scholar]
- 3.Thakkar SK, Giuffrida F, Cristina CH, et al. Dynamics of human milk nutrient composition of women from Singapore with a special focus on lipids. Am J Hum Biol. 2013;25:770–9. doi: 10.1002/ajhb.22446. [DOI] [PubMed] [Google Scholar]
- 4.Dewettinck K, Rombaut R, Thienpont N, Trung Le T, Messens K, Van Camp J. Nutritional and technological aspects of milk fat globule membrane material. Int Dairy J. 2008;18:436–57. [Google Scholar]
- 5.Garcia C, Innis S. Structure of the human milk fat globule. Lipid Technol. 2013;25(10):223–6. [Google Scholar]
- 6.Timby N, Domellöf E, Hernell O, Lönnerdal B, Domellöf M. Neurodevelopment, nutrition, and growth until 12 mo of age in infants fed a low-energy, low-protein formula supplemented with bovine milk fat globule membranes: a randomized controlled trial. Am J Clin Nutr. 2014;99(4):860–8. doi: 10.3945/ajcn.113.064295. [DOI] [PubMed] [Google Scholar]
- 7.Zavaleta N, Kvistgaard AS, Graverholt G, et al. Efficacy of an MFGM-enriched complementary food in diarrhea, anemia, and micronutrient status in infants. J Pediatr Gastroenterol Nutr. 2011;53(5):561–8. doi: 10.1097/MPG.0b013e318225cdaf. [DOI] [PubMed] [Google Scholar]
- 8.Danthine S, Blecker C, Paquot M, et al. Progress in milk fat globule membrane research: a review. Lait. 2000;80:209–22. [Google Scholar]
- 9.Deeth HC. The role of phospholipids in the stability of milk fat globules. Aust J Dairy Technol. 1997;52:44–6. [Google Scholar]
- 10.Fong BY, Norris CS, MacGibbon AKH. Protein and lipid composition of bovine milk-fat-globule membrane. Int Dairy J. 2007;17:275–88. [Google Scholar]
- 11.Riccio P. The proteins of the milk fat globule membrane in the balance. Trends Food Sci Technol. 2004;15:458–61. [Google Scholar]
- 12.Lönnerdal B. Infant formula and infant nutrition: bioactive proteins of human milk and implications for composition of infant formulas. Am J Clin Nutr. 2014;99(suppl):712S–7S. doi: 10.3945/ajcn.113.071993. [DOI] [PubMed] [Google Scholar]
- 13.Liao Y, Alvarado R, Phinney B, Lönnerdal B. Proteomic characterization of human milk fat globule membrane proteins during a 12 month lactation period. J Proteome Res. 2011;10(8):3530–41. doi: 10.1021/pr200149t. [DOI] [PubMed] [Google Scholar]
- 14.Garcia C, Lutz NW, Confort-Gouny S, Cozzone PJ, Armand M, Bernard M. Phospholipid fingerprints of milk from different mammalians determined by 31P NMR: towards specific interest in human health. Food Chem. 2012;135(3):1777–83. doi: 10.1016/j.foodchem.2012.05.111. [DOI] [PubMed] [Google Scholar]
- 15.Zou X, Huang J, Jin Q, et al. Lipid composition analysis of milk fats from different mammalian species: potential for use as human milk fat substitutes. J Agric Food Chem. 2013;61(29):7070–80. doi: 10.1021/jf401452y. [DOI] [PubMed] [Google Scholar]
- 16.Bode L, Beermann C, Mank M, Kohn G, Boehm G. Human and bovine milk gangliosides differ in their fatty acid composition. J Nutr. 2004;134(11):3016–20. doi: 10.1093/jn/134.11.3016. [DOI] [PubMed] [Google Scholar]
- 17.Oshida K, Shimizu T, Takase M, Tamura Y, Shimizu T, Yamashiro Y. Effects of dietary sphingomyelin on central nervous system myelination in developing rats. Pediatr Res. 2003;53(4):589–93. doi: 10.1203/01.PDR.0000054654.73826.AC. [DOI] [PubMed] [Google Scholar]
- 18.Gurnida DA, Rowan AM, Idjradinata P, Muchtadi D, Sekarwana N. Association of complex lipids containing gangliosides with cognitive development of 6-month-old infants. Early Hum Dev. 2012;88(8):595–601. doi: 10.1016/j.earlhumdev.2012.01.003. [DOI] [PubMed] [Google Scholar]
- 19.Zeisel SH, Char D, Sheard NF. Choline, phosphatidylcholine and sphingomyelin in human and bovine milk and infant formulas. J Nutr. 1986;116:50–8. doi: 10.1093/jn/116.1.50. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka K, Hosozawa M, Kudo N, et al. The pilot study: sphingomyelin-fortified milk has a positive association with the neurobehavioural development of very low birth weight infants during infancy, randomized control trial. Brain Dev. 2013;35(1):45–52. doi: 10.1016/j.braindev.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 21.US Food and Drug Administration Clinical Testing of Infant Formulas With Respect to Nutritional Suitability for Term Infants. Jun, 1988. [Accessed August 16, 2014]. Available at http://www.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/InfantFormula/ucm170649.htm.
- 22.Maqbool A, Olsen IE, Stallings VA. Clinical assessment of nutritional status. In: Duggan C, et al., editors. Nutrition in Pediatrics. 4th ed. Hamilton, ON, Canada: BC Decker Inc; 2008. pp. 5–13. [Google Scholar]
- 23.Billeaud C, Bouglé D, Sarda P, et al. Effects of preterm infant formula supplementation with alpha-linolenic acid with a linoleate/alpha-linolenate ratio of 6: a multicentric study. Eur J Clin Nutr. 1997;51(8):520–6. doi: 10.1038/sj.ejcn.1600436. [DOI] [PubMed] [Google Scholar]
- 24.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipids from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 25.Morrison WR, Smith LM. Preparation of fatty acid methyl esters and dimethylacetals with boron fluoride methanol. J Lipid Res. 1964;5:600–8. [PubMed] [Google Scholar]
- 26.Spitsberg VL. Invited review: bovine milk fat globule membrane as a potential nutraceutical. J Dairy Sci. 2005;88(7):2289–94. doi: 10.3168/jds.S0022-0302(05)72906-4. [DOI] [PubMed] [Google Scholar]
- 27.Yolken RH, Peterson JA, Vonderfecht SL, Fouts ET, Midthun K, Newburg DS. Human milk mucin inhibits rotavirus replication and prevents experimental gastroenteritis. J Clin Invest. 1992;90:1984–91. doi: 10.1172/JCI116078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Laegreid A, Otnaess ABK, Fuglesang J. Human and bovine milk: comparison of ganglioside composition and enterotoxin-inhibitory activity. Pediatr Res. 1986;20(5):416–21. doi: 10.1203/00006450-198605000-00008. [DOI] [PubMed] [Google Scholar]
- 29.Rueda R, Sabatel JL, Maldonado J, Molina-Font JA, Gil A. Addition of gangliosides to an adapted milk formula modifies levels of fecal Escherichia coli in preterm newborn infants. J Pediatr. 1998;133(1):90–4. doi: 10.1016/s0022-3476(98)70184-2. [DOI] [PubMed] [Google Scholar]
- 30.Wallensteen M, Lindblad BS, Zetterström R, Persson B. Acute C-peptide, insulin and branched chain amino acid response to feeding in formula and breast fed infants. Acta Paediatr Scand. 1991;80(2):143–8. doi: 10.1111/j.1651-2227.1991.tb11824.x. [DOI] [PubMed] [Google Scholar]