ABSTRACT
Objectives
This study aims to evaluate bone response to an implant surface modified by 1α,25-dihydroxyvitamin D3 [1.25-(OH)2D3] in vivo and the potential link between 1.25-(OH) 2D3 surface concentration and bone response.
Material and Methods
Twenty-eight implants were divided into 4 groups (1 uncoated control, 3 groups coated with 1.25-(OH)2D3 in concentrations of 10-8, 10-7 and 10-6 M respectively), placed in the rabbit tibia for 6 weeks. Topographical analyses were carried out on coated and uncoated discs using interferometer and atomic-force-microscope (AFM). Twenty-eight implants were histologically observed (bone-to-implant-contact [BIC] and new-bone-area [NBA]).
Results
The results showed that the 1.25-(OH)2D3 coated implants presented a tendency to osseointegrate better than the non-coated surfaces, the differences were not significant (P > 0.05).
Conclusions
The effect of 1.25-(OH)2D3 coating to implants suggested possible dose dependent effects, however no statistical differences could be found. It is thought that the base substrate topography (turned) could not sustain sufficient amount of 1.25-(OH)2D3 enough to present significant biologic responses. Thus, development a base substrate that can sustain 1.25-(OH)2D3 for a long period is necessary in future studies.
Keywords: dental implants, vitamin D, drug dose response relationship, histological techniques, bone formation
INTRODUCTION
Implant surface features plays a key role in the quality and rate of osseointegration [1]. Recent investigations have reported that along with the surface topographical modifications, the application of bioactive agents may result in enhanced osteogenic properties to the implant surface [2-4]. Bioactive implants have been reported to possibly develop a biochemical bonding between the bone tissue and the titanium implant surface rather than a merely physical one [5,6]. A bioactive implant surface is defined as one that has the potential to promote numerous molecular interactions, potentially forming a chemical bond between bone and implant surface [6]. Previous studies have demonstrated that proteins or peptides with bioactive capacity such as bone morphogenetic proteins (BMPs), fibronectin, type I collagen, fibroblast growth factor (FGF), and arginine-glycine-aspartic acid (RDG-peptide) are promising bioactive molecular candidates with a high osteogenic potential [7-9]. However, their fabrication and economic feasibility along with technical and regulatory issues have led researchers to explore alternative bioactive molecules such as the bone mobilizing hormone - vitamin D.
Vitamin D has been shown to play an essential role in bone mineral homeostasis and in its active form, 1α,25-dihydroxyvitamin D3 [1.25-(OH)2D3], may act as a bioactive protein promoting new bone formation [10,11]. The most important role of 1.25-(OH)2D3 is that it regulates the intestinal adsorption of calcium and phosphate, resulting in increased plasma concentrations [12]. However, to date, the exact role of 1.25-(OH)2D3 in osteogenesis has yet to be fully explored since the hormone effects multiple cellular pathways [13,14].
Previous studies have reported that 1.25-(OH)2D3 significantly promoted the expression of osteogenic markers, in addition, 1.25-(OH)2D3 deficiency negatively impacts osseointegration [16,17]. It has been suggested that 1.25-(OH)2D3 has a direct effect on osteogenesis since vitamin D receptors (VDR) are present on osteoblasts and osteoclast precursors, with activation leading to RANKL expression [17,18]. Masuyama et al. [16] reported in their in vivo study using mice that 1.25-(OH)2D3 exerted a regulatory effect on osteoblast and osteoclast chemotaxis during increasing vascular tissue infiltration. Furthermore, Masuyama et al. [16] showed that 1.25-(OH)2D3 could regulate collagen modification and maturation in an osteoblastic cell culture, which has been proven to be important in early bone formation [19]. Numerous studies have also suggested that these intriguing osteogenic influences of 1.25-(OH)2D3 are dose dependent [17,20,21].
Even though previous in vitro studies of 1.25-(OH)2D3 suggest its promising effects on osteogenesis, the in vivo biologic responses have not yet been confirmed especially when attached to implantable materials. The aim of this study was to histologically evaluate the osteogenic effect of 1.25-(OH)2D3 coatings to endosteal implant surfaces and to determine if the biologic response would be doses dependent of 1.25-(OH)2D3 concentration on the surface.
MATERIAL AND METHODS
Surface preparations
Twenty-eight commercially pure machine turned titanium implants (Grade 4, Neodent® Curitiba, Brazil), 7 mm in length and 3.75 mm in diameter were used in this study. The implants were divided into 4 groups with 7 implants in each group, with one functioning as an uncoated control group. The remaining groups were coated with 1.25-(OH)2D3 in concentrations of 10-8, 10-7 and 10-6 M respectively, based on a previous in vitro investigation [18]. 1.25-(OH)2D3 was diluted in ≥ 99.5% ethanol until desired concentrations were achieved. The implants were soaked in the respective solution for 1 h and thereafter were gently rinsed with phosphate buffered saline (Invitrogen, GIBCO, Sweden) and finally were air-dried in a 24-well plate. The well was covered with lab-foil and stored in a freezer prior to surgery. For topographical investigation, 3 discs (8 mm in diameter, 1mm in thickness) of turned commercially pure titanium (grade 4) were soaked in each solution (a total of 9 discs) following the same procedure as when soaking the implants.
Topographic analysis
An optical interferometer (MicroXam; ADE Phase Shift Technology, Inc., Tucson, AZ) was used to characterize the topography of 3 uncoated implants. All twelve discs were also measured with interferometer at 3 randomly selected sites and thereafter topographically compared to each other. The following topographical parameters were used: Sa (µm) = average height deviation from a mean plane, Sds (µm-2) = density of summits and Sdr (%) = developed surface ratio [22]. Before the parametrical calculation could be evaluated, the waviness from the surface was removed using a 50 × 50 µm Gaussian filter. For each selected scan area, the mean value and standard deviation of the parameters were obtained from 9 scans of each group (a total of thirty-six measurements), from random sites on the surface.
In order to obtain the surface roughness in the nanometer length scale, atomic-force-microscopy (AFM, XE-100, Park Systems Corp, Suwon, Korea) was utilized on the same twelve discs. For the measurements, 3 different scanning areas were selected (10 × 10 µm, 5 × 5 µm and 1 × 1 µm). The images obtained by AFM were subjected to levelling and applied Gaussian filtering with a cut-off of 2.5 µm (for 10 × 10 µm scans), 1 µm (for 5 × 5 µm scans), and 0.25 µm (for 1 × 1 µm scans), using the software MontainsMap 6 (Digital Surf, Besançon, France) and the same three-dimensional parameters used for the interferometer (Sa, Sdr, Sds) were evaluated to correlate to the micro roughness. For each selected scan area, the mean value and standard deviation of the parameters were obtained from 9 scans of each group (a total of thirty-six measurements), from random sites on the surface.
Animal preparation
Twelve New Zealand white rabbits (mean body weight 4.7 kg [range 3.8 - 5.2 kg]) were used in this study. The study was approved by the Ethics Committee for Animal Research at the École Nationale Vétérinaire d’Alfort (Maisons-Alfort, Val-de-Marne, France). All surgical procedures were performed under general anaesthesia. The pre-anaesthetic procedure comprised an intra-muscular administration of atropine sulfate (0.044 mg/kg) and xylazine chlorate (8 mg/kg). General anaesthesia was then obtained following an intra-muscular injection of ketamine chlorate (15 mg/kg). Thereafter, the hind legs were shaved and disinfected with iodine solution. After anaesthetic and disinfection procedures, the proximal tibiae on both sides were exposed and 4 osteotomy sites (2 in each leg) were prepared according to the manufacturer’s instructions for placement of implants in dense bone, i.e., to avoid excessive torque.
Histological preparation and analyses
After 6 weeks of healing, the animals were euthanized with anaesthesia overdose and the implants were removed en bloc and thereafter were placed in 4 % formaldehyde for 24 h. After fixation, the samples were subjected to dehydration in a series of ethanol (70 - 100%) and infiltration in resin (30 - 100%) under constant vacuuming and thereafter were embedded in light curing-resin (Technovit 7200 VLC; Heraeus Kulzer, Wehrheim, Germany). The embedded resin blocks were subjected to non-decalcified cut and grind sectioning. In brief, a central section of each sample were prepared using the EXAKTTM cutting and grinding equipment to a final thickness of 15 µm. After polishing to exclude scratches, the sections were finally stained with a mixed solution of toluidine blue and pyronin G.
The histological analyses were performed using a light microscope (Eclipse ME600; Nikon, Japan) and the histomorphological data was analyzed with image analysis software (Image J v. 1.43u; National Institute of Health). Calculation of bone-to-implant contact (BIC) ratio along the implants surfaces and new-bone-area (NBA) within the threads were made using a x10 magnification objective. Histology and histomorphometry were both conducted in a blind manner.
Statistical analysis
The statistical analyses of the mean values of the discs topography were composed and compared using One-Way Analysis of Variance (ANOVA). The statistical significance level was set at P ≤ 0.05. Histological multiple group comparisons were performed by computer software SPSS for Macintosh (SPSS Inc., Chicago, IL, USA). The results from the histomorphometric measurements were expressed as means and standard deviations (M [SD]). The different treatment groups were compared using Kruskal Wallis with the significance level set at P ≤ 0.05.
RESULTS
Topographical analyses
Topographical analyses of the discs with the interferometer showed a statistical significant difference (P = 0.049) regarding the density of summits in µm-2 (Sds), as presented in Table 1.
Table 1.
Mean values for Sa, Sdr, Sds (standard deviation) for topographical analyses of discs with interferometer and P-values for one-way ANOVA comparisons
|
Concentration of 1.25-(OH)2D3 (M) |
Sa (μm) | Sdr (%) | Sds (1/μm2) | Quantity |
|---|---|---|---|---|
| 0 | 0.3 (0.02) | 6 (0.1) | 129339 (1374) | 3 |
| 10-8 | 0.3 (0.03) | 8.1 (1.8) | 143980.5 (4187. 4) | 3 |
| 10-7 | 0.3 (0.06) | 5.5 (1.3) | 131965.2 (4142.4) | 3 |
| 10-6 | 0.3 (0.07) | 5.9 (1.3) | 131705.8 (9496.7) | 3 |
| P-value | 0.503 | 0.074 | 0.049 |
The 1.25-(OH)2D3 coating did not yield any significant differences in surface topography parameters in the micro level for the average height derivation in µm (Sa) or for the developed surface ratio in % (Sdr) (both dependent variables at P > 0.05).
Topographical analyses in the nanometer level with AFM showed no significant differences (P > 0.05) between all groups tested (Table 2 - 4).
Table 2.
Mean values for Sa for µm (standard deviation) for topographical analyses of discs with AFM in 3 different magnifications and P-values for one-way ANOVA comparisons
|
Concentration of 1.25-(OH)2D3 (M) |
10 x 10 μm | 5 x 5 μm | 1 x 1 μm | Quantity |
|---|---|---|---|---|
| 0 | 0.05 (0.006) | 0.02 (0.005) | 0.005 (0.0005) | 9 |
| 10-8 | 0.05 (0.006) | 0.02 (0.0007) | 0.007 (0.001) | 9 |
| 10-7 | 0.05 (0.003) | 0.02 (0.03) | 0.007 (0.002) | 9 |
| 10-6 | 0.05 (0.005) | 0.02 (0.005) | 0.009 (0.003) | 9 |
| P-value | 0.932 | 0.938 | 0.126 |
Table 3.
Mean values for Sdr in % (standard deviation) for topographical analyses of discs with AFM in three different magnifications and P-values for one-way ANOVA comparisons
|
Concentration of 1.25-(OH)2D3 (M) |
10 x 10 μm | 5 x 5 μm | 1 x 1 μm | Quantity |
|---|---|---|---|---|
| 0 | 11.7 (1.8) | 10.3 (4.6) | 36.3 (48.1) | 9 |
| 10-8 | 11.4 (2.9) | 10.8 (1.6) | 46.9 (32.1) | 9 |
| 10-7 | 13.4 (0.7) | 12.3 (2.9) | 48 (48) | 9 |
| 10-6 | 13.3 (3.3) | 13.9 (5) | 39.5 (24) | 9 |
| P-value | 0.65 | 0.66 | 0.978 |
Table 4.
Mean values for Sds in µm-2 (standard deviation) for topographical analyses of discs with AFM in three different magnifications and P-values for one-way ANOVA comparisons
|
Concentration of 1.25-(OH)2D3 (M) |
10 x 10 μm | 5 x 5 μm | 1 x 1 μm | Quantity |
|---|---|---|---|---|
| 0 | 12.9 (6.4) | 33.9 (15.6) | 2434.6 (2111.6) | 9 |
| 10-8 | 15.9 (4.1) | 49.6 (18.9) | 2472.6 (1327) | 9 |
| 10-7 | 18.6 (7.8) | 53.2 (27.1) | 2725.9 (888.1) | 9 |
| 10-6 | 13.6 (1.6) | 39.3 (4.3) | 2262.2 (1677.8) | 9 |
| P-value | 0.602 | 0.575 | 0.978 |
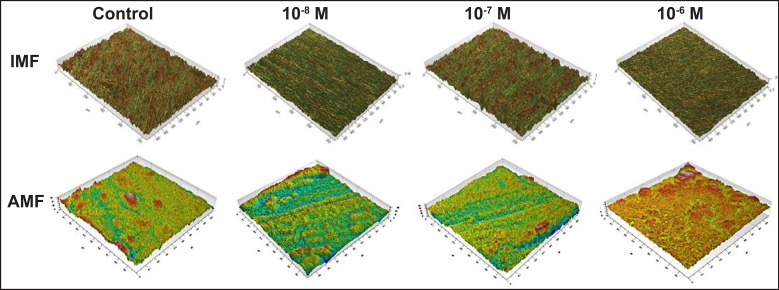
The descriptive images for both interferometer and AFM are presented in Figure 1. The investigation of samples by interferometer showed no qualitative differences in the morphology of the surface between the control and test groups. The image scans obtained from AFM also showed no qualitatively distinct differences in the surface morphology between all groups (only some examples of scans 1 x 1 µm are shown).
Figure 1.
Descriptive IFM and AFM three-dimensionally reconstructed images of the groups tested in the study.
Histological evaluation
The animals recovered without complications and no signs of infection were noted upon clinical examination at any time during the observation period.
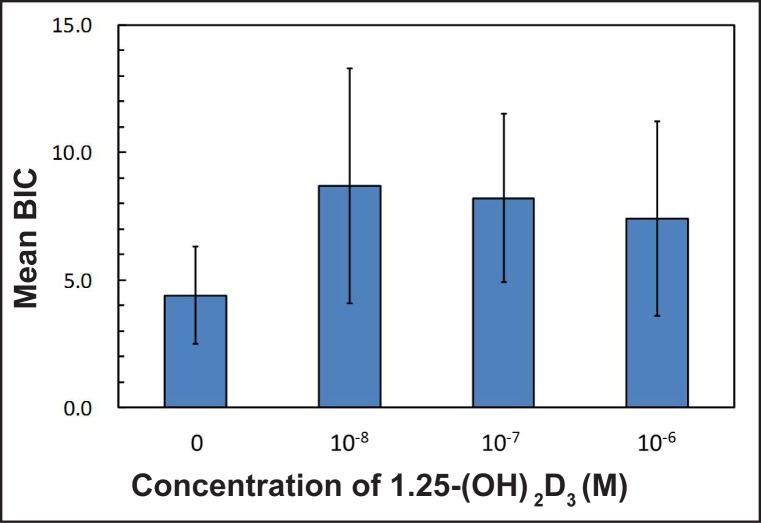
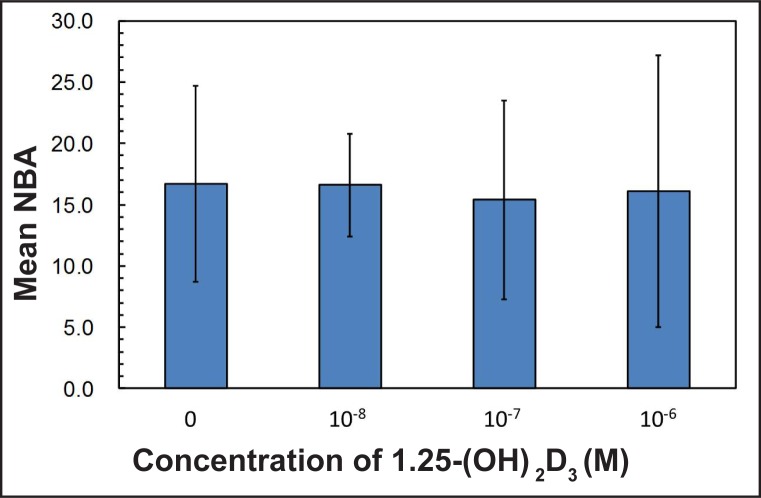
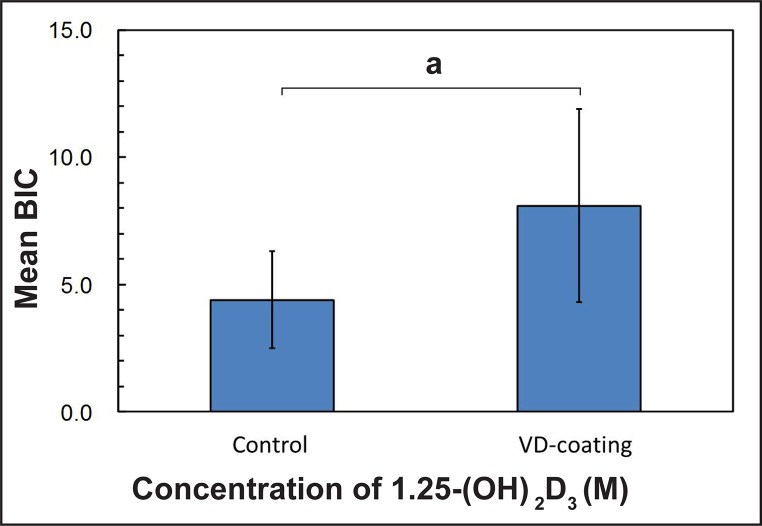
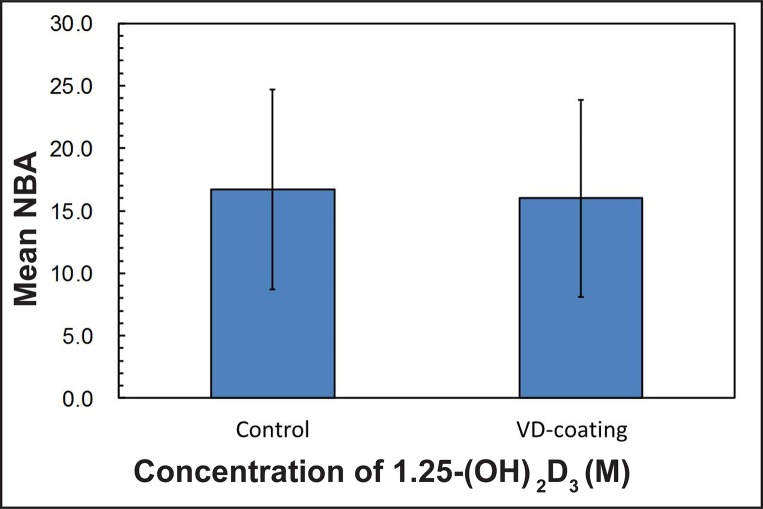
The mean BIC in percentage was 4.4 (1.9) for the control group (0 M), 8.7 (4.6) for group I (10-8 M), 8.2 (3.3) for group II (10-7 M) and 7.4 (3.8) for group III (10-6 M), respectively. The experimental group showed a trend toward higher bone contact, however the results were not statistically significant (Figure 2, Table 5). The means for NBA in percentage were 16.7 (8) for the control group, 16.5 (4.2) for group I, 15.4 (8.1) for group II and 16.1 (11.1) for group III, respectively, with no significant differences between each of the groups (Figure 3, Table 5). When the effect of dosage was collapsed and statistically compared to the control, there was a statistically significant effect of the vitamin D coating in BIC (P < 0.05) (Figure 4, Table 6). However, no significant differences were found in NBA (P > 0.05) (Figure 5, Table 6).
Figure 2.
Mean values in percentage (%) of bone-to-implant-contact (BIC) around the total dental implant for each tested group.
Table 5.
Mean values in percentage (standard deviation) for histological analyses and P-values for one-way ANOVA
|
Concentration of 1.25-(OH)2D3 (M) |
BIC total | NBA total | Quantity |
|---|---|---|---|
| 0 | 4.4 (1.9) | 16.7 (8) | 7 |
| 10-8 | 8.7 (4.6) | 16.5 (4.2) | 7 |
| 10-7 | 8.2 (3.3) | 15.4 (8.1) | 7 |
| 10-6 | 7.4 (3.8) | 16.1 (11.1) | 7 |
| P-value | 0.162 | 0.775 |
BIC = bone-to-implant-contact; NBA = new bone area.
Figure 3.
Mean values in percentage (%) of new bone area (NBA) around the total dental implant for each tested group.
Figure 4.
Mean values (non-coating vs coating) in percentage (%) of bone-to-implant-contact (BIC) around the total dental implant.
aStatistical significance (P < 0.05).
Table 6.
Mean values in percentage (standard deviation) on the comparison between control vs coated groups (the effect of dose was collapsed) and P-values for one-way ANOVA
| BIC total | NBA total | Quantity | |
|---|---|---|---|
| Control | 4.4 (1.9) | 16.7 (8) | 7 |
| VD-coating | 8.1 (3.8) | 16 (7.9) | 21 |
| P-value | 0.27 | 0.756 |
BIC = bone-to-implant-contact NBA = new bone area.
Figure 5.
Mean values (non-coating vs coating) in percentage (%) of new bone area (NBA) around the total dental implant.
Figure 6.
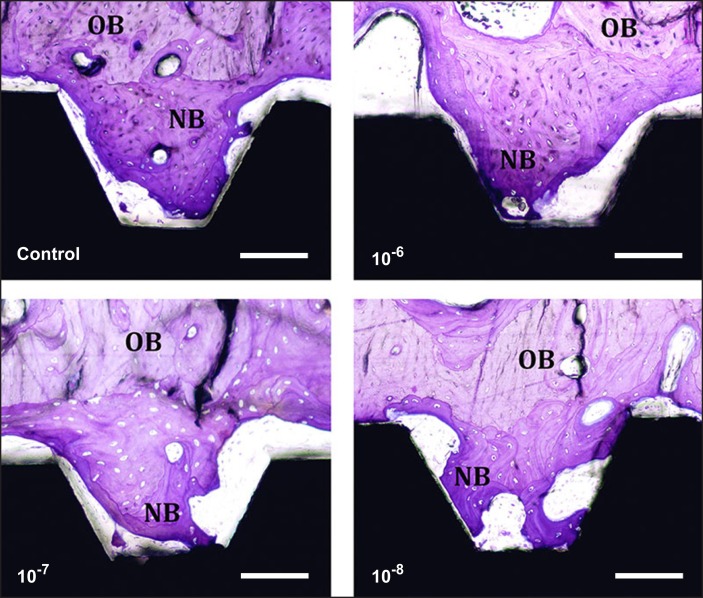
Histological photographs of the bone tissue formed around all tested groups after 6 weeks of implantation. Original magnification x10, Toluidine blue staining. Scale bar: 100 μm. NB = new bone; OB = old bone.
DISCUSSION
This study aimed to histologically evaluate the biologic effect of 1.25-(OH)2D3 coated onto implant surfaces. It was hypothesized that the biologic response would be distinct depending on the dosage of 1.25-(OH)2D3 applied to the implant surface.
It has been suggested that the optimal Sa value to obtain good bone response is between 1 - 1.5 µm, which is the so-called moderately roughened implant surface [23]. While surface roughness has been shown to affect bone response we utilized a smooth turned metal surface to examine the effect of the 1.25-(OH)2D3 coating without underlying implant roughness. This methodology has been used in other studies experimentally attempting to determine the effect of various protein or calcium phosphate coatings on the bone-to-implant response [8,24,25]. While the surface roughness values between all groups tested were similar, there were significant differences in the density of summits (Sds) between groups (P = 0.049). This significance is probably due to the different 1.25-(OH)2D3 coating concentrations, as the test groups showed higher mean values than control group (Table 1), thus the coatings may have resulted in changes to the surface topography. Interestingly, the AFM measurements demonstrated no statistical differences between all groups tested in the nanometer length scale (Table 2 - 4), despite each group presenting different qualitative topographical features as seen in the three-dimensional reconstructed images. Overall, the interferometer and AFM results indicate that coatings of 1.25-(OH)2D3 slightly altered the surface topography in a dose dependent manner, which may have influenced the host biological responses.
Previous studies have shown that vitamin D is dose dependent in serum [17,21] but it should also be noted that vitamin D also has a bone resorbing effect, especially with high therapeutic doses [17]. Thus, the selection of the optimal concentration must be determined based on further evidence. Moreover, it remains to be identified how much coating agent remains on the implant because the specimens were coated with the traditionally utilized dip coating, furthermore, turned surface implants were the base substrates. It is thought that one reason for the small differences between all groups in terms of histomorphometry was that the dip coating on the turned surface did not assure stable protein adsorption to the surface and the 1.25-(OH)2D3 simply remained after air drying. Thus, naturally, the release of the protein was a rapid process, which could not provide a sufficient bone forming effect. In future studies, the amount of protein adsorption to a modified textured surface, and its release rate should be determined in order to obtain the optimal surface for protein incorporation.
This study intended to investigate a possibly bioactive implant surface with the use of different 1.25-(OH)2D3 concentrations. Although there was a tendency for the 1.25-(OH)2D3 coated implants to show better bone responses, the results were not significant. Thus, an implant surface that can sustain the 1.25-(OH)2D3 over a long period of time should be considered.
CONCLUSIONS
The current study demonstrated that there may be dose dependent biologic effects of the 1.25-(OH)2D3 in vivo, however the differences were insignificant within the limitation of the study.
Acknowledgments
ACKNOWLEDGMENTS AND DISCLOSURE STATEMENTS
The authors declare no conflict of interests.
REFERENCES
- 1.Wennerberg A, Albrektsson T. On implant surfaces: a review of current knowledge and opinions. Int J Oral Maxillofac Implants. 2010 Jan-Feb;25(1):63-74. Review. [PubMed]
- 2.Bougas K, Jimbo R, Vandeweghe S, Tovar N, Baldassarri M, Alenezi A, Janal M, Coelho PG, Wennerberg A. In Vivo Evaluation of a Novel Implant Coating Agent: Laminin-1. Clin Implant Dent Relat Res. 2013 Jan 11. [Epub ahead of print] [DOI] [PubMed]
- 3.Park JW, Lee SG, Choi BJ, Suh JY. Effects of a cell adhesion molecule coating on the blasted surface of titanium implants on bone healing in the rabbit femur. Int J Oral Maxillofac Implants. 2007 Jul-Aug;22(4):533-41. [PubMed]
- 4.Schwartz-Filho HO, Bougas K, Coelho PG, Xue Y, Hayashi M, Faeda RS, Marcantonio RA, Ono D, Kobayashi F, Mustafa K, Wennerberg A, Jimbo R. The effect of laminin-1-doped nanoroughened implant surfaces: gene expression and morphological evaluation. Int J Biomater. 2012;2012:305638. Epub 2012 Dec 12. [DOI] [PMC free article] [PubMed]
- 5.Sul YT, Kwon DH, Kang BS, Oh SJ, Johansson C. Experimental evidence for interfacial biochemical bonding in osseointegrated titanium implants. Clin Oral Implants Res. 2013 Aug;24 Suppl A100:8-19. Epub 2011 Nov 14. [DOI] [PubMed]
- 6.Sul YT, Johansson C, Albrektsson T. A novel in vivo method for quantifying the interfacial biochemical bond strength of bone implants. J R Soc Interface. 2010 Jan 6;7(42):81-90. Epub 2009 Apr 15. [DOI] [PMC free article] [PubMed]
- 7.Franke Stenport V, Johansson CB, Sawase T, Yamasaki Y, Oida S. FGF-4 and titanium implants: a pilot study in rabbit bone. Clin Oral Implants Res. 2003 Jun;14(3):363-8. [DOI] [PubMed]
- 8.Jimbo R, Sawase T, Shibata Y, Hirata K, Hishikawa Y, Tanaka Y, Bessho K, Ikeda T, Atsuta M. Enhanced osseointegration by the chemotactic activity of plasma fibronectin for cellular fibronectin positive cells. Biomaterials. 2007 Aug;28(24):3469-77. Epub 2007 May 3. [DOI] [PubMed]
- 9.Matsuura T, Hosokawa R, Okamoto K, Kimoto T, Akagawa Y. Diverse mechanisms of osteoblast spreading on hydroxyapatite and titanium. Biomaterials. 2000 Jun;21(11):1121-7. [DOI] [PubMed]
- 10.Li YC, Amling M, Pirro AE, Priemel M, Meuse J, Baron R, Delling G, Demay MB. Normalization of mineral ion homeostasis by dietary means prevents hyperparathyroidism, rickets, and osteomalacia, but not alopecia in vitamin D receptor-ablated mice. Endocrinology. 1998 Oct;139(10):4391-6. [DOI] [PubMed]
- 11.Stoffels K, Overbergh L, Giulietti A, Verlinden L, Bouillon R, Mathieu C. Immune regulation of 25-hydroxyvitamin-D3-1alpha-hydroxylase in human monocytes. J Bone Miner Res. 2006 Jan;21(1):37-47. Epub 2005 Sep 19. [DOI] [PubMed]
- 12.Nemere I, Garbi N, Hammerling G, Hintze KJ. Role of the 1,25D3-MARRS receptor in the 1,25(OH)2D3-stimulated uptake of calcium and phosphate in intestinal cells. Steroids. 2012 Aug;77(10):897-902. Epub 2012 Apr 21. [DOI] [PubMed]
- 13.Nagaoka H, Mochida Y, Atsawasuwan P, Kaku M, Kondoh T, Yamauchi M. 1,25(OH)2D3 regulates collagen quality in an osteoblastic cell culture system. Biochem Biophys Res Commun. 2008 Dec 12;377(2):674-8. Epub 2008 Oct 18. [DOI] [PubMed]
- 14.St-Arnaud R. The direct role of vitamin D on bone homeostasis. Arch Biochem Biophys. 2008 May 15;473(2):225-30. Epub 2008 Apr 6. Review. [DOI] [PubMed]
- 15.Kelly J, Lin A, Wang CJ, Park S, Nishimura I. Vitamin D and bone physiology: demonstration of vitamin D deficiency in an implant osseointegration rat model. J Prosthodont. 2009 Aug;18(6):473-8. Epub 2009 Mar 26. [DOI] [PubMed]
- 16.Masuyama R, Stockmans I, Torrekens S, Van Looveren R, Maes C, Carmeliet P, Bouillon R, Carmeliet G. Vitamin D receptor in chondrocytes promotes osteoclastogenesis and regulates FGF23 production in osteoblasts. J Clin Invest. 2006 Dec;116(12):3150-9. Epub 2006 Nov 9. [DOI] [PMC free article] [PubMed]
- 17.Suda T, Takahashi F, Takahashi N. Bone effects of vitamin D – Discrepancies between in vivo and in vitro studies. Arch Biochem Biophys. 2012 Jul 1;523(1):22-9. Epub 2011 Nov 15. Review. [DOI] [PubMed]
- 18.Tang X, Meng H. Osteogenic induction and 1,25-dihydroxyvitamin D3 oppositely regulate the proliferation and expression of RANKL and the vitamin D receptor of human periodontal ligament cells. Arch Oral Biol. 2009 Jul;54(7):625-33. Epub 2009 May 22. [DOI] [PubMed]
- 19.Spagnoli DB, Marx RE. Dental implants and the use of rhBMP-2. Oral Maxillofac Surg Clin North Am. 2011 May;23(2):347-61 vii. Review. [DOI] [PubMed]
- 20.Huang Y, Ishizuka T, Miura A, Kajita K, Ishizawa M, Kimura M, Yamamoto Y, Kawai Y, Morita H, Uno Y, Yasuda K. Effect of 1 alpha,25-dihydroxy vitamin D3 and vitamin E on insulin-induced glucose uptake in rat adipocytes. Diabetes Res Clin Pract. 2002 Mar;55(3):175-83. [DOI] [PubMed]
- 21.Song I, Kim BS, Kim CS, Im GI. Effects of BMP-2 and vitamin D3 on the osteogenic differentiation of adipose stem cells. Biochem Biophys Res Commun. 2011 Apr 29;408(1):126-31. doi: 10.1016/j.bbrc.2011.03.135. Epub 2011 Apr 2. [DOI] [PubMed]
- 22.Wennerberg A, Albrektsson T, Lausmaa J. Torque and histomorphometric evaluation of c.p. titanium screws blasted with 25- and 75-microns-sized particles of Al2O3. J Biomed Mater Res. 1996 Feb;30(2):251-60. [DOI] [PubMed]
- 23.Albrektsson T, Wennerberg A. Oral implant surfaces: Part 1--review focusing on topographic and chemical properties of different surfaces and in vivo responses to them. Int J Prosthodont. 2004 Sep-Oct;17(5):536-43. [PubMed]
- 24.Bougas K, Jimbo R, Vandeweghe S, Hayashi M, Bryington M, Kozai Y, Schwartz-Filho HO, Tovar N, Adolfsson E, Ono D, Coelho PG, Wennerberg A. Bone apposition to laminin-1 coated implants: histologic and 3D evaluation. Int J Oral Maxillofac Surg. 2013 May;42(5):677-82. Epub 2012 Dec 8. [DOI] [PubMed]
- 25.Jimbo R, Coelho PG, Vandeweghe S, Schwartz-Filho HO, Hayashi M, Ono D, Andersson M, Wennerberg A. Histological and three-dimensional evaluation of osseointegration to nanostructured calcium phosphate-coated implants. Acta Biomater. 2011 Dec;7(12):4229-34. Epub 2011 Jul 21. [DOI] [PubMed]