Abstract
Although past research has suggested a link between chronic stress and both physical and mental well-being in older adults, less is known about the degree to which neuroendocrine markers of stress are associated with higher-order cognitive processes such as decision-making. In a sample of healthy older adults (55–85 years), we tested the degree to which variation in the diurnal cortisol rhythm, an index of hypothalamic-adrenal-pituitary axis dynamics, was related to differences in risky decision making. We found that diurnal cortisol fall predicted performance on the Cups Task, a risky decision making task that independently tests risk taking to achieve gains and risk taking to avoid losses. For potential gains, we found that greater risk-taking was associated with lower diurnal cortisol fall, independent of age or sex of the participant. For risks to avoid potential losses, we found that lower diurnal fall selectively was associated with suboptimal decision-making for men only. Compared to males with more typical diurnal fall, those who displayed lower diurnal fall made more risky choices and demonstrated lower sensitivity to the expected value of the risky choice. We integrate these results with the extant literature on the effects of stress on cognitive aging.
Keywords: stress, cortisol, aging, decision making, risk taking
Many of the decisions that older adults make involve uncertainty even under ideal conditions, ranging from health insurance choices to treatment options for themselves or a spouse regarding how to invest money. Thus, maintaining the ability to make independent, sound, and advantageous decisions in the face of uncertain outcomes can be viewed as a hallmark of successful cognitive aging. However, to date, research investigating the effects of aging on cognition, particularly for decision making processes, has been mixed regarding the extent of decline observed in elderly adults (Peters, Dieckmann, & Weller, 2011). For example, research has shown that older adults, compared to middle-aged cohorts, are less likely to base their decisions on the relative expected value between choice options (Weller, Levin, and Denburg, 2011). Such differences may be associated with age-related cognitive declines in processing speed and working memory (Henninger, Madden, & Huettel, 2010; Li, Baldassi, Johnson, & Weber, 2013; Salthouse, 2010). Yet, the majority of older adults independently function in everyday life, suggesting that the ability to make decisions remains intact (Carstensen, 2001). Moreover, some research has demonstrated that some types of problem solving actually improve with age (e.g., Blanchard-Fields, Mienaltowski, & Seay, 2007; Cornelius & Caspi, 1987).
It follows that a more thorough investigation of risk factors that may predict individual differences in decision-making abilities among older adults is needed. We propose that stress may be one such risk factor. It is well-established that the cumulative effects of stress have deleterious effects on health and cognitive processes across the lifespan (e.g., Lupien, McEwen, Gunnar, & Heim, 2009; Sapolsky, 1996). For older adults who may be experiencing general cognitive decline associated with normal aging, the impact of chronic stress could be especially detrimental to decision-making abilities, as stress is believed to accelerate age-related cognitive decline in neural structures responsible for learning, memory, and executive function (e.g., Evans et al., 2011; McEwen & Sapolsky, 1995; Stawski et al., 2011). Further, the adverse effects of stress on physical health (e.g., hypertension, heart disease) may directly affect neuropsychological function (Waldstein & Wendell, 2010), or indirectly increase the likelihood that an individual will have to make an important medical decision, for example, that subsequently involves confronting risk.
Although research has documented the effects of acute stressors on decision making (Mather et al., 2012; Starcke & Brand, 2012), little is known about how the neurobiological indicators of chronic stress may be associated with decision-making processes. The diurnal cycle of cortisol is believed to be an indicator of one of the main stress-response systems, the hypothalamic-pituitary-adrenal (HPA) axis. Research has found that the diurnal cortisol rhythm is associated with the experience of chronic stressors (see McEwen, 2012 and Miller, Chen, & Zhou, 2007 for review).1 In the current study, we investigate the degree to which the diurnal cortisol rhythm is associated with risky decision-making (i.e., choosing an option with greater outcome variability) in a sample of older adults between the ages of 55 and 88 years.
Risky Decision Making in Older Adults
Although early research conceptualized older adults as being risk-averse compared to younger cohorts (Okun, 1976), this proposition has received mixed support in laboratory-based studies of risk. For instance, Deakin, Aitken, Robbins, and Sahakian (2004) found that older adults made more conservative bets (i.e., more risk averse). In contrast, Zamarian, Sinz, Bonatti, Gamboz, and Delazer (2008) found that older adults did not differ from younger adults in a risky decision making task. Similarly, studies using the Iowa Gambling Task (IGT; Bechara, Damasio, Tranel, & Damasio, 1997), report that up to one-third of older adults display suboptimal decision making skills (Denburg, Recknor, Bechara, & Tranel, 2006; Denburg, Tranel, & Bechara, 2005; Denburg et al., 2007, 2009; Fein, McGillivray, & Finn, 2007; MacPherson, Phillips, & Della Sala, 2002), whereas other studies using the IGT reported no differences in terms of overall performance (Kovalchik, Camerer, Grether, Plott, & Allman, 2005; although see Wood, Busemeyer, Koling, Cox, & Davis, 2005). Still, other studies find that age-related differences in risky-decision making may vary as a function of contextual factors such as the decision outcome’s valence (i.e., gains vs. losses; Lauriola & Levin, 2001; Mather et al., 2012; Weller et al., 2011). For example, Lauriola and Levin (2001) found that age was positively associated with risk aversion for gains, but greater risk-seeking when potential losses were involved. Weller et al. (2011) found that older adults showed reduced levels of risk-taking for achieving risky gains compared to younger adults (44 years and younger), but older adults also showed a relatively consistent pattern of risk-taking to avoid losses. Mather et al. (2012) replicated this effect for potential gains, but similar to Lauriola and Levin (2001) found that older adults took more risks to avoid losses. These findings not only highlight the variability in older adults’ risk preferences, compared to younger adults, but also reinforce the need to examine factors that may account for this heterogeneity across older adults. Typically, researchers have examined cognitive factors such as processing speed, memory, and fluid intelligence as predictors of risky decision making in elderly populations (Henninger et al., 2010; Li et al., 2013). However, noncognitive factors such as personality traits also predict decision-making deficits. For instance, Denburg et al. (2009) found that older adults who showed greater levels of neuroticism, a trait related to the experience of negative emotions and stress reactivity, performed more poorly on the IGT, compared to both older adults lower in neuroticism and younger adults. Similarly, Williams, Suchy, and Kraybill (2010) found that neuroticism, measured by the NEO-PI-R (Costa & McCrae, 1992) was inversely associated with executive functioning in older adults.
Neurobiological Stress Response System
In the current study, we focus our inquiry on the degree to which individual differences in HPA axis dynamics may predict differences in older adults’ decision making. Studies involving animal models and humans have examined a number of physiological systems that are affected by chronic stress, including the HPA axis, the sympathetic nervous system (SNS), and the immune system (e.g., Heim, Newport, Bonsall, Miller, & Nemeroff, 2001). Glucocorticoid (GC; cortisol in humans) secretion is reflective of HPA axis activity and is a necessary factor in human metabolism processes, exerting multiple effects throughout the body (c.f., Bremner & Vermetten, 2001; De Bellis et al., 2001; Heim, Owen, Plotsky, & Nemeroff, 1997; Teicher, Andersen, Polcarri, Anderson, & Navalta, 2002). While acutely elevated GCs and SNS markers are necessary for the short-term metabolic demands brought on by stress, chronic activation of these systems threaten neuronal viability and can lead to a host of stress-related disorders (De Kloet, Rots, & Cools, 1996; McEwen, 2000). Indices of the diurnal rhythm of cortisol serve as an indicator of the integrity of HPA axis activity. Measurement of the diurnal cycle of cortisol captures dynamic intra-day activity that is altered by hyper-activation of the system and may serve as an index of chronic stress (McEwen, 2012).
From a neuropsychological perspective, the stress response system is interconnected with structures implicated in decision-making processes, including the amygdala, hippocampus and the medial prefrontal cortex (e.g., Bechara et al., 1997; Mohr, Biele, & Heekeren, 2010). Specifically, the hippocampus and the medial prefrontal cortex play an added role in determining stressor intensity and ultimately in turning off the HPA system through GC-mediated negative feedback (Diorio, Viau, & Meaney, 1993; Herman, Ostrander, Mueller, & Figueiredo, 2005; Sullivan & Gratton, 2002). Moreover, evidence suggests that chronic stress can result in structural changes to these neural structures. For instance, chronic stress is believed to result in dendritic expansion of the orbitofrontal cortex and dendritic shrinkage throughout the medial prefrontal cortex (Liston et al., 2006). The intimate connection between neural structures necessary for control of stress physiology and decision making suggest that these structures may represent a common denominator of age-related variability in these domains.
Stress and Cognition
Using acute stressor paradigms, stress-induced cortisol has been associated with decreased performance on a number of cognitive measures including memory retrieval (Buchanan, Tranel, & Adolphs, 2006; Buchanan & Tranel, 2008), working memory (al’Absi, Hugdahl, & Lovallo, 2002; Elzinga & Roelofs, 2005), and decision making (see Mather & Lighthall, 2012 and Starke & Brand, 2012, for reviews). However, considerable work has also demonstrated that diurnal measures of cortisol are associated with reduced cognitive performance (Lupien et al., 1994; 1998; Power, Li, & Hertzman, 2008; Evans et al., 2011). The dynamics of the diurnal cycle include the cortisol awakening response (Pruessner et al., 1997; Clow, Thorn, Evans, & Hucklebridge, 2004) and the ensuing diurnal fall in cortisol from the peak after awakening to the nadir of the cycle in the evening. Changes in the dynamics of the diurnal cycle of cortisol have been documented in a number of clinical populations, including breast cancer (Abercrombie et al., 2004), post-traumatic stress disorder (Rohleder, Joksimovic, Wolf, & Kirschbaum, 2004), and individuals with hippocampal damage (Buchanan, Kern, Allen, Tranel, & Kirschbaum, 2004). Evans et al. (2011) showed that a less steep diurnal fall (indicative of a less dynamic, less healthy HPA axis), but not basal cortisol levels, was associated with poorer overall cognitive performance in an aging population. Interestingly, this effect was independent of age, suggesting that integrity of the HPA axis, as measured by the diurnal fall in cortisol, may affect cognition throughout the lifespan.
Stress, HPA Dynamics, and Risky Decision Making in Older Adulthood
Although evidence from the acute stress literature has largely demonstrated a negative impact of stress on decision making (see Starke & Brand, 2012), research investigating the association between HPA axis dynamics and decision making is scant. Structural changes caused by prolonged stress exposure are believed to be associated with deficits in cognitive flexibility and the emotional processing of potential rewards and punishments. For instance, Dias-Ferreira et al. (2009) found that chronically-stressed rats became insensitive to changes in outcome value on a decision-making task and relied more on habitual responses than nonstressed rats. Moreover, Porcelli and Delgado (2009) found that an acute stress manipulation increased the classic “preference shift” effect (Levin, Gaeth, Schreiber, & Lauriola, 2002), in which individuals demonstrated more risk averse preferences for potential gains than losses.
Porcelli and Delgado (2009) suggested that while under stress, decision makers fall back on automatic reactions to risk that they would be better able to avoid in an unstressed state. However, goals and motivations are likely to vary across the lifespan. According to Carstensen and colleagues’ Socioemotional Selectivity Theory (SST; Carstensen, 2001; Reed & Carstensen, 2012), motivational goals shift from exploratory, knowledge-acquisition goals to emotional regulation goals in older adulthood. According to SST, this shift coincides with a positivity effect, in which older adults weigh positive emotional information more prominently than negative emotional information. This rationale is consistent with findings that older adults are more risk-averse when asked to make a decision between a sure and risky option (Lauriola & Levin, 2001; Mather et al., 2012; Weller et al., 2011).
However, implementation of these emotional regulation goals appears to be somewhat dependent on cognitive control capabilities (Mather & Knight, 2005). This research implies that the relatively greater influence of positive over negative information on older-adult decisions will occur only for those older adults who have the resources necessary (due to ability, motivation, or time) to meet the motivational goals hypothesized in SST. In the absence of these resources, an individual may fall back upon prior learned goals and behaviors. Thus, given the demonstrated impact of stress on cognitive flexibility (Plessow, Kiesel, & Kirschbaum, 2012), we would expect that older adults who had flattened diurnal cortisol profiles may be more likely to demonstrate increased risk-taking, especially for potential gains, compared to those with typical diurnal cortisol rhythms.
The Current Study
The current study tested whether the diurnal cortisol rhythm was associated with older adults’ performance on a risky-decision making task that involved both decisions to achieve potential gains and to avoid potential losses. First, we collected cortisol samples at three time points throughout the day. Then, on the following day, participants completed a behavioral assessment, including the expanded “Cups” Task (Weller, Levin, Shiv, & Bechara, 2007), which independently assesses risky decision making for potential gains and losses. This task also offers the opportunity to examine how stress may impact older adults’ ability to make judgments that are sensitive to the relative expected value (EV) between choice objects. Whereas the risk taking (number of risky choices made) provides an index of risk preference, making the EV-consistent judgments is a reflection of advantageous decision-making in the sense that it demonstrates the tendency to select options that offer more favorable consequences over the long run.
Method
Participants
Healthy adults who lived independently in the community were recruited from churches, organizations, and clubs, including a local senior center. The health of each participant was confirmed via a semi-structured health interview that assessed neurological status, current medications, alcohol/drug consumption, and mood (after Tranel, Benton, & Olson, 1997). We originally sampled 74 participants. One participant failed to return the saliva samples, one did not adhere to dietary restrictions (see below), and three participants’ cortisol output was not measurable at more than two assessments; hence, these five participants were excluded, leaving a total of 69 adults (Median age = 71 years, range 55–88 years; 58% female).2
Procedure
The study consisted of two separate phases: the salivary cortisol collection phase and the behavioral assessment phase.
Salivary Cortisol Collection
Each participant was provided with a saliva collection kit that included detailed written instructions and three Sorbette collection devices (Salimetrics, LLC, State College, PA). The use of these devices provides a well-established, noninvasive method to obtain saliva for the measurement of cortisol output (see Granger et al., 2007). Salivary cortisol collections occurred three times over the course of the day before the behavioral assessment session: 30 minutes after awakening, 8 hours after awakening, and 30 minutes before going to bed. A research assistant demonstrated saliva collection procedures to all participants; participants were given as much time as they needed to ask questions about the saliva sampling procedures. Prior to the first sample and before the evening assessment, participants were asked to abstain from brushing their teeth. They were also asked to abstain from alcohol, nicotine, and caffeine 1 hour prior to each saliva collection. Participants were asked to label the exact time and date of collection with a black permanent marker. Immediately, after each collection, participants replaced the cap on each Sorbette tube snugly and placed the saliva samples in their home freezer. Further, since cortisol levels can be influenced by numerous environmental, pharmacological, and internal factors, many safeguards were included in order to minimize the effects of potential confounds. We used the following inclusion criteria: a normal medical (i.e., no history of chronic illnesses such as diabetes, HIV/AIDS, cancer, etc.) and psychiatric history (i.e., no diagnosis of post-traumatic stress disorder, mood or anxiety disorder, or schizophrenia within the past six months), drinking less than two alcoholic beverages per day, smoking less than 10 cigarettes per day, and not taking any corticosteroids or other medications which might affect cortisol levels or interfere with assay. At the behavioral assessment session the next day, participants delivered the saliva samples to the laboratory. The samples were then stored at −20° C until shipped (on dry ice) for assay. Samples were assayed by an independent laboratory (Salimetrics, Inc., State College, PA). Intraassay and interassay coefficients of variation were less than 10%.
Although additional saliva samples across the day are needed to fully characterize the diurnal rhythm of cortisol, we decided to collect only three samples to focus on the measure of diurnal fall of cortisol and to reduce participant burden (see Clow et al., 2004). The selected sampling times permitted the examination of peak cortisol levels (morning), intermediate cortisol levels (8 hours post peak), and the nadir of cortisol levels (30 minutes pre-bedtime), allowing for the measure of individual differences in diurnal cortisol output. These data were reduced to determine two primary measures of interest: 1) mean cortisol across all three samples regardless of the time of day to provide an individual metric reflecting total cortisol output; and 2) the diurnal fall in cortisol across all three collection points to provide an individual measure of the diurnal dynamics in cortisol across one day (see Evans et al., 2011). For analyses involving the mean cortisol level variable, we first conducted a natural log transformation of each assessment and then averaged the log-transformed values to create a composite. The diurnal fall is assessed via the ‘area under the curve with respect to ground’ (AUCG; Pruessner, Kirschbaum, Meinlschmid, & Hellhammer, 2003). Note that the diurnal fall is depicted in negative numbers, and so reflects an ‘index of decrease’ rather than a true area under the curve (see Pruessner et al., 2003). For ease of interpretation in our inferential analyses, we mean-centered this value; thus greater values indicate a greater diurnal fall.
Behavioral Assessment
Upon arrival at the laboratory the day after salivary cortisol collection, participants were first asked to confirm compliance with dietary and collection schedule guidelines. Then, as part of a larger project, participants were asked to complete the Cups Task, a measure of decision-making under risk, and basic neurocognitive functioning involving mental status screening, premorbid intellectual ability, current intellectual functioning, and verbal and nonverbal anterograde memory, in addition to a self-report measure of neuroticism, a trait related to the dispositional tendency to experience stress, worry, and anxiety.
Cups Task
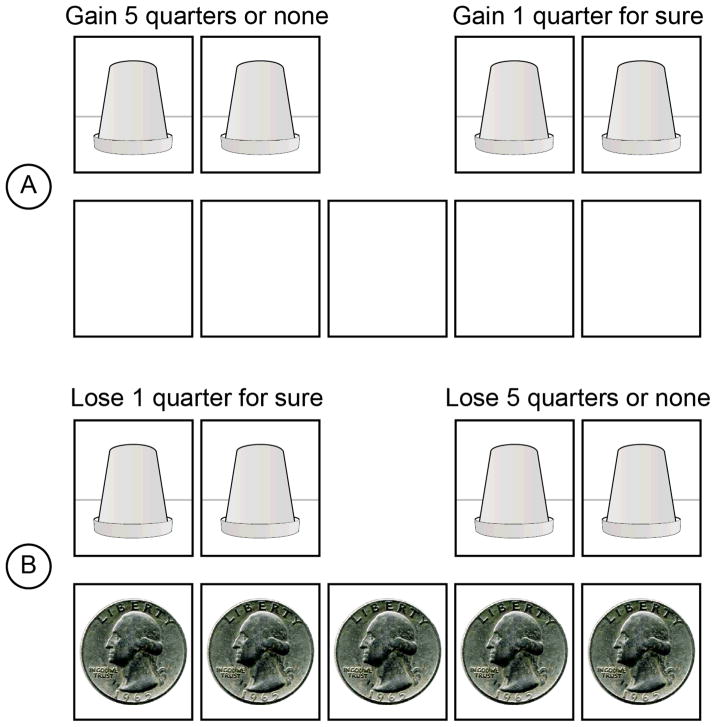
Participants completed a computerized version of the Cups task (Figure 1; see Weller et al., 2007, for a more detailed explanation). The Cups task consists of 54 trials representing 3 trials each of all combinations of two domains (gain/loss), 3 levels of probability (.20/.33/.50) and 3 levels of outcome magnitude for the risky option ($.50/.75/1.25) compared to one quarter for the riskless option. By independently manipulating probability and the outcome magnitude of the risky choice, the Cups task allows the examination of how individuals adjust their choices based on a comparison of the relative expected value (EV) between choice options. In each risk domain, nine trials offered choices that had equivalent EVs (Equal EV) for the risky and riskless options, nine trials involved decisions in which the EV for the risky option was more favorable than the riskless option (RA trials), and nine trials involved decisions in which the EV for the riskless option was more favorable than that of the risky option (RD trials). Thus, the tendency to choose the riskless option on RD trials and the risky option on RA trials represents an index of advantageous decision making (i.e., choosing the option with a more favorable EV will yield more positive outcomes over the long run).
Figure 1.
Cups Task paradigm. Note. In the Cups Task, participants see two arrays of cups. One array is designated as the riskless side. If chosen, the participant wins/loses one quarter. The other array is risky; the outcome is uncertain. Based on their choices, participants receive immediate feedback regarding the amount that they won or lost on that trial. Panel A displays an example of a risk-advantageous trial in the gain domain (EV of the risky choice is greater than the EV of the riskless choice). Panel B displays an example of a risk-disadvantageous trial in the loss domain (EV of the risky choice is less than the EV of the riskless choice). Although participants see an empty bank at the beginning of each gain trial, participants begin all loss trials with the amount of quarters that they could possibly lose.
Participants selected a cup from the side that they chose (i.e., riskless or risky). At the bottom of the screen was a depiction of a bank where coins were shown being added to (subtracted from) the decision maker’s monetary account. A random process with p = 1/(number of cups) determined whether the risky choice led to a gain (loss). When the participant completed all 54 trials, their total amount won appeared on the screen. Gain and loss trials were presented as blocks, counterbalanced in order across all participants.
Basic Neurocognitive Functioning and Neuroticism
Mental status screening was conducted with the Mini-Mental State Examination (MMSE; Folstein, Folstein, & McHugh, 1975), a 30-point, verbally administered questionnaire sensitive to impairment in attention, orientation, word recall, calculation, language, and visuospatial ability. Premorbid intellectual ability was measured using the Wide Range Achievement Test-3 Reading subtest (WRAT-3; Wilkinson, 1993), a 42-item single-word reading task. Reading performance is highly correlated with Wechsler vocabulary performance (Wilkinson, 1993). Current intellectual ability was measured using the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999), which consists of four subtests (two verbal and two nonverbal): Vocabulary, Similarities, Block Design, and Matrix Reasoning. The WASI subtests are newly created but designed to parallel their lengthier and well-validated Wechsler Adult Intelligence Scale (WAIS-III; Wechsler, 1997) counterpart. For this study, Full Scale IQ served as our measure of current intelligence. As a proxy for hippocampal integrity, verbal memory was tested with the Rey-Auditory Verbal Learning Test (Rey, 1964). Additionally, nonverbal anterograde memory was assessed with the Rey-Osterrieth Complex Figure Test (Rey, 1941), in which the participant is asked to copy the figure with the stimulus left exposed, and then to incidentally recall the figure after a 30-minute delay. Finally, we administered the Neuroticism scale of the well-validated NEO-FFI (Costa & McCrae, 1992).
Results
Neurocognitive Functioning
As seen in Table 1, participants’ performance on the neuropsychological instruments reflected normal, age-appropriate functioning (Lezak, Howieson, Bigler, & Tranel, 2012). To illustrate, MMSE scores were close to ceiling. Additionally, Full Scale IQ and WRAT scores fell in the Average to High Average range of intellectual functioning. Verbal and nonverbal memory scores met normative expectations.
Table 1.
Descriptive Statistics
| Variable | M | SD |
|---|---|---|
| MMSE | 29.26 | 1.00 |
| WASI Full Scale IQ | 119.31 | 11.10 |
| WRAT Reading: Age-corrected Standardized Score | 109.93 | 6.61 |
| Rey-O: | ||
| Copy | 32.07 | 2.92 |
| 30-min Delay | 16.44 | 6.25 |
| AVLT: | ||
| Immediate Recall (Trial 5) | 11.86 | 2.50 |
| 30-min Delay | 9.86 | 2.95 |
| Mean Cortisol Output | .22 | .22 |
| Cortisol 30m post-wake | .42 | .43 |
| Cortisol 8h post-waking | .15 | .21 |
| Cortisol 30m, before sleep | .08 | .11 |
| Diurnal Cortisol Fall (AUCg) | −.42 | .41 |
| Total Risk Taking Gains | 14.03 | 6.87 |
| Total Risk Taking Losses | 17.55 | 7.67 |
Note. MMSE = Mini-Mental State Examination; WASI = Wechsler Abbreviated Scale of Intelligence; WRAT = Wide Range Achievement Test; Rey-O = Rey-Osterrieth Complex Figure Test; AVLT = Rey Auditory Verbal Learning Test.
Diurnal Cortisol Levels
Cortisol values, including means of raw values at all three time points, the overall mean cortisol output, and the AUCG reflection of the diurnal fall are presented in Table 1. Overall, participants showed the expected diurnal pattern of cortisol secretion, including a pronounced diurnal fall from the awakening peak to bedtime, F (2,86) = 39.0, p < .0001. There were no sex differences for either diurnal cortisol fall, t (67) = .98, p= .33, or mean cortisol output, t (67) = 1.55, p= .13.
Correlations between Study Variables
Mean cortisol output was positively associated with diurnal cortisol fall, suggesting that the older adults who showed lower cortisol fall also demonstrated lower mean output. There were no significant associations between age and either of the cortisol variables, WASI Full Scale IQ or WRAT reading score. Additionally, we did not find significant associations between mean cortisol output and risk-taking to avoid losses, as measured by the Cups Task. However, we observed a significant inverse correlation between diurnal cortisol fall and risk-taking to achieve gains. Specifically, those who demonstrated a flattened diurnal cortisol fall throughout the day made more risky choices (see Table 2).
Table 2.
Intercorrelations Between Study Variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Mean Cortisol Output | — | |||||||||||
| 2. Diurnal Fall AUC (Centered) | .53** | — | ||||||||||
| 3. Age When Tested | .03 | −.04 | — | |||||||||
| 4. NEO Neuroticism | −.08 | −.09 | −.25* | — | ||||||||
| 5. WASI Full Scale IQ | .07 | .11 | .08 | .16 | — | |||||||
| 6. WRAT Reading | −.02 | .03 | .11 | −.06 | .48** | — | ||||||
| 7. Rey-O Copy | −.13 | −.16 | −.08 | −.05 | .12 | .27 | — | |||||
| 8. Rey-O 30min delay | −.16 | .09 | −.13 | .00 | .20 | −.09 | .26* | — | ||||
| 9. AVLT Trial 5 | −.10 | −.03 | −.29* | .11 | .25* | .15 | .12 | .33** | — | |||
| 10. AVLT 30min delay | −.22 | −.08 | −.27* | .08 | .24* | .29* | .22 | .29* | .80** | — | ||
| 11. Total Risk Taking Gains | −.18 | −.24* | −.02 | .04 | .08 | −.12 | −.03 | .04 | −.07 | −.12 | — | |
| 12. Total Risk Taking Losses | −.19 | −.11 | −.06 | −.10 | .01 | .03 | −.07 | .04 | −.15 | .04 | .39** | — |
Note. WASI = Wechsler Abbreviated Scale of Intelligence; WRAT = Wide Range Achievement Test; Rey-O = Rey-Osterrieth Complex Figure Test; AVLT = Rey Auditory Verbal Learning Test.
p < .05.
p < .01.
Cups Task Performance
To test the associations among diurnal cortisol fall, sex, and risky decision making, we conducted a Generalized Estimating Equation (GEE; Liang & Zeger, 1986) analysis that allows for a within-subjects analysis of participants’ decision behavior for each trial. We fit a binomial response model using a logit-link function using each choice (0 = safe, 1 = risky) as the dependent measure. Analyses were conducted with a compound symmetry working correlation matrix, which assumes homogenous correlations between within-subject responses. Parameter estimates were achieved using hybrid maximum likelihood estimation.
We began the analyses with a full-factorial model for domain (gain/loss), EV (relative EV between the sure and risky options; EVrisky − EVriskless), participant’s sex, and diurnal cortisol fall. EV, sex, and diurnal fall were mean-centered. WASI scores, neuroticism scores, and participant’s age were standardized and entered as control variables. As shown in Table 3, we observed several significant model effects. Overall, the probability that an individual made a risky choice became greater as the relative EV between choice options more strongly favored the risky option, and vice versa. Similarly, consistent with the “reflection” or “preference shift” effects (Levin et al., 2002; Kahneman & Tversky, 1979), we found that participants took more risks to avoid losses than to achieve gains. Participant age, Neuroticism, and WASI Full Scale IQ scores did not account for variance in risk-taking, holding other variables constant.
Table 3.
Overall Cups Task Performance, GEE Analysis
| Parameter | B | Std. Error | 95% CI
|
Wald χ2 | Odds Ratio | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| EV Level | 4.21 | .35 | 3.53 | 4.89 | 147.24** | 67.22 |
| Sex | .01 | .12 | −.23 | .25 | .00 | 1.01 |
| Diurnal Fall | −.43 | .29 | −1.01 | .14 | 2.17 | .65 |
| Age | −.08 | .12 | −.32 | .16 | .40 | .93 |
| NEO Neuroticism | −.11 | .13 | −.37 | .14 | .77 | .89 |
| Full Scale IQ | −.02 | .12 | −.26 | .23 | .02 | .98 |
| Domain (Gain = 0; Loss = 1) | .83 | .07 | .69 | .96 | 141.49** | 2.29 |
| Sex x Domain | .22 | .07 | .09 | .36 | 10.43** | 1.25 |
| Sex x EV Level | −1.24 | .35 | −1.93 | −.55 | 12.38** | .29 |
| Domain x EV Level | −1.17 | .47 | −2.08 | −.25 | 6.26** | .31 |
| Diurnal Fall x Sex | .12 | .31 | −.49 | .73 | .15 | 1.13 |
| Diurnal Fall x Domain | .27 | .17 | −.06 | .61 | 2.56 | 1.31 |
| Diurnal Fall x EV Level | −.09 | .81 | −1.69 | 1.51 | .01 | .91 |
| Diurnal Fall x Sex x Domain | −.10 | .17 | −.44 | .23 | .38 | .90 |
| Diurnal Fall x Sex x EV Level | −.54 | .82 | −2.15 | 1.06 | .44 | .58 |
| Diurnal Fall x Domain x EV Level | 1.31 | 1.18 | −1.00 | 3.62 | 1.23 | 3.71 |
| Sex x Domain x EV Level | .79 | .47 | −.14 | 1.72 | 2.79 | 2.20 |
| Diurnal Fall x Sex x Domain x EV Level | −2.47 | 1.19 | −4.80 | −.13 | 4.27* | .09 |
p < .05.
p < .01.
Though we did not find a main effect for participant’s sex, we did observe a significant Sex X EV Level interaction. Specifically, men demonstrated greater EV Sensitivity across domains than women. Additionally, a Sex × Domain interaction suggested that women were more likely to show greater risk taking for losses than for gains (i.e., a preference shift effect), whereas men were more consistent in terms of their overall risk taking across domains. The increased preference shift observed in women, compared to men, appeared to be the result of an increased likelihood to choose a risky option to avoid a certain loss, but not so for the gain domain. These interactions must be considered in light of an observed four-way Sex × Cortisol Fall × EV Level × Domain interaction. In order to decompose this effect, we conducted two follow-up GEE analyses, separately for risk-taking in the gain and loss domains (see Tables 4 and 5).
Table 4.
Risk-Taking to Achieve Gains, GEE Analysis
| Parameter | B | Std. Error | 95% CI
|
Wald χ2 | Odds Ratio | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| EV Level | 4.192 | .38 | 3.45 | 4.93 | 124.22** | 66.13 |
| Sex | .02 | .05 | −.08 | .13 | .17 | 1.02 |
| Diurnal Fall | −.49 | .13 | −.74 | −.23 | 14.24** | .62 |
| Age | −.01 | .05 | −.12 | .09 | .05 | .99 |
| NEO Neuroticism | −.04 | .05 | −.15 | .07 | .47 | .96 |
| Full Scale IQ | .07 | .05 | −.04 | .18 | 1.52 | 1.07 |
| Diurnal Fall x Sex | .07 | .38 | −.21 | .34 | .23 | 1.07 |
| Sex X EV Level | −1.25 | .38 | −2.00 | −.50 | 10.63** | .28 |
| Diurnal Fall x EV Level | −.09 | .89 | −1.84 | 1.66 | .01 | .91 |
| Diurnal Fall x Sex x EV Level | −.65 | .90 | −2.40 | 1.11 | .52 | .52 |
p< .05.
p < .01.
Table 5.
Risk Taking to Avoid Losses – GEE Analysis
| Parameter | Unstandardized B | Std Error | 95% CI
|
Wald χ2 | OR | |
|---|---|---|---|---|---|---|
| Lower | Upper | |||||
| EV Level | 3.08 | .56 | 1.99 | 4.17 | 30.58** | 21.70 |
| Sex | .31 | .18 | −.03 | .66 | 3.13 | 1.37 |
| Diurnal Fall | −.26 | .36 | −.97 | .44 | .53 | .77 |
| Age | −.20 | .19 | −.57 | .18 | 1.07 | .82 |
| NEO Neuroticism | −.20 | .23 | −.64 | .24 | .80 | .82 |
| Full Scale IQ | .11 | .17 | −.22 | .44 | .42 | 1.12 |
| Diurnal Fall x Sex | .27 | .39 | −.50 | 1.04 | .47 | 1.32 |
| Sex x EV Level | −.49 | .57 | −1.59 | .61 | .77 | .20 |
| Diurnal Fall x EV Level | 1.17 | 1.14 | −1.05 | 3.40 | 1.07 | 3.24 |
| Diurnal Fall x Sex x EV Level | −3.05 | 1.15 | −5.31 | −.79 | 6.99* | .05 |
p < .05.
p < .01.
Risky Decision Making, Gain Domain
As seen in Table 4, we found a significant main effect for EV Level. That is, compared to the sure option, as the EV for the risky choice became more favorable compared to the sure option, individuals took more risks, and individuals took fewer risks as the EV for the risky option became less favorable. Moreover, consistent with our correlational analysis, we found that lower diurnal cortisol fall predicted greater risk-taking, holding other variables constant. Additionally, we observed a significant Sex × EV Level interaction. In order to better interpret this interaction, we conducted GEE analyses, separately for men and women, which regressed risk taking on a given trial on EV Level.3 Men were 2.45 times more likely to make a risky choice on trials when the EV favored the risky option (i.e., RA trials) than when the EV favored the riskless option (i.e., RD trials; O.R. = 6.818). In comparison, woman were only 1.78 times more likely to make a risky choice on RA trials compared to RD trials (O.R. = 3.251).
Risky Decision Making, Loss Domain
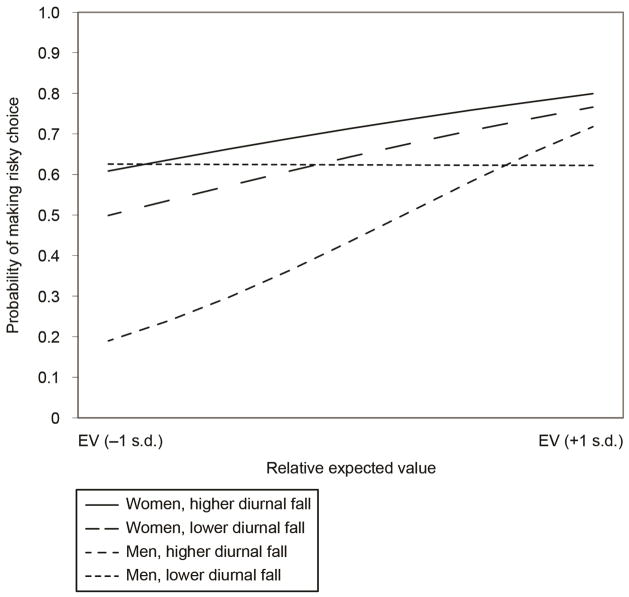
The results of the GEE analyses for risk-taking in the Loss domain are shown in Table 5. In contrast to the results observed for risk-taking to achieve potential gains, we observed a more complex pattern. As expected, we found a significant main effect for EV Level, such that individuals were sensitive to the relative EV between choice options. We did not observe significant main effects for Diurnal Fall or Sex. However, we observed a significant three-way EV Level × Diurnal Fall × Sex interaction (see Figure 2). Again, we decomposed these effects by conducting simple GEE analyses. In addition to the clustering of RA and RD trials, cortisol fall was dichotomized by a tertiary split in these simple effect analyses. Specifically, for those with higher diurnal fall, we observed that men were more sensitive to EV Level than women (see Weller, Levin, & Bechara, 2010 for similar results in a college-age population). Men with a greater diurnal fall were 1.90 times more likely to take a risk on a RA trial over a RD trial (O.R. = 4.03). By contrast, men with a lower diurnal cortisol fall were only 1.11 times more likely to do so (O.R. = 1.51), indicating a reduced sensitivity to EV. For women, there were smaller differences in EV sensitivity as a function of diurnal fall. Women with greater diurnal fall were 1.31 times more likely to take a risk on an RA trial than on a RD trial (O.R. = 2.41). In contrast, women with lower diurnal fall appeared to be slightly more sensitive to EV (R.R. = 1.51; O.R. = 4.31).
Figure 2.
Sex differences in the association between low cortisol fall and EV sensitivity for risks involving potential losses. Note. RD = risk-disadvantageous trials. RA = risk-advantageous trials Model-based estimates are represented.
Discussion
Past research has suggested that chronic stress, and its consequent dysregulation of the HPA-axis, is associated with reduced cognitive function, including decision making. To date, though, most research examining this relationship has focused on the effects of acute stressors on decision making performance. To our knowledge, this is the first study to directly examine the associations between an index of diurnal stress hormone dynamics and decision-making in an older adult population. Results from this study show that diurnal cortisol fall, a measure of HPA-axis dynamics, predicted risk propensity and EV sensitivity. These findings extend our knowledge about the role of stress in decision-making preferences in two primary ways. First, older adults who demonstrated a more normative pattern of diurnal cortisol fall (e.g., a steeper fall) were more likely to avoid risks in favor of a small, but certain gain than were those who demonstrated a flatter diurnal cortisol fall. Second, when the decisions involved potential losses, these effects became more gender-specific. Males displaying a flatter diurnal cortisol fall were less sensitive to differences in expected value between choice options and took more unnecessary risks for potential losses than those with more typical diurnal cortisol fall.
Sex Differences, Stress, and Decision Making
In this study, our results suggested a unique domain-specific pattern for the effects of sex-stress interactions on decision-making. Typically, men self-report greater risk-taking than women (Byrnes, Miller, & Schafer, 1999). However, in laboratory-based decision-making paradigms such as the IGT, women tend to perform worse than men in terms of making more long-term disadvantageous choices (e.g., Bolla, Eldreth, Matochik, & Cadet, 2004; Lejuez, Aklin, Zvolensky, & Pedulla, 2003; Overman et al., 2006; Reavis & Overman, 2001). Using the Cups task, Weller et al. (2010) also observed that women were more likely than men to take risks to avoid losses than to achieve gains. Moreover, women were less sensitive to relative differences in expected value between choice options, irrespective of domain. Broadly, the current results suggest that this pattern largely extends into older adulthood.
Notably, our sex-stress interaction results appear consistent with findings in the acute stress literature, which has suggested that stress impacts males and females differently (Lighthall, Mather, & Gorlick, 2009; Mather & Lighthall, 2012; Preston, Buchanan, Stansfield, & Bechara, 2007; Putnam, Antypa, Crysovergi, & van der Does, 2010; van den Bos, Harteveld, & Stoop, 2009). Specifically, acute stress has been shown to increase risk-taking in males, and decrease it in females. For instance, Preston et al. (2007) found that while stress decreased performance on the IGT in men and women, men exposed to a psychological stressor made even more choices from high-reward/high punishment decks compared to stressed women, a strategy that resulted in lower earnings. Using the Balloon Analogue Risk Task (Lejuez et al., 2003), Lighthall et al. (2009) found that stressed males took more risks compared to stressed females. In a study in which cortisol was experimentally administered, males exhibited increased risk-seeking, particularly for gambles with a high probability of loss and a large possible gain (Putman et al., 2010). However, these studies have all employed paradigms that concurrently involved potential gains and losses. Although men perform more advantageously on the Cups Task (Weller et al., 2010), the current study suggests that men may be particularly prone to the negative effects of stress on advantageous decision-making, especially when potential losses are involved.
Risk-Taking for Potential Gains and Losses
More broadly, these results extend a growing literature that has begun to investigate the differential antecedents and consequences of risky decision making to achieve gains and to avoid losses (Levin et al., 2012; Weller et al., 2007, 2010, 2011; Yechiam & Hochman, 2013; Yechiam & Telpaz, 2013). Yechiam and Hochman (2013) offer one potential alternative account for these findings. They posit that potential losses induce greater attentional resources, and therefore produce more consistency in response (Yechiam & Telpaz, 2013). One implication of this attention hypothesis might be that risk preferences to avoid losses should be less impacted by extraneous influences, including differences in HPA dynamics. Although we found that the main effect of diurnal fall predicted overall risk taking for gains but not losses, our results suggesting sex differences would indicate that decision-making to avoid potential losses may also be disrupted by factors not essential to the decision per se. Consistent with Yechiam and Hochman’s (2013) theory, we posit that potential losses may direct attention; however, this increased attention may differentially mobilize decision strategies for men and women. In a small study using the IGT, Cheng, Sheu, and Yen (2009) reported that women were more biased by loss information than men (see also Weller et al., 2010). In this light, when not experiencing chronic or acute stress, men may more readily adopt a strategy that more heavily recruits deliberative, “colder” cognitive strategies (i.e., solving the problem mathematically) than women, who may approach losses by adopting “hotter” strategies (i.e., overweighting the magnitude of the potential loss). Hence, with these “colder” processes generally declining as a function of general physiological aging, HPA axis dysregulation may further compromise the ability to recruit deliberative processes, leading to suboptimal decision-making. In contrast, because of a greater reliance on “hotter” strategies in the first place, the decision-making abilities for women with lower diurnal cortisol fall may not be as affected. Experimental manipulations of attention, stress or incidental emotions will ultimately help to disambiguate the underlying decision processes mediating gain and loss preferences.
Older Adults and the Positivity Effect
These findings also may be interpreted within the context of the well-documented “positivity effect” observed in older adults predicted by socioemotional selectivity theory (SST; Carstensen, 2001; Reed & Carstensen, 2012). There has been robust evidence in the literature suggesting that older adults report lower negative affect and are more motivated towards positive emotional information than younger adults (Charles, Mather, & Carstensen, 2003; Kennedy, Mather, & Carstensen, 2004; Mather & Carstensen, 2003). This pattern corresponds with mean-level reductions in neuroticism across the lifespan (Soto, John, Gosling, & Porter, 2011). An implication of a SST account is that cognitive resources are required to implement and execute these emotion regulation goals (Mather & Knight, 2005). Because risk aversion when considering potential gains appears to progressively increase over the lifespan (Weller et al., 2011), we speculate that this tendency may be a consequence of an emotional regulation goal. It follows that if the processing of this goal is disrupted or made less accessible, then it is more likely that the individual will demonstrate an elevated risk propensity. Research directly designed to manipulate the accessibility of emotion regulation goals and its subsequent effects on risk-taking would help to illuminate these issues.
Implications for Healthy Aging
Although older adults are believed to show biases toward processing positive emotional information, and/or inhibiting negative information, the current results suggest that the insidious effects of stress over the lifespan may result in deleterious effects on decision making. Individuals showing a diurnal cortisol fall characterized by a flatter slope are exposed to greater average cortisol levels over time compared to individuals showing a steeper slope of cortisol (Miller et al., 2007). A recent study assessing the dynamics of diurnal cortisol in a large sample of older participants showed that older age, male sex, and lower socioeconomic status predicted higher cortisol levels and a flatter diurnal cycle (Karlamangla, Friedman, Seeman, Stawski, & Almeida, 2013). This increased exposure may set the stage for vulnerability to the negative effects of stress on cognition, perhaps similar to those observed in acute studies of stress. Evans et al. (2011) suggest a specific dysfunction of the hippocampus, which leads to altered HPA dynamics and poor cognitive function in aging. Results from the current study provide preliminary evidence that these altered HPA dynamics may affect the function of brain regions beyond the hippocampus, specifically regions of the prefrontal cortex that are known to play a role in decision making. In addition to effects of cortisol exposure on cognition, this pattern most likely would result in physiological and neurological changes that could have deleterious effects on mental and physical health (see Lupien et al., 2009), making chronic stress a significant public health concern.
Although this study does not equate chronic stress with diurnal cortisol fall, we speculate that a potential implication of our findings is that older men who have experienced chronic stress throughout their lives may also demonstrate differences in other decision-making situations that involve losses. For instance, they may be more likely to show greater loss aversion and an increased tendency to honor sunk costs, biases that have the potential to lead to poor financial outcomes (e.g., Arkes & Blumer, 1985; Kahneman & Tversky, 1979). This research raises the possibility that the ability to manage stress effectively may especially help older men make prudent decisions later in life. This point is especially important given that the current generation of older men often perceive stigma associated with mental health treatment and diagnoses (Currin, Hayslip, Schneider, & Kooken, 1998; Ojeda & Bergstresser, 2008). Those who may have experienced or currently experience chronic stressors may be less willing to seek treatment. Interventions such as social integration, continued physical activity and healthy diet through older age may be effective methods to counteract the cumulative effects of stress on cognitive processing (McEwen, 2012). More research is needed to explore this issue, but such steps may positively contribute to not only physical and psychological well-being, but also the maintenance of advantageous decision-making skills.
Limitations
One limitation of this study is the small number of saliva samples that were collected to assess diurnal dynamics of the HPA axis. Previous work has suggested that the collection of multiple samples across several days is ideal in order to increase the reliability of estimates of the integrity of the HPA axis (see Smyth et al., 1997; Evans et al., 2007). Research that has examined the diurnal fall of cortisol across several days, using a similar sampling regimen employed here (Evans et al., 2007, 2011), have shown good consistency across days, however, suggesting that our one-day sampling regimen is a stable estimate of the diurnal dynamics of cortisol. Further, we have previously used a similar sampling regimen in a study including a neurological patient population as well as healthy age-matched comparison participants (Buchanan et al., 2004). In that study, we found high reliability of cortisol dynamics between two testing days (correlation coefficient between days: r = .70).
Future research would also benefit from examining the associations between the cortisol awakening response and risky decision-making. The awakening response has been associated with a number of cognitive and health outcomes in older adults (see Fries, Dettenborn, & Kirschbaum, 2009). Stawski et al. (2011) found that blunted morning rise in cortisol was associated with poorer cognitive function. We instead chose to focus on the diurnal fall in cortisol, which has previously been associated with cognitive performance in an older adult population (Evans et al., 2011); our results extend this association to very specific components of decision making. Finally, we did not use an objective measure to assess compliance with regard to the timing of saliva collection. Previous research has shown that the use of measures such as time stamped sample collection increases the reliability of sample timing (Kudielka, Broderick, & Kirschbaum, 2003). Our results should be interpreted with these limitations in mind.
Last, because this study was correlational in nature, we cannot speak to the causal effects of stress on decision making in this study. Acute stress paradigms provide laboratory-based assessments of the effects of cortisol reactivity on cognitive performance. Because diurnal cortisol fall and responsivity to an acute stressor are separate research issues, an experimental manipulation falls outside the scope of the current inquiry. Researchers have begun to study the effects of stress on decision-making (See Starcke & Brand, 2012 for a review), yet little research has been directed towards examining these effects in a lifespan/developmental context (although see Mather et al., 2012). Future research could expand on these studies to determine the degree to which both HPA dynamics and acute stress reactivity similarly, or differentially, impact decision-making abilities in older adults.
Although the effects of stress on physical and mental health in older adults have been well-documented, less is known about its effects on complex cognitive processes such as decision making. In this study, we found that diurnal cortisol fall was associated with increased risk-taking for decisions involving potential gains. Further, we observed that men with low diurnal cortisol fall showed deficits in the ability to make EV-sensitive judgments. We hope that these results facilitate future research that examines the neuro-endocrinal factors that may predict differences in social and economic decision making in older adults.
Acknowledgments
The authors gratefully acknowledge support from the following sources: National Science Foundation Grant (Weller, PI; SES08-20585); National Institute on Aging Career Development Award (Denburg, PI; K01 AG022033). We also would like to thank Maggie Eliot, Sara Shivapour, C. K. Mertz, and Leisha Wharfield for their assistance with data collection, data management, and manuscript preparation.
Footnotes
Note that we do not equate altered diurnal cortisol with chronic stress. Altered diurnal cortisol, and more specifically the diurnal fall in cortisol, merely serves as a proxy for the dynamics of the HPA axis. These dynamics may be changed by various disease states as well as chronic stress.
Instead of using a 65 + years of age cutoff as is commonly done in aging studies, we included adults 55 years and older. 73.2% of the sample was over 65 years of age, and only 4 participants were younger than 60 years. In a post-hoc analysis, we compared those younger than 65 years to those 65 years and older. The two age groups did not significantly differ in terms of mean cortisol output, AUC, or risk-taking. Thus, we do not discuss this issue further.
We calculated simple odds ratios and relative risk (R.R.) ratios (i.e., probability ratios) to demonstrate the nature of the interaction. For ease of interpretation, we dichotomized the EV variable by clustering all RA trials (= 1) and RD trials (= 0). In this respect, these analyses parallel linear contrast analyses that have been previously reported with the Cups Task using ANOVA and OLS regression methods.
Contributor Information
Joshua A. Weller, Decision Research and Idaho State University
Tony W. Buchanan, Saint Louis University
Crystal Shackleford, University of Oregon.
Arielle Morganstern, University of Oregon.
Joshua J. Hartman, University of Iowa Carver College of Medicine
Jonathan Yuska, University of Iowa Carver College of Medicine.
Natalie L. Denburg, University of Iowa Carver College of Medicine
References
- Abercrombie HC, Giese-Davis J, Sephton S, Epel ES, Turner-Cobb JM, Spiegel D. Flattened cortisol rhythms in metastatic breast cancer patients. Psychoneuroendocrinology. 2004;29:1082–1092. doi: 10.1016/j.psyneuen.2003.11.003. [DOI] [PubMed] [Google Scholar]
- al’Absi M, Hugdahl K, Lovallo WR. Adrenocortical stress responses and altered working memory performance. Psychophysiology. 2002;39:95–99. doi: 10.1017/S0048577202001543. [DOI] [PubMed] [Google Scholar]
- Arkes HR, Blumer C. The psychology of sunk cost. Organizational Behavior and Human Decision Processes. 1985;35:124–140. [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy. Science. 1997;275:1293–1295. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- Blanchard-Fields F, Mienaltowski A, Seay RB. Age differences in everyday problem-solving effectiveness: Older adults select more effective strategies for interpersonal problems. Journal of Gerontology: Psychological Sciences. 2007;62:P61–P64. doi: 10.1093/geronb/62.1.p61. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, Matochik JA, Cadet JL. Sex-related differences in a gambling task and its neurological correlates. Oxford Journals: Cerebral Cortex. 2004;14:1226–1232. doi: 10.1093/cercor/bhh083. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E. Stress and development: Behavioral and biological consequences. Development and Psychopathology. 2001;13:473–489. doi: 10.1017/S0954579401003042. [DOI] [PubMed] [Google Scholar]
- Buchanan TW, Kern S, Allen JS, Tranel D, Kirschbaum C. Circadian regulation of cortisol after hippocampal damage in humans. Biological Psychiatry. 2004;56:651–656. doi: 10.1016/j.biopsych.2004.08.014. [DOI] [PubMed] [Google Scholar]
- Buchanan TW, Tranel D. Stress and emotional retrieval: Effects of sex and cortisol response. Neurobiology, Learning, and Memory. 2008;89:134–141. doi: 10.1016/j.nlm.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan TW, Tranel D, Adolphs R. Impaired memory retrieval correlates with individual differences in cortisol response but not autonomic response. Learning & Memory. 2006;13:382–387. doi: 10.1101/lm.206306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrnes JP, Miller D, Schafer WD. Gender differences in risk taking: A meta-analysis. Psychological Bulletin. 1999;125:367–383. doi: 10.1037/0033-2909.125.3.367. [DOI] [Google Scholar]
- Carstensen LL. Emotion and aging. In: Maddox G, editor. Encyclopedia of aging. 3. New York, NY: Springer; 2001. pp. 327–329. [Google Scholar]
- Charles ST, Mather M, Carstensen LL. Aging and emotional memory: The forgettable nature of negative images for older adults. Journal of Experimental Psychology: General. 2003;132:310–324. doi: 10.1037/0096-3445.132.2.310. [DOI] [PubMed] [Google Scholar]
- Cheng CP, Sheu CF, Yen NS. A mixed-effects expectancy-valence model for the Iowa gambling task. Behavior Research Methods. 2009;41:657–663. doi: 10.3758/BRM.41.3.657. [DOI] [PubMed] [Google Scholar]
- Clow A, Thorn L, Evans P, Hucklebridge F. The awakening cortisol response: Methodological issues and significance. Stress. 2004;7:29–37. doi: 10.1080/10253890410001667205. [DOI] [PubMed] [Google Scholar]
- Cornelius SW, Caspi A. Everyday problem solving in adulthood and old age. Psychology and Aging. 1987;2:144–153. doi: 10.1037/0882-7974.2.2.144. [DOI] [PubMed] [Google Scholar]
- Costa PT, Jr, McCrae RR. Manual for the revised NEO personality inventory (NEO PI-R) and NEO five-factor inventory (NEO-FFI) Odessa, FL: Psychological Assessment Resources; 1992. [Google Scholar]
- Currin JB, Hayslip B, Jr, Schneider L, Kooken RA. Cohort differences in attitudes toward mental health services among older persons. Psychotherapy. 1998;35:506–518. [Google Scholar]
- Deakin J, Aitken M, Robbins T, Sahakian BJ. Risk taking during decision-making in normal volunteers: Changes with age. Journal of the International Neuropsychological Society. 2004;10:590–598. doi: 10.1017/S1355617704104104. [DOI] [PubMed] [Google Scholar]
- De Bellis A, Falorni A, Laureti S, Perinno S, Coronella C, Forini F, Bellastella A. Time course of 21-hydroxylase antibodies and long-term remission of subclinical autoimmune adrenalitis after corticosteroid therapy: Case report. The Journal of Clinical Endocrinology & Metabolism. 2001;86:675–678. doi: 10.1210/jc.86.2.675. [DOI] [PubMed] [Google Scholar]
- De Kloet ER, Rots NY, Cools AR. Brain corticosteroid hormone dialogue: Slow and persistent. Celluar and Molecular Neurobiology. 1996;16:345–356. doi: 10.1007/BF02088100. [DOI] [PubMed] [Google Scholar]
- Denburg NL, Cole CA, Hernandez M, Yamada TH, Tranel D, Bechara A, Wallace RB. The orbitofrontal cortex, real-world decision making, and normal aging. Annals of the New York Academy of Sciences. 2007;1121:480–498. doi: 10.1196/annals.1401.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denburg NL, Recknor EC, Bechara A, Tranel D. Psychophysiological anticipation of positive outcomes promotes advantageous decision-making in normal older persons. International Journal of Psychophysiology. 2006;61:19–25. doi: 10.1016/j.ijpsycho.2005.10.021. [DOI] [PubMed] [Google Scholar]
- Denburg NL, Tranel D, Bechara A. The ability to decide advantageously declines in some normal older persons. Neuropsychologia. 2005;43:1099–1106. doi: 10.1016/j.neuropsychologia.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Denburg NL, Weller JA, Yamada TH, Shivapour DM, Kaup AR, LaLoggia A, Bechara A. Poor decision making among older adults is related to elevated levels of neuroticism. Annals of Behavioral Medicine. 2009;37:164–172. doi: 10.1007/s12160-009-9094-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dias-Ferreira E, Sousa JC, Melo I, Morgado P, Mesquita AR, Cerquiera JJ, Sousa N. Chronic stress causes frontostriatal reorganization and affects decision-making. Science. 2009;325:621–625. doi: 10.1126/science.1171203. [DOI] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. The Journal of Neuroscience. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elzinga BM, Roelofs K. Cortisol-induced impairments of working memory require acute sympathetic activation. Behavioral Neuroscience. 2005;119:98–103. doi: 10.1037/0735-7044.119.1.98. [DOI] [PubMed] [Google Scholar]
- Evans P, Forte D, Jacobs C, Fredhoi C, Aitchison E, Hucklebridge F, Clow A. Cortisol secretory activity in older people in relation to positive and negative well-being. Psychoneuroendocrinology. 2007;32:922–930. doi: 10.1016/j.psyneuen.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Evans P, Fredhoi C, Loveday C, Hucklebridge F, Aitchison E, Forte D, Clow A. The diurnal cortisol cycle and cognitive performance in the healthy old. International Journal of Psychophysiology. 2011;79:371–377. doi: 10.1016/j.ijpsycho.2010.12.006. [DOI] [PubMed] [Google Scholar]
- Fein G, McGillivray S, Finn P. Older adults make less advantageous decisions than younger adults: Cognitive and psychological correlates. Journal of the International Neuropsychological Society. 2007;13:480–489. doi: 10.1017/S135561770707052X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. Mini-mental state: A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Fries E, Dettenborn L, Kirschbaum C. The cortisol awakening response (CAR): Facts and future directions. International Journal of Psychophysiology. 2009;72:67–73. doi: 10.1016/j.ijpsycho.2008.03.014. [DOI] [PubMed] [Google Scholar]
- Granger DA, Kivlighan KT, Fortunato C, Harmon AG, Hibel LC, Schwartz EB, Whembolua GL. Integration of salivary biomarkers into developmental and behaviorally-oriented research: problems and solutions for collecting specimens. Physiol Behav. 2007;92(4):583–590. doi: 10.1016/j.physbeh.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. American Journal of Psychiatry. 2001;158:575–581. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- Heim C, Owens MJ, Plotsky PM, Nemeroff CB. Persistent changes in corticotropin-releasing factor systems due to early life stress: Relationship to the pathophysiology of major depression and post-traumatic stress disorder. Psychopharmacology Bulletin. 1997;33(4):185–192. [PubMed] [Google Scholar]
- Henninger DE, Madden DJ, Huettel SA. Processing speed and memory mediate age-related differences in decision making. Psychology and Aging. 2010;25:262–270. doi: 10.1037/a0019096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: Hypothalamo-pituitary-adrenocortical axis. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2005;29:1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- Kahneman D, Tversky A. Prospect theory: An analysis of decision under risk. Econometrica. 1979;47:263–291. [Google Scholar]
- Karlamangla AS, Friedman EM, Seeman TE, Stawksi RS, Almeida DM. Daytime trajectories of cortisol: Demographic and socioeconomic differences—Findings from the National Study of Daily Experiences. Psychoneuroendocrinology. 2013 doi: 10.1016/j.psyneuen.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy Q, Mather M, Carstensen LL. The role of motivation in the age-related positivity effect in autobiographical memory. Psychological Science. 2004;15:208–214. doi: 10.1111/j.0956-7976.2004.01503011.x. [DOI] [PubMed] [Google Scholar]
- Kovalchik S, Camerer CF, Grether DM, Plott CR, Allman JM. Aging and decision making: A comparison between neurologically healthy elderly and young individuals. Journal of Economic Behavior and Organization. 2005;58:79–94. doi: 10.1016/j.jebo.2003.12.001. [DOI] [Google Scholar]
- Kudielka BM, Broderick JE, Kirschbaum C. Compliance with saliva sampling protocols: electronic monitoring reveals invalid cortisol daytime profiles in noncompliant subjects. Psychosom Med. 2003;65(2):313–319. doi: 10.1097/01.psy.0000058374.50240.bf. [DOI] [PubMed] [Google Scholar]
- Lauriola M, Levin IP. Relating individual differences in attitude toward ambiguity to risky choices. Journal of Behavioral Decision Making. 2001;14:107–122. doi: 10.1002/bdm.368. [DOI] [Google Scholar]
- Lejuez CW, Aklin WM, Zvolensky MJ, Pedulla CM. Evaluation of the Balloon Analogue Risk Task (BART) as a predictor of adolescent real-world risk-taking behaviours. Journal of Adolescence. 2003;26:475–479. doi: 10.1016/S0140-1971(03)00036-8. [DOI] [PubMed] [Google Scholar]
- Levin IP, Gaeth GJ, Schreiber J, Lauriola M. A new look at framing effects: Distribution of effect sizes, individual differences, and independence of types of effects. Organizational Behavior and Human Decision Processes. 2002;88:411–429. [Google Scholar]
- Levin IP, Xue G, Weller JA, Reimann M, Lauriola M, Bechara A. A neuropsychological approach to understanding risk-taking for potential gains and losses. Frontiers in Neuroscience. 2012;6(15) doi: 10.3389/fnins.2012.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Bigler ED, Tranel D. Neuropsychological Assessment. 5. New York: Oxford University Press; 2012. [Google Scholar]
- Li Y, Baldassi M, Johnson EJ, Weber E. Complementary cognitive abilities, economic decision making, and aging. Psychology and Aging. 2013;28:595–613. doi: 10.1037/a0034172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang K, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- Lighthall NR, Mather M, Gorlick MA. Acute stress increases sex differences in risk seeking in the balloon analogue risk task. PLoS ONE. 2009;4(7):e6002. doi: 10.1371/journal.pone.0006002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, McEwen BS. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. Journal of Neuroscience. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, Lecours AR, Lussier I, Schwartz G, Nair NP, Meaney MJ. Basal cortisol levels and cognitive deficits in human aging. The Journal of Neuroscience. 1994;14:2893–2903. doi: 10.1523/JNEUROSCI.14-05-02893.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, de Leon M, de Santi S, Convit A, Tarshish C, Nair NPV, Meaney MJ. Cortisol levels during human aging predict hippocampal atrophy and memory deficits. Nature Neuroscience. 1998;1:69–73. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C. Effects of stress throughout the lifespan on the brain, behavior and cognition. Nature Reviews Neuroscience. 2009;10:434–445. doi: 10.1038/nrn2639. [DOI] [PubMed] [Google Scholar]
- MacPherson SE, Phillips LH, Della Sala S. Age, executive function, and social decision making: A dorsolateral prefrontal theory of cognitive aging. Psychology and Aging. 2002;17:598–609. doi: 10.1037/0882-7974.17.4.598. [DOI] [PubMed] [Google Scholar]
- Mather M, Carstensen LL. Aging and attentional biases for emotional faces. Psychological Science. 2003;14:409–415. doi: 10.1111/1467-9280.01455. [DOI] [PubMed] [Google Scholar]
- Mather M, Knight M. Goal-directed memory: The role of cognitive control in older adults’ emotional memory. Psychology and Aging. 2005;20:554–570. doi: 10.1037/0882-7974.20.4.554. [DOI] [PubMed] [Google Scholar]
- Mather M, Lighthall NR. Risk and reward are processed differently in decisions made under stress. Current Directions in Psychological Science. 2012;21:36–41. doi: 10.1177/0963721411429452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Mazar N, Gorlick MA, Lighthall NR, Burgeno J, Schoeke A, Ariely D. Risk preferences and aging: The “certainty effect” in older adults’ decision making. Psychology and Aging. 2012;27:801–816. doi: 10.1037/a0030174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. The neurobiology of stress: From serendipity to clinical relevance. Brain Research. 2000;886:172–189. doi: 10.1016/s0006-8993(00)02950-4. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Brain on stress: How the social environment gets under the skin. PNAS Proceedings of the National Academy of Sciences of the United States of America. 2012;109:17180–17185. doi: 10.1073/pnas.1121254109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Sapolsky RM. Stress and cognitive function. Current Opinion in Neurobiology. 1995;5:205–216. doi: 10.1016/0959-4388(95)80028-x. [DOI] [PubMed] [Google Scholar]
- Miller GE, Chen E, Zhou ES. If it goes up, must it come down? Chronic stress and the hypothalamic-pituitary-adrenocortical axis in humans. Psychological Bulletin. 2007;133:25–45. doi: 10.1037/0033/2909.133.1.25. [DOI] [PubMed] [Google Scholar]
- Mohr PNC, Biele G, Heekeren HR. Neural processing of risk. The Journal of Neuroscience. 2010;30:6613–6619. doi: 10.1523/JNEUROSCI.0003-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeda VD, Bergstresser SM. Gender, race-ethnicity, and psychosocial barriers to mental health care: An examination of perceptions and attitudes among adults reporting unmet need. Journal of Health and Social Behavior. 2008;49:317–334. doi: 10.1177/002214650804900306. [DOI] [PubMed] [Google Scholar]
- Okun M. Adult age and cautiousness in decision: A review of the literature. Human Development. 1976;19:220–233. doi: 10.1159/000271530. [DOI] [PubMed] [Google Scholar]
- Overman W, Graham L, Redmond A, Eubank R, Boettcher L, Samplawski O, Walsh K. Contemplation of moral dilemmas eliminates sex differences on the Iowa Gambling Task. Behavioral Neuroscience. 2006;120:817–825. doi: 10.1037/0735-7044.120.4.817. [DOI] [PubMed] [Google Scholar]
- Peters E, Dieckmann NF, Weller JA. Age differences in complex decision making. In: Schaie KW, Willis SL, editors. Handbook of the psychology of aging. 7. Burlington, MA: Academic Press; 2011. pp. 133–151. [Google Scholar]
- Plessow F, Kiesel A, Kirschbaum C. The stressed prefrontal cortex and goal-directed behavior: acute psychosocial stress impairs the flexible implementation of task goals. Experimental Brain Research. 2012;216:397–408. doi: 10.1007/s00221-011-2943-1. [DOI] [PubMed] [Google Scholar]
- Porcelli AJ, Delgado MR. Acute stress modulates risk taking in financial decision making. Psychological Science. 2009;20:278–283. doi: 10.1111/j.1467-9280.2009.02288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power D, Li L, Hertzman C. Cognitive development and cortisol patterns in mid-life: Findings from a British birth cohort. Psychoneuroendocrinology. 2008;33:530–539. doi: 10.1016/j.psyneuen.2008.01.017. [DOI] [PubMed] [Google Scholar]
- Preston SD, Buchanan TW, Stansfield RB, Bechara A. Effects of anticipatory stress on decision making in a gambling task. Behavioral Neuroscience. 2007;121:257–263. doi: 10.1037/0735-7044.121.2.257. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Wolf OT, Hellhammer DH, Buske-Kirschbaum A, von Auer K, Jobst S, Kirschbaum C. Free cortisol levels after awakening: A reliable biological marker for the assessment of adrenocortical activity. Life Sciences. 1997;61:2539–2549. doi: 10.1016/s0024-3205(97)01008-4. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Putnam P, Antypa N, Crysovergi P, van der Does WAJ. Exogenous cortisol acutely influences motivated decision making in healthy young men. Psychopharmacology. 2010;208:257–263. doi: 10.1007/s00213-009-1725-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reavis R, Overman WH. Adult sex differences on a decision-making task previously shown to depend on the orbital prefrontal cortex. Behavioral Neuroscience. 2001;115:196–206. doi: 10.1037/0735-7044.115.1.196. [DOI] [PubMed] [Google Scholar]
- Reed A, Carstensen L. The theory behind the age-related positivity effect. Frontiers in Psychology. 2012;3:1–9. doi: 10.3389/fpsyg.2012.00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rey A. L’examen psychologique dans les cas d’encephalopathie traumatique. Archives de Psychologie. 1941;28:286–340. [Google Scholar]
- Rey A. L’examen Clinique en Psychologie. Paris, France: Presses Universitaires de France; 1964. [Google Scholar]
- Rohleder N, Joksimovic L, Wolf JM, Kirschbaum C. Hypocortisolism and increased glucocorticoid sensitivity of pro-inflammatory cytokine production in Bosnian war refugees with posttraumatic stress disorder. Biological Psychiatry. 2004;55:745–751. doi: 10.1016/j.biopsych.2003.11.018. [DOI] [PubMed] [Google Scholar]
- Salthouse T. Major issues in cognitive aging. New York, NY: Oxford University Press; 2010. [Google Scholar]
- Sapolsky RM. Why stress is bad for your brain. Science. 1996;273:749–750. doi: 10.1126/science.273.5276.749. [DOI] [PubMed] [Google Scholar]
- Smyth JM, Ockenfels MC, Gorin AA, Catley D, Porter LS, Kirschbaum C, Stone AA. Individual differences in the diurnal cycle of cortisol. Psychoneuroendocrinology. 1997;22:89–105. doi: 10.1016/s0306-4530(96)00039-x. [DOI] [PubMed] [Google Scholar]
- Soto C, John OP, Gosling SD, Porter J. Age differences in personality traits from 10 to 65: Big Five domains and facets in a large cross-sectional sample. Journal of Personality and Social Psychology. 2011;100:330–348. doi: 10.1037/a0021717. [DOI] [PubMed] [Google Scholar]
- Starcke K, Brand M. Decision making under stress: A selective review. Neuroscience and Biobehavioral Reviews. 2012;36:1228–1248. doi: 10.1016/j.neubiorev.2012.02.003. [DOI] [PubMed] [Google Scholar]
- Stawski RS, Almeida DM, Lachman ME, Tun PA, Rosnick CB, Seeman T. Associations between cognitive function and naturally occurring daily cortisol during middle adulthood: Timing is everything. Journal of Gerontology: Psychological Sciences. 2011;66B:i71–i81. doi: 10.1093/geronb/gbq094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan RM, Gratton A. Prefrontal cortical regulation of hypothalamic-pituitary-adrenal function in the rat and implications for psychopathology: Side matters. Psychoneuroendocrinology. 2002;27:99–114. doi: 10.1016/s0306-4530(01)00038-5. [DOI] [PubMed] [Google Scholar]
- Teicher MH, Andersen SL, Polcari A, Anderson CM, Navalta CP. Developmental neurobiology of childhood stress and trauma. Psychiatric Clinics of North America. 2002;25:397–426. doi: 10.1016/s0193-953x(01)00003-x. [DOI] [PubMed] [Google Scholar]
- Tranel D, Benton A, Olson K. A 10-year longitudinal study of cognitive changes in elderly persons. Developmental Neuropsychology. 1997;13:87–96. [Google Scholar]
- van den Bos R, Harteveld M, Stoop H. Stress and decision-making in humans: Performance is related to cortisol reactivity, albeit differently in men and women. Psychoneuroendocrinology. 2009;34:1449–1458. doi: 10.1016/j.psyneuen.2009.04.016. [DOI] [PubMed] [Google Scholar]
- Waldstein SR, Wendell CR. Neurocognitive function and cardiovascular disease. Journal of Alzheimer’s Disease. 2010;20:833–842. doi: 10.3233/JAD-2010-091591. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler adult intelligence scale. 3. New York: Psychological Corporation; 1997. [Google Scholar]
- Wechsler DA. Wechsler abbreviated scale of intelligence. New York, NY: Psychological Corporation; 1999. [Google Scholar]
- Weller JA, Levin IP, Bechara A. Do individual differences in Iowa Gambling Task performance predict adaptive decision making for risky gains and losses? Journal of Clinical and Experimental Neuropsychology. 2010;32:141–150. doi: 10.1080/13803390902881926. [DOI] [PubMed] [Google Scholar]
- Weller JA, Levin IP, Denburg NL. Trajectory of risky decision making for potential gains and losses from ages 5 to 85. Journal of Behavioral Decision Making. 2011;24:331–344. doi: 10.1002/bdm.690m. [DOI] [Google Scholar]
- Weller JA, Levin IP, Shiv B, Bechara A. Neural correlates of adaptive decision making for risky gains and losses. Psychological Science. 2007;18:958–964. doi: 10.1111/j.1467-9280.2007.02009.x. [DOI] [PubMed] [Google Scholar]
- Wilkinson GS. Wide Range Achievement Test: Revision 3. Wilmington, DE: Jastak; 1993. [Google Scholar]
- Williams PG, Suchy Y, Kraybill ML. Five-Factor Model personality traits and executive functioning among older adults. Journal of Research in Personality. 2010;44:485–491. [Google Scholar]
- Wood S, Busemeyer JR, Koling A, Cox CR, Davis H. Older adults as adaptive decision makers: Evidence from the Iowa gambling task. Psychology and Aging. 2005;20:220–225. doi: 10.1037/0882-7974.20.2.220. [DOI] [PubMed] [Google Scholar]
- Yechiam E, Hochman G. Losses as modulators of attention: Review and analysis of the unique effects of losses over gains. Psychological Bulletin. 2013;139:497–518. doi: 10.1037/a0029383. [DOI] [PubMed] [Google Scholar]
- Yechiam E, Telpaz A. Losses induce consistency in risk taking even without loss aversion. Journal of Behavioral Decision Making. 2013;26:31–40. doi: 10.1002/bdm.758. [DOI] [Google Scholar]
- Zamarian L, Sinz H, Bonatti E, Gamboz N, Delazer M. Normal aging affects decisions under ambiguity, but not decisions under risk. Neuropsychology. 2008;22:645–657. doi: 10.1037/0894-4105.22.5.645. [DOI] [PubMed] [Google Scholar]