Abstract
Consumers of marijuana typically feel a strong compulsive desire to consume food. Although past research revealed that the CB1 cannabinoid receptor is a potent regulator of food intake, the functional presence of neuronal CB2 cannabinoid receptors in the brain had been controversial. The role of CB2 receptors in food and alcohol consumption and the behavioral effects of CB2 receptor ligands are not well characterized. This is because CB2 cannabinoid receptors were thought to be absent from the brain and expressed primarily in immune cells and in the periphery. We tested the effects of peripheral injections of CB2 antagonist AM 630, CB2 agonist PEA and CB1 antagonist AM 251 on male C57BL/6, Balb/c, and DBA/2 mice at the beginning of the night cycle and after overnight 12-hour fasts. We also investigated the effects of the putative CB2 agonist, JWH015, and CB2 antagonist SR144528 in mouse motor function tests and in the two compartment black and white box. Under standard conditions, the CB2 antagonist AM 630 inhibited food consumption in C57BL/6 mice and DBA/2 mice, but failed to block food intake of Balb/c mice. The CB2 agonist PEA had no significant effect on food consumption in Balb/c mice, and reduced food intake in C57BL/6 and DBA mice. The CB1 antagonist AM 251 inhibited food ingestion in the three mouse strains at variable times. After 12-hour food deprivation, the CB2 antagonist AM 630 increased food consumption in C57Bl/6 mice, but failed to produce significant changes in food intake for Balb/c and DBA/2 mice. The CB2 agonist PEA also reduced food consumption in all three mice strains at variable times. In comparison to the CB2 ligands, CB1 antagonist AM 251 inhibited food ingestion in the mouse strains. A general pattern of depression in locomotor activity was induced by JWH 015 in both males and females in the three mouse strains tested as the dose was increased. The development and enhancement of alcohol preference was observed following chronic treatment with CB2 agonist JWH 015 in stressed mice but not in controls. Using the DBA/2 strain the spontaneous locomotor activity and stereotype behavior was enhanced by acute administration of low doses of SR144528. There was a reduction in CNR2 gene expression in the ventral mid-brain region of mice that developed alcohol preference but not in those that did not develop alcohol preference. These effects of CB2 cannabinoid receptor ligands in in vivo behavioral tests are provided as functional evidence that CB2-Rs in the brain plays a role in food and alcohol consumption and in the modification of mouse behavior.
Keywords: Food intake, alcohol consumption, cannabinoids, CB1 and CB2 cannabinoid receptors, CB2-R gene expression, locomotor activity, stereotypy
Introduction
Users of marijuana and cannabinoid (CB) related substances often experience a compulsive craving for food. The appetite-stimulating effects of cannabinoids, CBs, have been known for centuries.1 Thus, cannabinoid agonists increase in appetite in various animal models and in humans is a basis for the clinical utility of CBs in cachexia in HIV-AIDS and in cancer chemotherapy. Interestingly, the CB1-R inverse agonist, like accomplia is used to treat obesity and metabolic syndrome.2 Currently, there are two well characterized CB-Rs with known splice variants: CB1-Rs and CB2-Rs.3 CB1-Rs are known to be expressed in the brain and in peripheral tissues. Although CB2-Rs was once believed to be restricted to immune cells, we and others have demonstrated that it is also functionally expressed in neurons albeit at lower levels than CB1-Rs.3, 4 While there have been extensive studies on CB1-Rs that support its role in regulating food intake, studies on CB2-Rs have not been conclusive. Some investigators found no significant changes in food intake within a 1 hour time span after administration of CB2 antagonist SR144528 in male ICR mice deprived of food for 24 hours.5 However, others noted a significant increase in food ingestion by male Lewis rats that received intracerebroventricular injections of CB2 antagonist AM 630 at 4 hours and 6 hours following a 12-hr fast.6 These findings while interesting did not systematically investigate the potential role of CB2-Rs in the pharmacological effects of cannabinoids on food intake and in appetite control and behavior.
In present study, we investigated the effects of the CB2 antagonist AM 630 and the putative CB2 agonist palmitoylethanolamide (PEA) in three mouse strains C57BL/6, Balb/c, and DBA/2 mice. By examining different mouse strains simultaneously, the consequences of genetic variation on the cannabinoid system can be determined. To confirm mouse strain differences, baseline experiments were performed to detect the basal food intake of the three mouse strains in natural settings and after 12-hr fasts. Following the baseline analysis, we examined the effects of AM 630 and PEA on the mouse strains at the start of the night cycle within a 24-hr time span, and after 12 hours of food deprivation. We compared the effects of CB2 ligands on food consumption to the effects of the CB1-R inverse agonist AM 251. In view of our finding of the functional expression of neuronal CB2-Rs in the brain, we also examined the possible role of CNS CNR2 gene in alcohol preference and consumption in mice.
Materials and Methods
Subjects
Male C57BL/6, Balb/c, and DBA/2 mouse strains were housed in individual cages with access to mouse chow 12 hr in the light and 12 hr in the dark. These mice were used in control and treatment groups for baseline analysis and food consumption experiments at the beginning of the night cycle and following 12 hours of food deprivation. The animal care for this experimental protocol was in accordance with the NIH guidelines for the Care and Use of Laboratory Animals and the principles presented in the Guidelines for the Use of Animals in Neuroscience Research by the Society for Neuroscience.
Baseline Analysis of food intake
Ten of each strain of C57BL/6, Balb/c, and DBA/2 mice were kept in individual cages with access to mouse chow and water ad libitum. For nine days, the mouse chow weight was recorded near the start of the night cycle 18:50 hr. Weighed mouse chow was added whenever the food supply was low. After two weeks, the mice were subjected to four consecutive 12-hour food deprivation trials with one day of rest between each trial. In each 12-hr fast trial, all mouse chow was removed at around the beginning of the night cycle 18:50 hr. At the start of the light cycle 7:50 hr, all mice were transported individually to a nearby lit room to receive a measured amount of mouse chow. Mouse food consumption was measured at 0.5, 1, 2, 4, 6, 12, and 24 hr after the start of the light cycle.
Normal food consumption tests
The weights of the mice were recorded prior to the beginning of the experiment. At the beginning of the night cycle, all mice were transported individually to the lit room to receive a measured amount of mouse chow. Five mice of each strain were injected with either AM 630 (10 mg/kg), PEA (10 mg/kg), or AM 251 (3 mg/kg). Following peripheral drug administration and addition of mouse chow, each mouse was transported back to the dark room. Mouse food consumption was measured at 0.5, 1, 2, 4, 12, and 24 hr after the start of the night cycle. After each trial, the injected mice were euthanized and replaced with new mice. These new mice became the control groups, while the previous control groups received injections in the subsequent trial.
12-hour food deprivation tests
Mice body weights were recorded prior to the beginning of the experiment. At the start of the night cycle 18:50 hr, all mouse chow was removed. The mice were deprived of food for about 12 hours, or until the start of the light cycle 7:50 hr. After the 12 hr fast, all mice were transported individually to the lit room to receive a measured amount of mouse chow. Five mice of each strain were injected with either AM 630 (10 mg/kg), PEA (10 mg/kg), or AM 251 (3 mg/kg). Following the peripheral drug administration and addition of mouse chow, each mouse was transported back to the holding room. Mouse food consumption was measured at 0.5, 1, 2, 4, 6, 12, and 24 hr after the start of the light cycle. After each trial, the injected mice were euthanized.
Subjects subjected to chronic mild stress
A total 40 BALB/c male mice housed 12 hr/12 hr in light/dark, age 24-28 weeks and were 25-30 g body weight. Chronic mild stress (CMS) was the stress paradigm used.7 These experimental animals were subjected to the weekly CMS regime consisting of three 10 hr periods of 45° cage tilt; 3 periods of overnight stroboscopic illumination, two 10 hr periods of empty water bottle; two periods of overnight food or water deprivation; two 10 hr periods of damp bedding. Anhedonia and weight changes were measured weekly. The CMS treated and non-stressed groups consisted of 20 mice each and were split into two subgroups, respectively. The first group was subjected to chronic daily administration of 20 mg/kg CB2-R agonist JWH015 (n= 10 each). The second group was subjected to saline injections (n = 10 each). All non-stressed groups were given food and water at all times, as well as comfortable cage surroundings, while the experimental group was housed in a different room. After 4 weeks of treatment in each group, ethanol consumption during 15 hr nighttime of mice subjected to chronic mild stress (CMS) and the effect of chronic daily administration with CB2-R agonist and antagonist on ethanol consumption in CMS and control animals were measured.
CNR2 expression and regulation by alcohol treatment
C57BL/6 male mice were housed 12 hr/12 hr in light/dark, age 8-10 weeks and 20-25 g body weight. A group of mice (n = 5), as the acute alcohol treatment model, were injected with 10 ml/kg of 20 % v/v ethanol (in saline) s.c., sacrificed 4 hours later after the injection, and brains were taken out for dissection to extract RNA. Another group of mice were housed in a cage separately and had access to water and ethanol during the night (n = 9) daily, and they had access to water during the rest of the period. Alcohol concentration was increased from 2 to 4, 8, 16, and 32 % every three days period. We measured water and ethanol consumption daily. 1 hour after the time of last access to ethanol bottle, mice were sacrificed for RNA extraction from brain dissections. Control group of mice (n = 5) did not have access to ethanol but only to water at this time of the experiment, and RNA was also extracted in the same way for comparison to the mice that developed ethanol preference. Brains were dissected into five regions, prefrontal cortex, striatum, thalamus, hippocampus, and ventral midbrain. RNA was extracted using RNeasy kit and cDNA was synthesized by Revertra Ace and oligo dT primer. The expression of CNR2 gene was compared by TaqMan real-time PCR with an ABI PRISM 7900 HT Sequence Detection System (Applied Biosystems, Foster City, CA, USA), using the TaqMan gene expression assay for Cnr2 (Mm0043826_m1).
Behavioral effects of CB2-R ligands
In the elevated plus-maze test, the behavioral effects in mice following intracerebroventricular (ICV) microinjection of CB2 antisense oligos, 20 μg twice daily for 3 d, and the time spent in the open arms of the plus-maze was compared to control mice injected with sense and mismatch oligos. The behavioral effects in naïve and prenatally exposed mice to capsaicin were analyzed and compared to a separate group of these animals challenged by the mixed CB1/CB2 agonist WIN55212-2. The spontaneous locomotor activity of male and female mouse strains placed in individual activity monitors after treatment with the CB2 cannabinoid agonists or antagonists were recorded. The pretreatment time for the agonists was 10 mins and 30 mins for the antagonist. The locomotor activities and stereotype behavior were recorded over a 20 min period following the intra-peritoneal (ip) administration. The behavioral effects of CB2 cannabinoid receptor agonist, JWH015 or the antagonists, SR144528 were evaluated in the two- compartment black and white test box in selected mouse strains. In this automated two chambered black and white test box, with an interconnecting opening, the locomotor activities and time spent in both chambers were also recorded.
Results
Influence of CB2-R ligands on food intake
We compared food consumption in mice following administration of CB2 agonist and antagonist to that of a known CB1 antagonist AM 251. Baseline food consumption varied according to mouse strains used and whether food was available or during deprivation. When food was available ad libitum, the suppressant effect of CB2 ligands used were strain and time dependent. This effect was similar to that of CB1 receptor antagonist AM 251. However, during a 12-hr food deprivation, AM 630 and PEA increased and suppressed food consumption respectively in a strain and time dependent manner, whereas AM 251 suppressed food intake in all strains used in a time dependent fashion (figure 1). CNR2 gene expression was down regulated in the striatum of mice that developed ethanol preference and acute injection of ethanol down regulated CNR2 gene expression in the ventral midbrain region of mice (figure 2). CNR2 gene down regulation in ventral midbrain did not occur in non-alcohol preferring mice, under the same condition of access to alcohol drinking. Stress moderately increased alcohol consumption in mice, while CB2-R agonist did not. However, CB2-R agonist JWH 015 enhanced alcohol consumption whereas CB2-R antagonist AM 630 reduced alcohol consumption in stressed mice respectively as we previously reported.8
Figure 1. Effect of CB2-R ligands and AM 251 on mouse food intake.
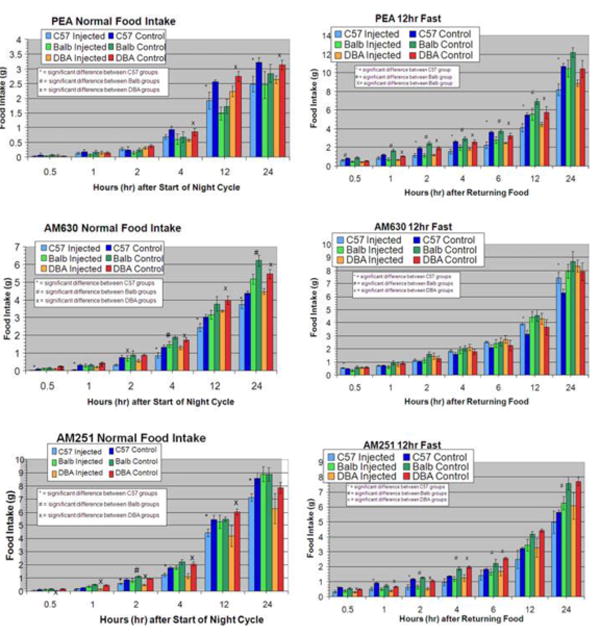
Food intake was evaluated in 3 mouse strains, C57BL/6, DBA/2 and in Balb/c mice following the administration of PEA (10 mg/kg), AM 630 (10 mg/kg) in comparison with AM 251 (3 mg/kg). Normal food intake and food consumption following 12-hr food deprivation was evaluated over a 24-hr period after drug or vehicle administration. Significant differences in food intake are indicated (p<0.05).
Fig 2. Development of alcohol preference and CNR2 expression.
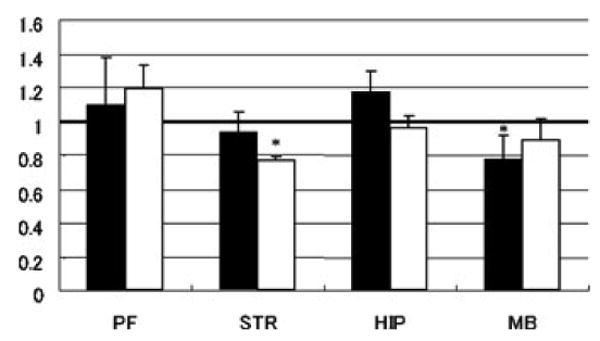
Groups of mice in the acute alcohol injection and those in the alcohol preference paradigm and the control group that had access to water were used. Mice were sacrificed at the end of the session and the brains were quickly dissected to extract RNA for CNR2 gene expression by real-time PCR system. The Y-axis indicates relative expression levels of CNR2 gene, compared with the control mice (n = 5) that had no access to ethanol. CNR2 gene expression was regulated in two regions of brain showing ethanol effects in mice. Black bars indicate regulation of gene expression following acute ethanol injection. White bars indicate regulation of gene expression in developing ethanol preference in mice. PF: prefrontal cortex, STR: striatum, HIP: hippocampus, MB: ventral midbrain. * in the figure indicates significant CNR2 down regulation from control subjects: p = 0.01.
Modification of mouse behavior by CB2 ligands
The data and figures showing mouse behavioral modification in the motor function and emotionality tests is presented elsewhere in this monograph. Briefly, acute treatment with the putative CB2 agonist JWH015 (1-20 mg/kg) altered mouse spontaneous locomotor activities in a strain and gender dependent fashion with a general pattern of decrease in activity as the dose was increased. However, lower doses of acute administration of CB2 antagonist SR144528 enhanced spontaneous activity and stereotype behavior dependent on gender and mouse strain used. An inconsistent profile of acute treatment precipitating an anxiogenic response with increasing doses of JWH015 while chronic treatment induced an anxiolytic profile of response in control but not in mice subjected to chronic mild stress for 4 weeks, was recorded.
Discussion
Our major finding is that CB2-R ligands in-vivo modified food and alcohol consumption and induced changes in mouse behavioral tests of motor function and emotionality. These effects of CB2-R ligands are provided as additional functional evidence for the presence of CB2 cannabinoid receptors in the brain.9 We compared food consumption in mice following administration of CB2 agonist and antagonist to that of a known CB1 inverse agonist AM 251 in previous reports.2, 10, 11 We report, 1). That baseline food consumption varied according to mouse strains used and whether food was available ad libitum or during deprivation. 2). When food was available ad libitum, the suppressant effect of the CB2 ligands used were strain and time dependent. This effect was similar to that of AM251, the CB1 receptor inverse agonist. 3). However, during a 12-hr food deprivation, AM630 and PEA increased and decreased food intake respectively, in a strain and time dependent fashion. 4). The behavioral effects of the CB2 ligands in the motor function and emotionality tests were not dependent on the mouse strain and gender, but was also dependent on acute or chronic administration of the CB2- ligands. 5). CNR2 gene was down regulated in the striatum of mice that developed alcohol preference and acute injection of alcohol down regulated CNR2 gene expression in the ventral midbrain region of mice as previously reported.8 The significant differences in food intake between C57BL/6 mice and Balb/c mice, as well as between Balb/c mice and DBA/2 mice were not surprising. Our previous data indicated differential sensitivities to cannabinoid induced behaviors may be associated with the different levels of CB-R gene expression in these mouse strains and depending on gender and ethnic background in the human population.12, 13, 14
The CB2 antagonist AM630 inhibited food intake in C57BL/6 mice and DBA/2 mice throughout the normal food consumption trials, but did not suppress food intake consistently in Balb/c mice. Overall, the inverse agonistic effect of AM630 induced an inhibitory effect resulting in a decrease in food consumption. However, the CB2-R ligands had unpredictable effects on the food intake of mice deprived of food for 12 hours. Thus, AM630 stimulated food ingestion in a time and strain dependent manner, similar to reports of increased food consumption following the intracerebroventricular injections of AM 630 in rats.6 On the other hand the putative CB2-R agonist PEA suppressed food consumption in general for all three mice strains. In addition, one would expect PEA to increase appetite if PEA acted as a CB2-R agonist for controlling food consumption behavior. These results are surprising but PEA has been noted to have a weak affinity for CB2-Rs, 15 which may explain the weak influence on food intake by PEA as well as the amount of time to observe significant changes in food consumption. Some studies have suggested that PEA also acts on a non-CB1/CB2 cannabinoid receptor.16,17 The differences between strains may imply genetic variability of CNR2 gene mentioned above. However, the mixed results of CB2-R activation and inhibition on food intake are difficult to interpret. But unlike CB2-R ligands used in this study, the CB1 inverse agonist AM 251 consistently reduced food intake in mouse strain specific and time dependent manner with the C57BL/6 and DBA/2 more sensitive than the Balb/c mice. Nevertheless, AM251 suppressed food intake of C57BL/6 mice and DBA/2 mice in this study is in agreement with data from previous studies using different feeding paradigms.18 These results suggest that the activity of the CB1-R ligands do not change following a significant period of food deprivation. Thus, the CB1 ligands may primarily function in controlling behaviors such as hunger, and demonstrate a strong potential for regulating appetite in various physiological conditions. The interaction between CB1 and CB2 receptors in feeding is speculative, but one possible explanation from our results may be that CB2-Rs and CB1-Rs work independently and/or cooperatively to regulate food intake. The CB1-Rs probably control food intake by directly affecting the desire for food. The CB2-Rs may likely act on the immune system, which indirectly alters appetite through changes in the activity of the digestive system.
We and others have now shown the functional expression of neuronal CB2-Rs, 3,4,8,9 which traditionally have been associated primarily with immune function.17 It is therefore tempting to speculate that activation or inhibition of immune function may be implicated in the effects of CB2-R ligands on appetite as marijuana use or treatment with synthetic cannabinoids are know to enhance appetite in HIV-AIDS patients.19 However, the involvement of CB2-Rs in food consumption in conditions of anorexia and in HIV-AIDS and in other conditions where the immune system is compromised requires further investigation. Although our experimental design appeared adequate based on the baseline results, nevertheless, the mechanisms associated with the effects of CB2-R ligands remains to be determined. By considering the mechanisms of CB2-R involvement in appetite regulation and investigating simultaneously other aspects that may regulate food consumption, such as the immune system and CB1-R activity, the physiological role of neuronal CB2-Rs beyond immunocannabinoid activity can be determined.
Acknowledgments
ESO acknowledges financial support from William Paterson University center for research, the Dean's Student Worker Funds and the Provost office for release time for research. We are indebted to Biology department Chair, Dr. Eileen Gardner for animals and maintenance supplies of the animals used in our research.
Abbreviations
- CBs
Cannabinoids
- CB2-Rs
cannabinoid receptors 2
- CB1-Rs
cannabinoid receptors 1
- eCBs
endocannabinoids
- PEA
palmitoylethanolamide
- CMS
chronic mild stress
References
- 1.Abel EL. Cannabis: effects on hunger and thirst. Behav Biol. 1975;15:255–281. doi: 10.1016/s0091-6773(75)91684-3. [DOI] [PubMed] [Google Scholar]
- 2.Salamone JD, Mclaughin PJ, et al. Cannabinoid CB1 receptor inverse agonists and neutral antagonists: Effects on food intake, food-reinforced behavior and food aversions. Physiol Behav. 2007;91:383–388. doi: 10.1016/j.physbeh.2007.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Onaivi ES, Ishiguro H, et al. Discovery of the presence and functional expression of cannabinoid CB2 receptors in brain. Ann N Y Acad Sci. 2006;1074:514–536. doi: 10.1196/annals.1369.052. [DOI] [PubMed] [Google Scholar]
- 4.Van Sickle MD, Duncan M, et al. Identification and functional characterization of brainstem cannabinoid CB2 receptors. Science. 2005;310:329–332. doi: 10.1126/science.1115740. [DOI] [PubMed] [Google Scholar]
- 5.Wiley JL, Burston JJ, et al. CB1 cannabinoid receptor-mediated modulation of food intake in mice. Brit J Pharmacol. 2005;145:293–300. doi: 10.1038/sj.bjp.0706157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Werner NA, Koch JE. Effects of the cannabinoid antagonists AM281 and AM630 on deprivation-induced intake in Lewis rats. Brain Research. 2003;967:290–292. doi: 10.1016/s0006-8993(02)04274-9. [DOI] [PubMed] [Google Scholar]
- 7.Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- 8.Ishiguro H, Iwasaki S, et al. Involvement of cannabinoid CB2 receptor in alcohol preference in mice and alcoholism in humans. The Pharmacogenomics J. 2007;7:380–385. doi: 10.1038/sj.tpj.6500431. [DOI] [PubMed] [Google Scholar]
- 9.Onaivi ES, Ishiguro H, et al. Methods to study the behavioral effects and expression of CB2 cannabinoid receptors and its gene transcripts in chronic mild stress model of depression. In: Onaivi EmmanuelS., editor. Marijuana and cannabinoid research: Methods and Protocols. Humana press Inc; Totowa, NJ: 2006. [DOI] [PubMed] [Google Scholar]
- 10.South T, Deng C, Huang XF. AM251 and B-Funaltrexamine reduce fat intake in a fat-preferring strain of mouse. Behav Brain Res. 2007;181:153–157. doi: 10.1016/j.bbr.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 11.Ho J, Cox JM, Wagner EJ. Cannabinoid-induced hyperphagia: Correlation with inhibition of proopiomelanocortin neurons? Physiol & Behav. 2007;92:507–519. doi: 10.1016/j.physbeh.2007.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Onaivi ES, Chaudhuri G, et al. Expression of cannabinoid receptors and their gene transcripts in human blood cells. Prog-Neuro-Psychopharmacol & Biol Psychiatry. 1999;13:963–976. doi: 10.1016/s0278-5846(99)00052-4. [DOI] [PubMed] [Google Scholar]
- 13.Zhang PW, Ishiguro H, et al. Human cannabinoid receptor 1: 5′ exons, candidate regulatory regions, polymorphisms, haplotypes and association with polysubstance abuse. Molecular Psychiatry. 2004;9:916–931. doi: 10.1038/sj.mp.4001560. [DOI] [PubMed] [Google Scholar]
- 14.Onaivi ES, Chakrabarti A, et al. Neurobehabioral effects of delta 9-THC and cannabinoid (CB1) receptor gene expression in mice. Behav Brain Res. 1995;72:115–125. doi: 10.1016/0166-4328(96)00139-8. [DOI] [PubMed] [Google Scholar]
- 15.Dimarzo V, Melck D, et al. Palmitolethanolamide inhibits the expression of fatty acid amide hydrolase and enhances the anti-proliferative effect of anandamide in human breast cancer cells. Biochemistry Journal. 2001;358:249–255. doi: 10.1042/0264-6021:3580249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Franklin A, Parmeniter-Batteur S, et al. Palmitoylehanolamide increases after Focal Cerebral Ischemia and Potentiates Microglial Cell Motility. The Journal of Neuroscience. 2003;23:7767–7775. doi: 10.1523/JNEUROSCI.23-21-07767.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mackie K, Stella N. Cannabinoid Receptors and Endocannabinoids: Evidence for New Players. AAPS Journal. 2006;8:E298–E306. doi: 10.1007/BF02854900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chambers AP, Koopmans HS, et al. AM 251 produces sustained reductions in food intake and body weight that are resistant to tolerance and conditioned taste aversion. Brit J Pharmacol. 2006;147:109–116. doi: 10.1038/sj.bjp.0706439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haney M, Rabkin J, et al. Dronabinol and marijuana in HIV(+) marijuana smokers: acute effects on caloric intake and mood. Psychopharmacology. 2005;181:170–178. doi: 10.1007/s00213-005-2242-2. [DOI] [PubMed] [Google Scholar]


