Abstract
Background: Magnesium is the second most abundant intracellular cation. It plays an important role in insulin homeostasis and glucose metabolism through multiple enzymatic reactions. With increasing data on magnesium deficiency in diabetic patients and epidemiological studies demonstrating magnesium deficiency as a risk factor for diabetes, it is logical to search for its possible beneficial effects on diabetes control and prevention. We aimed to determine whether oral magnesium supplementation improves metabolic control, lipid profile and blood pressure in patients with type II diabetes.
Methods: Fifty four patients with type II diabetes were included in a randomized double blind placebocontrolled clinical trial.Patients received either placebo or 300 mg elemental magnesium (as magnesium sulfate -MgSo4-) daily, for 3 months. Metabolic control, lipid profile, blood pressure, magnesium status, hepatic enzymes, hemoglobin concentration, and anthropometric indices were determined in the beginning and at the end of the study.
Results: Daily administration of 300 mg elemental magnesium for 3 months, significantly improved fasting blood glucose (183.9±15.43 to 125.8±6.52 vs. 196.5±28.12 to 136.5±7.94, p< 0.0001), 2-hour post prandial glucose (239.1±74.75 to 189.1±60mg/dl vs. 246.4±97.37 to 247.8±86.74mg/dl, p< 0.01), lipid profile, blood pressure and hepatic enzymes.
Conclusion: Oral magnesium supplementation with proper dosage has beneficial effects on blood glucose, lipid profile, and blood pressure in patients with type II diabetes.
Keywords: Magnesium, Diabetes, Blood glucose, Lipid profile, Blood pressure
Introduction
Magnesium (Mg) is the second most abundant intracellular cation (1). Mg homeostasis and metabolism is affected by multiple factors but it is in part, a determinant of cellular ionic environment and cellular responsiveness to external stimuli such as insulin and glucose (2). Mg is a cofactor in the cell membrane glucose-transporting mechanism, as well as in various enzymes in carbohydrate oxidation. It is also involved in insulin secretion, binding, and activity at various levels (3). It is suggested that Mg depletion contributes to states of insulin resistance such as hypertension, metabolic syndrome and type II diabetes (2).
Some studies have shown that hypomagnesaemia, which is a frequent condition in patients with diabetes, could be involved in the development of complications related to diabetes (4). However total serum Mg concentrations do not reflect the Mg status or intracellular pool, and intracellular or serum ionized Mg. Mg depletion can be seen with normal total serum Mg concentration (2). Previous studies have shown different results for oral Mg administration in patients with diabetes. Purvis and colleagues found that the administration of 386 mg magnesium chloride for 6 weeks could significantly decrease systolic blood pressure, without any effect on metabolic indices or lipid profile (5). Other studies have shown different effects of Mg supplementation on blood pressure (6, 7). Paolisso and co-workers found that the administration of 15.8mmol magnesium pidolate for 4 weeks improved insulin stimulated glucose uptake and glucose oxidation (8). Lima and colleagues compared supplementation with 20.7 or 41.4mmol magnesium oxide and placebo for 30 days; they found that supplementation with 20.7mmol Mg did not show any beneficial effects, but a dosage of 41.4 mmol significantly reduced fructosamine (9). Klitgard and colleagues used 500 mg of magnesium oxide daily for 24 weeks and reported a significant reduction in total cholesterol (TC), low density cholesterol (LDL C.), and Apo lipoprotein ß, but no effect on glycosylated hemoglobin (HbA1c) or fasting blood glucose (FBG) (10). Yokota and co-workers stated that Mg supplementation with a natural preparation from a water lake had reduced homeostasis model assessment index (HOMA-IR), but not FBG or HbA1c (11).
In previous studies we found that oral MgSO4 administration in diabetic animal models could decrease blood glucose and blood pressure (12). We also reported that MgSo4 had a beneficial effect on animal model diabetic vessel complications (13). Based on our previous studies and increasing data on Mg deficiency in patients with diabetes (12-17), as well as epidemiological studies reporting Mg deficiency as a risk factor for diabetes (18-22), it is logical to search for its possible beneficial effects on diabetes control and prevention.
We aimed to determine whether oral Mg supplementation improves blood glucose, lipid profile, blood pressure, hepatic enzymes, hemoglobin concentration, and anthropometric indices, as control parameters for patients with type II diabetes.
Methods
Patients
In this randomized double-blind controlled trial we included patients with type II diabetes who did not have obvious disturbances in Mg metabolism, with any level of diabetes control, and were available and willing to participate. Therefore, patients with type II diabetes aged 20–60 years were recruited from an out-patient primary level medical care office, specific for diabetic patients in Bandar-Abbas, southern Iran.
Before the study all patients completed a questionnaire. Also, they were clinically evaluated and blood samples were withdrawn in order to exclude patients who were using insulin, calcium channel blockers, and Mg or calcium (Ca) containing supplements. Moreover, patients who had experienced renal insufficiency (serum creatinine levels more than 1.3 mg/dl in women and more than 1.5 mg/dl in men), elevated hepatic enzymes (more than 3 folds over normal values), recent infections (less than one month prior to study) and chronic inflammatory diseases, cerebrovascular accident (CVA) and acute coronary syndrome (ACS) less than one month prior to the study and were alcoholic, pregnant, or drug abusers were excluded.
After obtaining informed consent, 54 patients with type II diabetes were ultimately enrolled in the study and randomly allocated to receive either Mg supplementation or placebo for 3 moths using the simple randomization method. The study flow diagram is shown in figure 1. The patients and personnel were blind to group assignment. Only the study analyst saw unblended data, but was not in contact with the participants.
Fig. 1 .
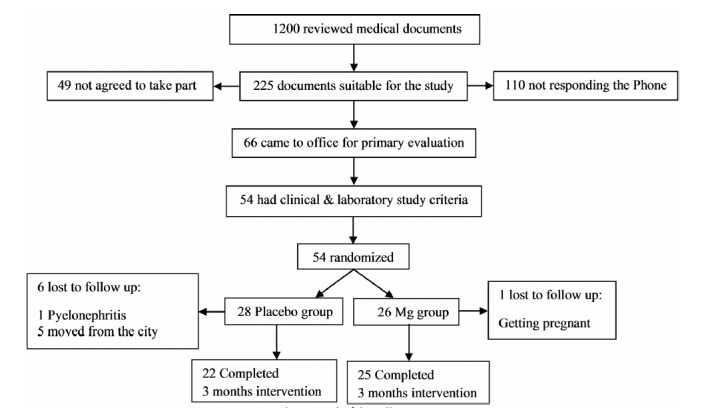
Study felow diagram.
The present study was a randomized double-blind placebo-controlled clinical trial. All patients and researcher except the one who was responsible for randomization, were blind to the study protocol.
The study was approved by the Ethics Committee of the Faculty of Medicine of Bandar Abbas University of Medical Sciences, and registered in the Iranian registry of clinical trials (IRCT), with a membership number of 1707.
Initially, 10ml blood was withdrawn from the antecubital vein of all participants to measure variables including FBG as the primary outcome, 2-hour post prandial blood glucose (2hr PP BS), HbA1C, fasting plasma insulin (FPI) as indicators of metabolic control, hemoglobin concentration (Hb), triglycerides (TG), TC, LDL C., high density cholesterol (HDL C.) as indicators of diabetes complications, serum Ca and Mg levels, urine Mg level, and Ca /Mg ratio as indicators of diabetes-related vascular complications (23). Hepatic enzymes (Alanine transferase [ALT] and Aspartate transferase [AST]) were also measured as indicators of hepatic side effects of Mg. Blood pressure was measured at baseline using the standard method (24). HOMA-IR index (25) was calculated at the baseline and at the end of the study using FBG and FPI; it was then used as an indicator for insulin resistance and metabolic control.
All patients were visited by an endocrinologist, who was blind to the grouping, and their medications were adjusted. Glibenclamide, metformin, atorvastatin, and enalapril were used for treatment. After the initiation of the intervention, the patients were visited every month to be evaluated for adherence to pharmacological treatment. Blood pressure (Microlife BPAG1-20, Microlife, Swiss) and FBG (glucometer, EasyGluco, Infopia CO., Korea) were measured monthly during the study period and all laboratory measurements were done completely. All participants were visited by the same endocrinologist every month and their drugs were readjusted. At the end of the intervention the changes in glucose lowering drug dosage were recorded and used as indicators of metabolic control.
Drugs
Capsules containing MgSo4 were used as magnesium supplement. Each capsule contained 100 mg elemental Mg. Each patient used 3 capsules per day; one with each meal adding up to a daily supplementation of 300 mg elemental Mg, according to our pilot study. The placebo capsules were similar to the Mg capsules and were used 3 times a day. Mg and placebo capsules were prepared at the Herbal Drugs Research Center of Tehran University of Medical Sciences, Tehran, Iran.
Measurements
Height and weight were measured using standard protocols when the patients were wearing light clothing without shoes. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared (24).
FBG was measured every three weeks using the glucose–oxidase method (Glucose - monoreagent kit, Kimia Pajhouhan, Iran) at baseline and every month thereafter during the study period. Two-hr PP BS was also measured using the glucose–oxidase method. Enzymatic methods were used for the measurement of TC (CHOD-PAP cholesterol kit, Pars Azmun, Iran), LDL C. (LDL- C kit, Pars Azmun, Iran), HDL C. (CHOD-PAP cholesterol kit, Pars Azmun, Iran), and TG (GPO-PAP triglycerides kit, Pars Azmun, Iran). FPI levels were measured using Elisa test (Monobind insulin microplate Elisa test, Monobind Inc., USA). Serum Mg concentrations (Colorimetric Magnesium kit, Pars Azmun, Iran) and Hb concentration (Colorimetric Hb kit, Pars Azmun, Iran) were measured with the colorimetric method. Nycocard HbA1c kit (Axis-shild, UK) was used for HbA1C measurement. Cresol phethalein complex method (Pars Azmun, Iran) was used to measure serum Ca and monoreagent method (Pars Azmun, Iran) was used for the measurement of AST and ALT.
Insulin resistance was determined by the homeostasis model assessment index (defined as HOMA–IR= FBG(mg/dl)×FPI (µu/ml) ∕405) (25).
Statistical analyses
Data were expressed as mean ± SD. The normal distribution of our populations (Gaussian distributions) was tested using the Kolmogorov and Smirnov method. Comparisons between groups were analyzed using the T test. Paired-T test was used to analyze difference between means of each group before and after the intervention and different multiple statistical tests such as Bonferroni and Newman-Keuls tests were used to adjust for the effect of other medications changes. SPSS software, version 13, was used for the analyses. p<0.05 was considered as statistically significant.
Results
Fifty four patients were enrolled in our study, and were randomly allocated into Mg (n=26) and placebo (n=28) groups. The Mg group received 300 mg elemental Mg daily and the placebo group received placebo capsules. One woman in the placebo group developed pyelonephritis during the study and was excluded from the study to receive appropriate treatment. Five other participants in the placebo group were lost to follow-up, because they moved from Bandar Abbas. In the Mg group, one patient was excluded because of becoming pregnant. Twenty five patients in the Mg group and twenty two patients in the placebo group completed the three-month intervention successfully (Fig. 1). Mg supplement was well tolerated and did not cause any adverse effects in the participants apart from slight diarrhea in two patients without the need for terminating the treatment.
The results of this study before the intervention are shown in Table 1. Table 2 demonstrates the study results in both groups after the intervention.
Table 1 . Study variables in Mg treated and placebo group before intervention.
| MgSO 4 group (n =25) | Placebo group (n = 22) | ||
| Baseline | Baseline | p | |
| Age (years) | 46.76 ± 9 | 50.15 ± 6 | Not significant |
| Sex (male/female) | 7/18 | 6/16 | Not significant |
| Duration of diabetes (years) | 4.11 ± 4.23 | 5.35 ± 4.01 | Not significant |
| BMI (kg/m2) | 26.19 ± 2.86 | 26.89 ± 5.23 | Not significant |
| Systolic blood pressure ( mm Hg) | 117.9 ± 14.99 | 120.8 ± 18.31 | Not significant |
| Diastolic blood pressure ( mm Hg) | 72.91 ± 8.10 | 73.75 ± 8.82 | Not significant |
| Serum Mg (mg/dl) | 2.15 ± 0.33 | 2.03 ± 0.58 | Not significant |
| Urine Mg (mg/dl) | 5.67 ± 1.77 | 5.54 ± 1.56 | Not significant |
| Calcium to magnesium ratio | 4.58 ± 0.68 | 4.79 ± 1. | Not significant |
| Fasting blood glucose (mg/dl) | 183.9 ± 15.43 | 196.5 ± 28.12 | Not significant |
| 2hr PP blood glucose (mg/dl) | 239.1 ± 74.75 | 246.4 ± 97.37 | Not significant |
| HbA1C (%) | 8.33 ± 1.47 | 8.30 ± 1.99 | Not significant |
| HOMA-IR index | 2.47 ± 2.71 | 2.37 ± 3.02 | Not significant |
| Fasting plasma insulin (qIU/ml) | 7.12 ± 8.02 | 6.82 ± 8.60 | Not significant |
| Triglycerides (mg/dl) | 162.6 ± 68.92 | 188 ± 69.90 | Not significant |
| Total cholesterol (mg/dl) | 217.7±106.49 | 201±54.07 | Not significant |
| LDL cholesterol (mg/dl) | 118.3 ± 32.55 | 130.65 ± 33.52 | Not significant |
| HDL cholesterol (mg/dl) | 44.30 ± 10.20 | 47.45 ± 10.50 | Not significant |
| Non HDL cholesterol (mg/dl) | 149.35 ± 35.07 | 158.38 ± 26.92 | Not significant |
| Hemoglobin concentration (gr/dl) | 13.45 ± 1.26 | 13.32 ± 1.29 | Not significant |
| Aspartate transfrase (IU/ml) | 18 ± 5.40 | 21 ± 6.50 | Not significant |
| Alanin transfrase (IU/ml) | 22.80 ± 2.22 | 23.81 ± 1.89 | Not significant |
| Metformin dosage ( mg ) | 1045.45 ± 598.60 | 1136.36 ± 551.85 | Not significant |
| Glibenclamid dosage ( mg ) | 11.36 ± 5.04 | 11.75 ± 6.66 | Not significant |
| Enalapril dosage (mg) | 15 ± 4.56 | 15 ± 2.88 | Not significant |
| Atorvastatin dosage (mg) | 22.5 ± 2.5 | 23.33 ± 6.6 | Not significant |
Data are means ± SD.
Table 2 . Study variables in Mg treated and placebo group after 3 months intervention.
| MgSO 4 group (n =25) | Placebo group (n = 22) | ||
| End | End | p | |
| Age (years) | ________ | ________ | ________ |
| Sex (male/female) | ________ | ________ | ________ |
| Duration of diabetes (years) | ________ | ________ | ________ |
| BMI (kg/m2) | 26.41 ± 3.29 | 27.28 ± 5.13 | Not significant |
| Systolic blood pressure ( mm Hg) | 102.8 ± 9.51 | 117.2 ± 3.48 | <0.001 |
| Diastolic blood pressure ( mm Hg) | 62.85 ± 7.55 | 70 ± 7.07 | <0.05 |
| Serum Mg (mg/dl) | 2.16 ± 0.36 | 2.23 ± 0.50 | Not significant |
| Urine Mg (mg/dl) | 5.99 ± 2 | 4.56 ± 1.72 | <0.05 |
| Calcium to magnesium ratio | 4.64 ± 1.46 | 5.21 ± 1.65 | Not significant |
| Fasting blood glucose (mg/dl) | 125.8 ± 6.52 | 136.5 ± 7.94 | <0.0001 |
| 2hr PP blood glucose (mg/dl) | 189.1 ± 60.05 | 247.8 ± 86.74 | <0.01 |
| HbA1C (%) | 7.90 ± 1.68 | 7.61 ± 1.59 | Not significant |
| HOMA-IR index | 5.01 ± 2.47 | 4.65 ± 3.48 | Not significant |
| Fasting plasma insulin (qIU/ml) | 15.90 ± 8.02 | 13.35 ± 11.53 | Not significant |
| Triglycerides (mg/dl) | 158.7 ± 77.16 | 175.5 ± 107.5 | Not significant |
| Total cholesterol (mg/dl) | 176.2 ± 48.33 | 182.7 ± 56.23 | Not significant |
| LDL cholesterol (mg/dl) | 93.63 ± 24.58 | 120.4 ± 34.86 | <0.01 |
| HDL cholesterol (mg/dl) | 41.7 ± 8.79 | 43.65 ± 11.20 | Not significant |
| Non HDL cholesterol (mg/dl) | 125.30 ± 23.19 | 152.16 ± 37.05 | <0.001 |
| Hemoglobin concentration (gr/dl) | 13.46 ± 1.51 | 13.18 ± 1.71 | Not significant |
| Aspartate transfrase (IU/ml) | 18.15 ± 10.40 | 24.92 ± 12.12 | Not significant |
| Alanin transfrase (IU/ml) | 22.30 ± 2.83 | 33.93 ± 5.70 | <0.05 |
| Metformin dosage ( mg ) | 1371.42 ± 625.21 | 1750 ± 379.77 | <0.01 |
| Glibenclamid dosage ( mg ) | 10.76 ± 7.10 | 13.31 ± 6.18 | Not significant |
| Enalapril dosage (mg) | 13 ± 4.6 | 23 ± 6.6 | <0.01 |
| Atorvastatin dosage (mg) | 15 ± 1.88 | 17.5 ± 3.65 | Not significant |
Data are means ± SD.
We found that Mg supplementation could significantly decrease both systolic (p< 0.001) and diastolic (p< 0.05) blood pressure (Table 2). Differences in the blood pressure were significant, even when antihypertensive drugs were taken into account.
Urine Mg concentration was also measured, showing a significant increase in the Mg group compared with the placebo group (p<0.05). We measured the Ca/Mg ratio as an indicator for atherosclerosis; and observed that this ratio was higher in the placebo group at the end of the study but the difference between the two groups was not significant.
Blood glucose measurements showed reduced FBG and 2hr PP BS levels in the participants of both groups which was significantly lower in the Mg group compared with the placebo group (p<0.0001 for percentage of FBG reduction and p<0.01 for decrease in 2hr PP BS). At the end of the study, the dosage of metformin used in the placebo group was significantly higher than the group treated with Mg (p<0.01). As shown in table 2, we also found a significant decrease in plasma LDL C. levels in the Mg treated group compared with the placebo group (p<0.01). After oral Mg supplementation non-HDL cholesterol level showed a significant reduction in the Mg treated group compared with the placebo group (p<0.001).
AST levels showed no significant difference, but the interesting finding was related to the ALT levels. In the placebo group ALT levels were rising during the study, but in the Mg treated group these levels remained unchanged (p<0.05).
Discussion
We found that oral Mg supplementation could improve glycemic control, blood pressure, and lipid profile in patients with type II diabetes without having any important complications.
Since we had previously found beneficial effects in supplementation with oral Mg (as MgSo4) on streptozotocin induced diabetic rats (12, 13), we designed a double-blind placebo-controlled clinical trial with the best preparation and dosage of Mg according to our previous studies and other investigations. The purpose of this study was to determine whether oral Mg supplementation improves metabolic control, lipid profile, blood pressure, hepatic enzymes, hemoglobin concentration and anthropometric indices, as parameters of diabetes control in patients with type II diabetes.
Considering the increasing prevalence of diabetes and its complications throughout the world, researchers are trying hard to discover new prevention and treatment methods for diabetes (26, 27). Mg supplementation had proven to be effective in controlling type II diabetes (2), and numerous studies on animal models have found consistent results (12, 13). Recently, some clinical trials have studied the effectiveness of oral Mg supplementation in controlling type II diabetic patients and found favorable results; although there is not enough data to confirm these benefits yet (4-11).
Blood glucose measurements in this study indicated that the participants of both groups had reduced FBG and 2hr PP BS levels. This reduction is mainly due to the glucose lowering agents prescribed by the endocrinologist in monthly visits. Although the endocrinologist was blind to the group assignment, reduction of FBG and 2hrPP BS was significantly higher in the Mg supplemented group. To the best of our knowledge, almost all previous clinical trials have failed to show any changes in FBG (5-11). This failure in showing beneficial effects of Mg on FBG can be explained by the differences in Mg dosage and duration of supplementation. As in our previous studies on diabetic rat models we found that supplementation with just a dosage of 10 g/L MgSO4 in drinking water could improve glycemic control and the histological structure of the pancreas and aorta (12). Dosages less than 10 g/L, did not show beneficial effects and dosage more than 10 g/L, could reduce blood glucose but had deleterious effects on the histological structure of the pancreas (12).
Of course, the beneficial effects of Mg on FBG and 2hr PP BS in our study can be explained by their critical role in carbohydrate metabolism and post receptor reactions in insulin receptors (2). However, the important point is that although glycemic control (FBG & 2hr PP BS) was improved, we found no significant difference in HOMA-IR index and FPI levels between the two groups. This finding implies that the beneficial effects of Mg in diabetes control cannot be only due to improvement of insulin sensitivity or insulin secretion. Therefore we think that, one of the main sites of Mg action can be the muscles. It is possible that Mg can help, translocation of glucose transporter number 4 (GLUT 4) to the cell membrane, to take place.
On the other hand, it should be kept in mind that in our study both groups were blindly treated by oral hypoglycemic agents. The main used oral hypoglycemic agent was metformin in both groups. Metformin dosage was significantly lower in the Mg treated patients after three months of Mg supplementation compared with the placebo group.
The significant decrease in LDL cholesterol in the Mg treated patients in this study infers the beneficial effects of Mg on atherogenic lipid profile, as shown in our previous study in an animal model (13) and other similar studies (9, 10). This effect can be explained by the role of Mg in the activity of lipoprotein lipase enzyme as described by Rayssiguire and colleagues (28). Mg deficiency enhances catecholamine secretion which results in increased lipolysis. Enhancement of lipolysis and subsequent elevation of plasma free fatty acids may lead to an increase in VLDL and TG synthesis and secretion and elevated plasma TG concentration (28)
Our results were in agreement with our previous study, showing significant decrease in systolic, diastolic, and mean arterial blood pressure (13). These effects result from changes in intracellular ionic environment (2). When the intracellular Ca/Mg ratio increases as a result of Mg deficiency, vascular smooth muscle tone and smooth muscle cell response to external constrictor stimuli increases, which leads to vasoconstriction and as a result elevation of blood pressure (2). In our previous studies (13) we showed that the administration of magnesium can decrease mesenteric vascular bed sensitivity to phenylephrine and decrease Ca/Mg ratio. We also showed that magnesium decrease collagen thickness, intima/media thickness and the lumen/ media ratio in aorta (13). So it seems that administration of magnesium can decrease blood pressure due to prevent vascular morphological changes and decrease vascular sensitivity to neurotransmitter. ALT levels significantly increases in the placebo group during the study while there was no significant increase in ALT levels in the Mg group. The elevation of hepatic enzymes in the placebo group could be a sign of fatty liver disease. The higher increase in metformin dosage in the placebo group might be responsible for the significant rise of ALT levels in this group compared with the Mg supplemented group.
In patients supplemented with Mg, urinary Mg increased significantly compared with the placebo group. Increased urinary Mg without plasma Mg changes may be due to an increase in ionized or intracellular Mg in these patients.
Conclusion
According to the results of our study and the previous ones, we can conclude that not only Mg supplementation can be helpful in diabetes control, but also the effective dosage and duration of supplementation, and the patients who need the supplementation should be considered. Therefore, Mg is also recommended as an inexpensive, easy to use, natural adjuvant therapy for patients with type II diabetic.
Acknowledgments
The authors would like to thank the Deputy of Research of Hormozgan University of Medical Sciences and we are also deeply appreciative of the help provided by Dr Shahram Zare in the biostatistics department.
Cite this article as: Solati M, Ouspid E, Hosseini S, Soltani N, Keshavarz M, Dehghani M. Oral magnesium supplementation in type II diabetic patients. Med J Islam Repub Iran 2014 (15 July). Vol. 28:67.
References
- 1.Barbagallo M, Dominguez LJ, Galioto A, Ferlisi A, Cani C, Malfa AP, Pineo A, Busardo' A, Paolisso G. Role of magnesium in insulin action, diabetes and Cardio-metabolic syndrome X. Molecular Aspects of Medicin. 2003;24:39–52. doi: 10.1016/s0098-2997(02)00090-0. [DOI] [PubMed] [Google Scholar]
- 2.Barbagallo M, Dominguez JL. Magnesium metabolism in type 2 diabetes, metabolic syndrome and insulin resistance. Archives of Biochemistry and Biophysics. 2007;458:40–47. doi: 10.1016/j.abb.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Cheatan PH, Sialy R, Banzal D. Magnesium deficiency and diabetes mellitus. Curr Sci. 2002;83:1456–62. [Google Scholar]
- 4.Atabek ME, Kurtoglu S, Pirgon O, Baykara M. Arterial wall thickening and stiffening in children and adolescents with type 1 diabetes. Diabetes Research and Clinical Practice. 2006;74:33–40. doi: 10.1016/j.diabres.2006.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Purvis JR, Cummings DM, Landsman P, Carroll R, Barakat H, Bray J, Whitley C, Horner RD. Effect of Oral Magnesium Supplementation on Selected Cardiovascular Risk Factors in Noninsulin-Dependent Diabetics. Archives of Family Medicine. 1994;3:503–508. doi: 10.1001/archfami.3.6.503. [DOI] [PubMed] [Google Scholar]
- 6.Eriksson J, Kohvakka A. Magnesium and Ascorbic Acid Supplementation in Diabetes mellitus. Annals of Nutrition and Metabolism, Journal of Nutrition, Metabolic Diseases and Dietetics. 1995;39:217–223. doi: 10.1159/000177865. [DOI] [PubMed] [Google Scholar]
- 7.Eibl NL, Kopp H P, Nowak HR, Schnack CJ, Hopmeier PG, Schernthaner G. Hypomagnesemia in type II diabetes: Effect of a 3-month replacement therapy. Diabetes Care. 1995;18(2):188–192. doi: 10.2337/diacare.18.2.188. [DOI] [PubMed] [Google Scholar]
- 8.Paolisso G, Scheen A, Cozzolino D, Di Maro G, Varricchio M, D'Onofrio F, Lefebvre PJ. Changes in glucose turnover parameters and improvement of glucose oxidation after 4-week magnesium administration in elderly noninsulin-dependent (type II) diabetic patients. Journal of Clinical Endocrinology & Metabolism. 1994;78:1510–1514. doi: 10.1210/jcem.78.6.8200955. [DOI] [PubMed] [Google Scholar]
- 9.Lima MD, Cruz T, Pousada Jc, Rodrigues LE, Barbosa K, Cangucu V. The effect of magnesium Supplementation in increasing doses on the control of type 2 diabetes. Diabetes care. 1998;21:682–686. doi: 10.2337/diacare.21.5.682. [DOI] [PubMed] [Google Scholar]
- 10.Djurhuus MS, klitgaard NA, Pederson K, Blaabjerg B, Ltura BT, Altura MA, Henriksen JE. Magnesium reduces insulin- stimulated glucose uptake and serum lipid concentration in typ1 diabetes. Metabolism. 2001;50:1409–1417. doi: 10.1053/meta.2001.28072. [DOI] [PubMed] [Google Scholar]
- 11.Yokota K, Kato M, Lister F, Ii H, Hayakawa T, Kikuta T, Kageyama S, Tajima N. Clinical Efficacy of Magnesium Supplementation in Patients with Type 2 Diabetes. Journal of the American College of Nutrition. 2004;23:506S–509S. doi: 10.1080/07315724.2004.10719390. [DOI] [PubMed] [Google Scholar]
- 12.Soltani N, Keshavarz M, Minaii B, Mirershadi F, Zahedi Asl S, Dehpour A R. Effect of administration of oral Mg2+ on plasma glucose and pathological changes in the aorta and pancrease of diabetic rats. Clinical and Experemental Pharmacology and Physiology. 2005;32:604–610. doi: 10.1111/j.0305-1870.2005.04238.x. [DOI] [PubMed] [Google Scholar]
- 13.Soltani N, Keshavarz M, Dehpour AR. Effect of oral magnesium sulfate administration on blood pressure and lipid profile in streptozotocin diabetic rat. European Journal of Pharmacology. 2007;10 560(2-3):201–5. doi: 10.1016/j.ejphar.2006.12.020. [DOI] [PubMed] [Google Scholar]
- 14.Wälti MK, Zimmermann MB, Spinas GA, Hurrell RF. Low plasma magnesium in type 2 diabetes. Swiss Medical Weekly. 2003;133:289–292. doi: 10.4414/smw.2003.10170. [DOI] [PubMed] [Google Scholar]
- 15.McNair P, Christensen MS, Christiansen C, Madsbad S, Transbol I. Renal hypomagnesaemia in human diabetes mellitus: its relation to glucose homeostasis. European Journal of Clinical Investigation. 1982;12:81–85. doi: 10.1111/j.1365-2362.1982.tb00942.x. [DOI] [PubMed] [Google Scholar]
- 16.Guerrero-Romero F, Rodriguez-Moran M. Low serum magnesium levels and metabolic syndrome. Acta Diabetologia. 2002;39:209–213. doi: 10.1007/s005920200036. [DOI] [PubMed] [Google Scholar]
- 17.Resnick LM, Gupta RK, Gruenspan H, Alderman MH, Laragh JH. Hypertension and peripheral insulin resistancePossible mediating role of intracellular free magnesium. American Journal of Hypertension. 1990;3:373–379. doi: 10.1093/ajh/3.5.373. [DOI] [PubMed] [Google Scholar]
- 18.Colditz GA, Manson JE, Stampfer MJ, Rosner B, Willett WC, Speizer FE. Diet and risk of clinical diabetes in women. American Journal of Clinical Nutrition. 1992;55:1018–1023. doi: 10.1093/ajcn/55.5.1018. [DOI] [PubMed] [Google Scholar]
- 19.Kao WH, Folsom AR, Nieto FJ, Mo JP, Watson RL, Brancati FL. Serum and Dietary Magnesium and the Risk for Type 2 Diabetes Mellitus. Archives of Internal Medicine. 1999;159:2151–2159. doi: 10.1001/archinte.159.18.2151. [DOI] [PubMed] [Google Scholar]
- 20.Humphries S, Kushner H, Falkner B. Low dietary magnesium is associated with insulin resistance in a sample of young, no diabetic black Americans. American Journal of Hypertension. 1999;12:747–756. doi: 10.1016/s0895-7061(99)00041-2. [DOI] [PubMed] [Google Scholar]
- 21.Lopez-Rid aura R, Willett WC, Rimm EB, Liu S, Stampfer MJ, Manson JE, Hu FB. Magnesium Intake and Risk of Type 2 Diabetes in Men and Women. Diabetes Care. 2004;27:134–140. doi: 10.2337/diacare.27.1.134. [DOI] [PubMed] [Google Scholar]
- 22.Song Y, Manson JE, Buring JE, Liu S. Dietary Magnesium Intake in Relation to Plasma Insulin Levels and Risk of Type 2 Diabetes in Women. Diabetes Care. 2004;27:59–65. doi: 10.2337/diacare.27.1.59. [DOI] [PubMed] [Google Scholar]
- 23.Altura BM, Altura BT. Magnesium and cardiovascular biology an important link between cardiovascular risk factors and atherogenesisCellMolBiol. Res. 1995;41:347–59. [PubMed] [Google Scholar]
- 24.National institute of health; National heart, lung and blood institute. Clinical guidelines on the identification, evaluation and treatment of overweight and obesity in adults. The evidence reports observers. 1998;6:(suppl 2)515. [PubMed] [Google Scholar]
- 25.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β cell function from fasting plasma glucose and insulin concentration in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 26.Soltanian N, Amini A, Iraj B, Askari GH, Ebneyamin S, Ghias M, Hajian H, Zahed A, Amini M. Weight status of the first-degree relatives of patients with type 2 diabetes based on the glucose tolerance test. Res Med Sci. 2012;17(3):269–274. [PMC free article] [PubMed] [Google Scholar]
- 27.Babes E, Babes V, Popescu M, Ardelean A. Value of N-Terminal Pro-B-Type Natriuretic Peptide in Detecting Silent Ischemia and its Prognostic Role in Asymptomatic Patients with Type 2 Diabetes Mellitus. Acta Endo (Buc) 2011;7(2):209–218. [Google Scholar]
- 28.Rayssiguier Y, Gueux E. Magnesium and lipids in cardiovascular disease. Journal of American College of Nutrition. 1986;5:507–519. doi: 10.1080/07315724.1986.10720153. [DOI] [PubMed] [Google Scholar]


