Abstract
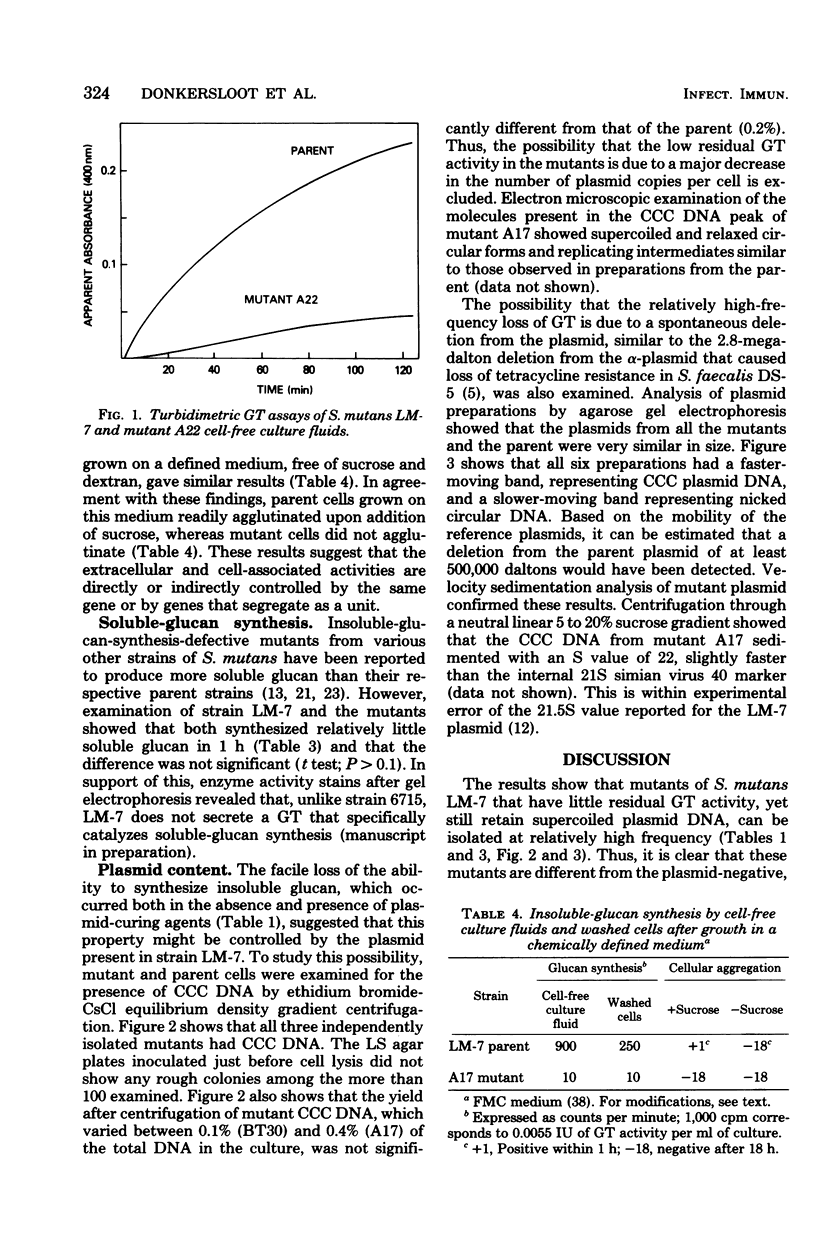
The possibility that glucosyltransferase (GT)-mediated insoluble-glucan synthesis from sucrose is controlled by the 3-megadalton plasmid pAM7 in Streptococcus mutans LM-7 has been examined. A low-sucrose agar medium was developed to readily detect and quantitate presumptive GT-negative mutants. Such mutants were isolated from Todd-Hewitt broth cultures grown either with or without sodium dodecyl sulfate (10 microgram/ml) or acriflavine (0.5 microgram/ml) at frequencies ranging from about 0.01 to 1%. Independently isolated mutants had the following characteristics: (i) cells were virtually devoid of cell-associated GT and did not aggregate upon addition of sucrose; (ii) cell-free culture fluids synthesized 10X less insoluble glucan than those of the parent; and (iii) cultures grown with sucrose did not form adherent deposits on the wall of the culture tube, as is typical of S. mutans. Both parent and mutants formed relatively little soluble glucan in 1-h assays. Three independently isolated mutants and the parent were found to contain similar amounts of plasmid DNA. Analysis by sucrose density gradient centrifugation and agarose gel electrophoresis did not reveal a size difference between the plasmids from parent and mutants. These results show that (i) S. mutans LM-7 generates GT-deficient mutants at relatively high frequency that still contain a 3-megadalton plasmid; (ii) both cell-associated and extracellular GT levels are depressed in the mutants, which suggests that these activities are directly or indirectly controlled by the same gene or by genes that segregate as a unit.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernsohn J., Stephanides L. M., Norgello H. Incorporation of [1-14C]linoleic acid into central nervous system of the adult cat after intracisternal administration. Brain Res. 1971 May 7;28(2):327–337. doi: 10.1016/0006-8993(71)90664-0. [DOI] [PubMed] [Google Scholar]
- Bratthall D. Demonstration of five serological groups of streptococcal strains resembling Streptococcus mutans. Odontol Revy. 1970;21(2):143–152. [PubMed] [Google Scholar]
- Chassy B. M., Beall J. R., Bielawski R. M., Porter E. V., Donkersloot J. A. Occurrence and distribution of sucrose-metabolizing enzymes in oral streptococci. Infect Immun. 1976 Aug;14(2):408–415. doi: 10.1128/iai.14.2.408-415.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciardi J. E., Beaman A. J., Wittenberger C. L. Purification, resolution, and interaction of the glucosyltransferases of Streptococcus mutans 6715. Infect Immun. 1977 Oct;18(1):237–246. doi: 10.1128/iai.18.1.237-246.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B., Yagi Y., Bauer B. Plasmid-determined tetracycline resistance in Streptococcus faecalis: evidence for gene amplification during growth in presence of tetracycline. Proc Natl Acad Sci U S A. 1975 May;72(5):1720–1724. doi: 10.1073/pnas.72.5.1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courvalin P. M., Carlier C., Chabbert Y. A. Plasmid-linked tetracycline and erythromycin resistance in group D "streptococcus". Ann Inst Pasteur (Paris) 1972 Dec;123(6):755–759. [PubMed] [Google Scholar]
- Cox E. C. Bacterial mutator genes and the control of spontaneous mutation. Annu Rev Genet. 1976;10:135–156. doi: 10.1146/annurev.ge.10.120176.001031. [DOI] [PubMed] [Google Scholar]
- Currier T. C., Nester E. W. Isolation of covalently closed circular DNA of high molecular weight from bacteria. Anal Biochem. 1976 Dec;76(2):431–441. doi: 10.1016/0003-2697(76)90338-9. [DOI] [PubMed] [Google Scholar]
- Donkersloot J. A., Robrish S. A., Krichevsky M. I. Fluorometric determination of deoxyribonucleic acid in bacteria with ethidium bromide. Appl Microbiol. 1972 Aug;24(2):179–183. doi: 10.1128/am.24.2.179-183.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunny G. M., Birch N., Hascall G., Clewell D. B. Isolation and characterization of plasmid deoxyribonucleic acid from Streptococcus mutans. J Bacteriol. 1973 Jun;114(3):1362–1364. doi: 10.1128/jb.114.3.1362-1364.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman M. L., Tanzer J. M. Dissociation of plaque formation from glucan-induced agglutination in mutants of Streptococcus mutans. Infect Immun. 1974 Jul;10(1):189–196. doi: 10.1128/iai.10.1.189-196.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germaine G. R., Harlander S. K., Leung W. L., Schachtele C. F. Streptococcus mutans dextransucrase: functioning of primer dextran and endogenous dextranase in water-soluble and water-insoluble glucan synthesis. Infect Immun. 1977 May;16(2):637–648. doi: 10.1128/iai.16.2.637-648.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Berman K. S., Knoettner P., Kapsimalis B. Dental caries and alveolar bone loss in gnotobiotic rats infected with capsule forming streptococci of human origin. Arch Oral Biol. 1966 Jun;11(6):549–560. doi: 10.1016/0003-9969(66)90220-2. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Nygaard M. Synthesis of insoluble dextran and its significance in the formation of gelatinous deposits by plaque-forming streptococci. Arch Oral Biol. 1968 Oct;13(10):1249–1262. doi: 10.1016/0003-9969(68)90081-2. [DOI] [PubMed] [Google Scholar]
- Guggenheim B., Newbrun E. Extracellular glucosyltransferase activity of an HS strain of Streptococcus mutans. Helv Odontol Acta. 1969 Oct;13(2):84–97. [PubMed] [Google Scholar]
- Guggenheim B., Schroeder H. E. Biochemical and morphological aspects of extracellular polysaccharides produced by cariogenic streptococci. Helv Odontol Acta. 1967 Oct;11(2):131–152. [PubMed] [Google Scholar]
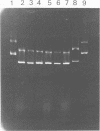
- Higuchi M., Araya S., Higuchi M. Plasmid DNA satellite bands seen in lysates of Streptococcus mutans that form insoluble extracellular polysaccharides. J Dent Res. 1976 Mar-Apr;55(2):266–271. doi: 10.1177/00220345760550021801. [DOI] [PubMed] [Google Scholar]
- Higuchi M., Endo K., Hoshino E., Araya S. Preferential induction of rough variants in Streptococcus mutans by ethidium bromide. J Dent Res. 1973 Sep-Oct;52(5):1070–1075. doi: 10.1177/00220345730520051401. [DOI] [PubMed] [Google Scholar]
- Higuchi M., Rhee G. H., Araya S., Higuchi M. Bacteriophage deoxyribonucleic acid-induced mutation of Streptococcus mutans. Infect Immun. 1977 Mar;15(3):938–944. doi: 10.1128/iai.15.3.938-944.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. C., Bozzola J. J., Shechmeister I. L. Morphological study of Streptococcus mutans and two extracellular polysaccharide mutants. J Bacteriol. 1974 Apr;118(1):304–311. doi: 10.1128/jb.118.1.304-311.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J. P., Frank R. M. Mise en évidence de virus dans les bactéries cariogénes de la plaque dentaire. J Biol Buccale. 1973 Mar;1(1):79–85. [PubMed] [Google Scholar]
- Klein J. P., Guinard M., Frank R. M. Polysaccharides extracellulaires et activité carieuse en système gnotobiote de streptocoques lysogènes et guéris. J Biol Buccale. 1975 Mar;3(1):65–75. [PubMed] [Google Scholar]
- Kuramitsu H. K. Properties of a mutant of Streptococcus mutans altered in glucosyltransferase activity. Infect Immun. 1976 Feb;13(2):345–353. doi: 10.1128/iai.13.2.345-353.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little R. V., Brubaker R. R. Characterization of deoxyribonucleic acid from Yersinia pestis by ethidium bromide-cesium chloride density gradient centrifugation. Infect Immun. 1972 Apr;5(4):630–631. doi: 10.1128/iai.5.4.630-631.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCabe R. M., Donkersloot J. A. Adherence of Veillonella species mediated by extracellular glucosyltransferase from Streptococcus salivarius. Infect Immun. 1977 Dec;18(3):726–734. doi: 10.1128/iai.18.3.726-734.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers J. A., Sanchez D., Elwell L. P., Falkow S. Simple agarose gel electrophoretic method for the identification and characterization of plasmid deoxyribonucleic acid. J Bacteriol. 1976 Sep;127(3):1529–1537. doi: 10.1128/jb.127.3.1529-1537.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of the Adherence of Streptococcus mutans to Smooth Surfaces III. Purification and Properties of the Enzyme Complex Responsible for Adherence. Infect Immun. 1974 Nov;10(5):1135–1145. doi: 10.1128/iai.10.5.1135-1145.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson G. A., Bleiweis A. S., Small P. A., Jr Adherence inhibition of Streptococcus mutans: an assay reflecting a possible role of antibody in dental caries prophylaxis. Infect Immun. 1972 Apr;5(4):419–427. doi: 10.1128/iai.5.4.419-427.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogolsky M., Wiley B. B., Glasgow L. A. Phage group II staphylococcal strains with chromosomal and extrachromosomal genes for exfoliative toxin production. Infect Immun. 1976 Jan;13(1):44–52. doi: 10.1128/iai.13.1.44-52.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith R. M., Parisi J. T., Vidal L., Baldwin J. N. Nature of the genetic determinant controlling encapsulation in Staphylococcus aureus Smith. Infect Immun. 1977 Jul;17(1):231–234. doi: 10.1128/iai.17.1.231-234.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonstein S. A., Baldwin J. N. Loss of the penicillinase plasmid after treatment of Staphylococcus aureus with sodium dodecyl sulfate. J Bacteriol. 1972 Jan;109(1):262–265. doi: 10.1128/jb.109.1.262-265.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinell D. M., Gibbons R. J. Influence of culture medium on the glucosyl transferase- and dextran-binding capacity of Streptococcus mutans 6715 cells. Infect Immun. 1974 Dec;10(6):1448–1451. doi: 10.1128/iai.10.6.1448-1451.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terleckyj B., Willett N. P., Shockman G. D. Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect Immun. 1975 Apr;11(4):649–655. doi: 10.1128/iai.11.4.649-655.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umesaki Y., Kawai Y., Mutai M. Effect of Tween 80 on glucosyltransferase production in Streptococcus mutans. Appl Environ Microbiol. 1977 Aug;34(2):115–119. doi: 10.1128/aem.34.2.115-119.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenberger C. L., Beaman A. J., Lee L. N. Tween 80 effect on glucosyltransferase synthesis by Streptococcus salivarius. J Bacteriol. 1978 Jan;133(1):231–239. doi: 10.1128/jb.133.1.231-239.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Stoppelaar J. D., König K. G., Plasschaert A. J., van der Hoeven J. S. Decreased cariogenicity of a mutant of Streptococcus mutans. Arch Oral Biol. 1971 Aug;16(8):971–975. doi: 10.1016/0003-9969(71)90186-5. [DOI] [PubMed] [Google Scholar]