Abstract
Purpose
To determine long-term oncologic outcomes of radical prostatectomy (RP) after neoadjuvant chemo-hormonal therapy for clinically localized, high-risk prostate cancer.
Methods
In this phase II multicenter trial of patients with high-risk prostate cancer (PSA>20ng/ml, Gleason ≥8, or clinical stage ≥T3), androgen deprivation therapy (goserelin acetate depot) and paclitaxel, carboplatin and estramustine were administered prior to RP. We report the long-term oncologic outcomes of these patients and compared them to a contemporary cohort who met oncologic inclusion criteria but received RP only.
Results
Thirty-four patients were enrolled in this study and followed for a median of 13.1 years. Within 10 years most patients experienced biochemical recurrence (BCR-free probability= 22%; 95% CI 10%, 37%). However the probability of disease-specific survival at 10 years was 84% (95% CI 66%, 93%) and overall survival was 78% (95% CI 60%, 89%). The chemohormonal therapy group had higher-risk features than the comparison group (N=123 patients) with an almost doubled risk of calculated preoperative 5-year BCR (69% vs 36%, p<0.0001). After adjusting for these imbalances the CHT group had trends toward improvement in BCR (HR 0.76, 95% CI 0.43, 1.34; p=0.3) and metastasis free survival (HR 0.55, 95% CI 0.24, 1.29; p=.2) although these were not significant.
Conclusion
Neoadjuvant chemohormonal therapy followed by RP was associated with lower observed rates of BCR and metastasis compared to a prostatectomy only group; however these results were not significant. Because this treatment strategy has known harms and unproven benefit, this strategy should only be instituted in the setting of a clinical trial.
Introduction
Although prostate cancer (PCa) often follows an indolent course, those with high-risk features are at significant risk for local and distant progression.1 Although there are many definitions for high-risk non-metastatic PCa2, the National Comprehensive Cancer Network (NCCN) defines it as having any of the following characteristics; PSA> 20 ng/ml, clinical stage ≥T3, or Gleason grade ≥8.3 Radical prostatectomy (RP) or radiation therapy alone may provide benefit but many develop recurrence and progress from their disease.2
Multi-modality approaches have been explored in attempts to reduce rates of failure after monotherapy. Trials combining radiation therapy with neoadjuvant, concurrent and adjuvant hormonal therapy have demonstrated survival advantages over radiation alone4 but similar results with RP have not been observed.5-7 Regardless, surgery remains an attractive option for high-risk patients because it may treat or prevent obstructive symptoms, it may more readily lend itself to salvage modalities, and it allows more accurate pathologic and nodal staging. Additionally approaches combining surgery with neoadjuvant chemotherapy is the favored treatment for various other urologic and non-urologic malignancies cancers such as bladder8, testicular9, breast10 and esphogeal11 cancer.
In an attempt to improve oncologic outcomes multiple small studies have investigated the feasibility and pathologic outcomes of combined chemohormonal therapy (CHT) followed by RP in patients with high-risk PCa.12-17 The goal of such an approach is to obtain optimal local control while also addressing the potential metastatic component of the disease. However because of its long natural history, the therapeutic effect of such trials may not be fully realized in these prior studies with short to intermediate follow-up. Furthermore such studies are limited due to the lack of a comparison group. Herein, we report long term follow up of a previously reported phase II trial of patients with high-risk PCa treated with neoadjuvant CHT followed by RP and compared their outcomes to a group of patients from a similar timeframe who met oncologic inclusion criteria but underwent RP only.18
METHODS
Study Design
We previously reported on 36 patients enrolled in an IRB approved, non-randomized, multicenter phase I/II study to determine the feasibility and safety of neoadjuvant estramustine, paclitaxel and carboplatin (4 to 6 cycles of paclitaxel and carboplatin and estramustine with concurrent goserelin acetate depot subcutaneously every 3 months during chemotherapy) followed by RP.18 The study was conducted between 1997 and 2000 and early outcomes were reported in 2004.18 In this current study we omitted two patients with a history of treatment for non-genitourinary pelvic malignancies prior to the start of CHT therapy, leaving a total of 34 patients in the analysis. Criteria for enrollment included the presence of noncastrate, non-metastatic PCa high-risk PCa, consistent with the NCCN guidelines and defined as any of the following; PSA> 20 ng/ml, clinical stage ≥T3, or Gleason grade ≥8. Complete inclusion/exclusion criteria are reported elsewhere.18
To determine if neoadjuvant CHT therapy improved outcomes in this cohort, we identified a comparison group of 123 consecutive patients who met the oncologic eligibility criteria in the original trial, but received no neoadjuvant therapy (neither chemotherapy nor androgen deprivation) and underwent RP only at Memorial Sloan-Kettering Cancer Center from 1996-2001. Because of the likely bias to enroll the highest risk patients into the CHT trial we further identified a subgroup within the comparison group at the top 30% of the Kattan risk score, as a higher-risk comparison group.19, 20 Patients in both the experimental and comparison group were offered additional adjuvant therapy and treated in a similar manner if they developed evidence of recurrent or systemic disease.
Statistical Methods
To test for differences in baseline patient and disease characteristics between patients treated with CHT plus RP and patients treated with RP alone Wilcoxon rank sum and Fisher's exact tests were used. Cox proportional hazard models were used to compare the risk of BCR and development of metastasis between the two groups with date of surgery as time zero, with adjustment for the Kattan nomogram risk score (predicted risk of BCR within 5 years) for risk adjustment. For this study BCR was defined as a PSA >0.05 ng/ml following radical prostatectomy. This model was repeated comparing the experimental cohort to the higher-risk comparison group. As an additional sensitivity analysis, we considered that patients in the CHT group had delayed surgery because of the time required for neoadjuvant therapy. Consider a patient with occult metastasis destined to produce sufficient PSA to be detectable two years after diagnosis irrespective of treatment. Such a patient would have an apparently shorter time from surgery to BCR if surgery was delayed by CHT. To control for this effect, we used a previously published methodology.21 The outcomes were dichotomized into event before or after five years, with patients censored before five years excluded. A logistic regression model was then built, adjusted for Kattan nomogram risk score. All analysis were conducted using Stata 12.0 (Stata Corp., College Station, Tx). Two patients with no follow-up were omitted from the biochemical recurrence analysis (one in each treatment group), and one patient with no follow-up (control group) was omitted from the metastatic disease analysis. All patients were included in overall survival analysis.
Results
Patient and disease characteristics for both the treatment group and the control group are presented in table 1. Patients receiving CHT were younger than those undergoing RP alone and at much higher baseline risk from their disease as reflected by a nomogram risk of BCR scores 33 percentage points higher than those treated with RP alone (p<0.0001). Median total PSA was 18.5 ng/ml higher than patients undergoing RP alone (p<0.0001) and 55% of those enrolling in the protocol were clinical stage ≥T3 versus 12% of those undergoing RP alone (p<0.0001). Even for a subset of control group at the top 30% of the Kattan risk score, important differences remained in total PSA levels and clinical stage. Pathologic comparisons demonstrate that despite worse pretreatment features, the CHT group had a lower proportion of positive surgical margins and lymph node invasion. Although a higher percentage of patients in this group had extraprostatic extension, seminal vesicle invasion, and bladder neck invasion.
Table 1.
Patient and disease characteristics of prostate cancer patients treated with chemohormonal therapy (CHP) and radical prostatectomy (RP) vs those eligible for enrollment but underwent RP only. CHT patients were also compared to a high-risk RP subset of the comparison group at the top 30% of the Kattan risk score. Data presented are median (IQR) or frequency (%).
| CHT + RP (n=34) | RP Only (n=123) | p-value | High Risk RP (N=37) | p-value | |
|---|---|---|---|---|---|
| Clinical Risk Factors | |||||
| Age at diagnosis | 56.0 (51.0, 61.0) | 60.8 (55.5, 64.9) | 0.002 | 57.6 (55.0, 62.7) | 0.2 |
| Total PSA | 27.7 (11.0, 43.2) | 9.2 (5.5, 20.0) | <0.0001 | 16.8 (10.1, 23.0) | 0.017 |
| Predicted 5-year risk of BCR* | 69.1 (54.5, 90.2) | 36.2 (25.2, 50.9) | <0.0001 | 68.1 (52.1, 72.2) | 0.091 |
| Clinical Stage | |||||
| T1c/T2a | 12 (35%) | 66 (54%) | <0.0001 | 6 (16%) | 0.002 |
| T2b/T2c | 4 (9%) | 42 (34%) | 17 (46%) | ||
| T3a/T3b | 18 (53%) | 15 (12%) | 14 (38%) | ||
| Biopsy Gleason Score | |||||
| <7 | 2 (6%) | 16 (13%) | 0.005 | 4 (11%) | 0.7 |
| 7 | 17 (53%) | 27 (22%) | 21 (57%) | ||
| >=8 | 14 (41%) | 80 (65%) | 12 (32%) | ||
| Pathologic Outcomes | |||||
| Extracapsular Extension | 34 (100%) | 70 (57%) | 25 (68%) | ||
| Seminal Vesicle Invasion | 20 (59%) | 31 (25%) | 14 (38%) | ||
| Bladder Neck Invasion | 8 (24%) | 10 (8.1%) | 6 (16%) | ||
| Positive Surgical Margins | 8 (24%) | 47 (38%) | 17 (46%) | ||
| Positive Nodes | 2 (5.9%) | 16 (13%) | 8 (22%) | ||
| Pathological stage | |||||
| T2a | 2 (5.9%) | 10 (8.1%) | 1 (2.7%) | ||
| T2b/T2c | 9 (26%) | 39 (32%) | 8 (22%) | ||
| T3a/T3b | 16 (47%) | 64 (52%) | 22 (59%) | ||
| T4 | 7 (21%) | 10 (8.1%) | 6 (16%) | ||
| Pathologic Gleason Score | |||||
| Not graded due to CHT | 23 (68%) | 0 | 0 | ||
| 6 | 1 (9.1%) | 8 (6.5%) | 2 (5.4%) | ||
| 7 | 4 (36%) | 62 (50%) | 20 (54%) | ||
| 8 | 1 (9.1%) | 28 (23%) | 7 (19%) | ||
| 9 | 5 (45%) | 25 (20%) | 8 (22%) | ||
| Postoperative Treatment | |||||
| Adjuvant Treatment | 3 (9.1%) | 1 (1.7%) | 1 (5.9%) | ||
| Salvage XRT within 5 years | 3 (8.8%) | 26 (21%) | 5 (14%) | ||
Based on the Kattan Nomogram (Gleason grade, clinical stage and PSA)
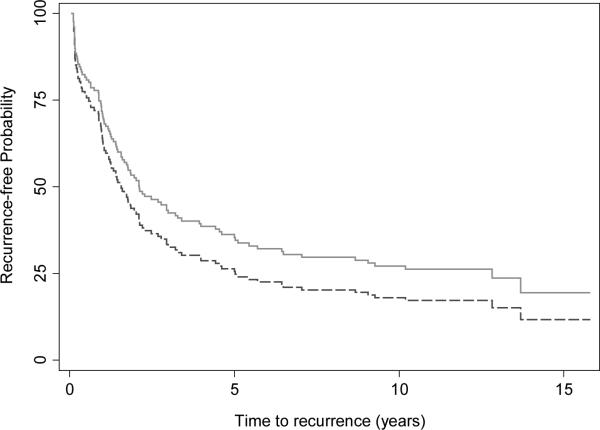
Median follow up for BCR-free patients was 11.2 and 12.3 years for those treated with RP alone and CHT plus RP, respectively. BCR was present in 73 of 122 patients in the RP only group and 24 of 33 patients in the CHT plus RP group during the entire follow up; 60 and 24 within 5 years respectively. Differences between groups were small and non-significant, and the direction of effect was sensitive to the statistical method used (Table 2). Similarly survival curves adjusted for Kattan risk failed to demonstrate significant differences between groups (Figure 1).
Table 2.
Outcomes for patients treated with chemohormonal therapy (CHT) and RP (N=34) compared with patients eligible for enrollment in the CHT trial but underwent RP only (N=123). Because of imbalances in the two groups the CHT were also compared to a subset of the comparison group at the top 30% of the Kattan risk score (N=37).
| Outcome | Comparison Group | Cox Proportional Hazard Model | 5-yr Logistic Regression Model | ||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p-value | OR | 95% CI | p-value | ||
| BCR | Complete | 0.76 | 0.43, 1.34 | 0.3 | 1.02 | 0.35, 2.95 | 1 |
| BCR | High-risk only | 0.83 | 0.47, 1.48 | 0.5 | 1.42 | 0.46, 4.40 | 0.5 |
| Metastatic Disease | Complete | 0.55 | 0.24, 1.29 | 0.2 | 0.37 | 0.10, 1.31 | 0.12 |
| Metastatic Disease | High-risk only | 0.59 | 0.25, 1.41 | 0.2 | 0.43 | 0.12, 1.47 | 0.2 |
| Overall Death | Complete | 0.98 | 0.41, 2.35 | 1 | 0.42 | 0.07, 2.62 | 0.4 |
| Overall Death | High-risk only | 0.99 | 0.40, 2.45 | 1 | 0.41 | 0.07, 2.32 | 0.3 |
* BCR = Biochemical Recurrence
Figure 1.
Biochemical recurrence-free probability for patients treated for high-risk prostate cancer treated with chemohormonal therapy and RP in a phase II multicenter trial (solid, n=33) compared to patients treated with RP alone (dashed, n=122), adjusted for the Kattan risk score (HR 0.76, 95% CI 0.43, 1.34; p=0.3).
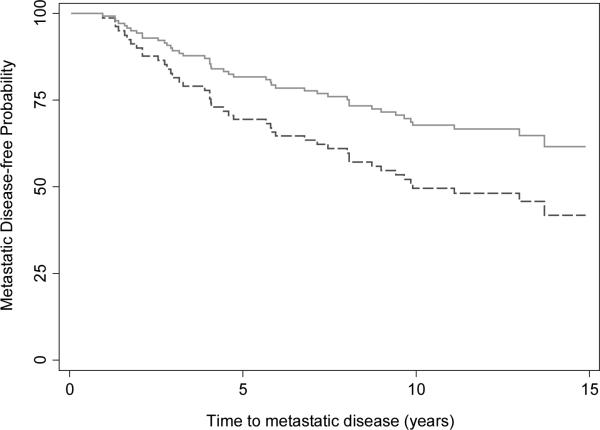
During follow-up 31 patients who underwent RP only developed metastatic PCa and 13 patients in the CHT plus RP developed metastatic disease; 19 and 6 within 5 years, respectively. The risk of metastasis was importantly lower in the CHT + RP group, with similar findings for the four different analyses. Nonetheless, confidence intervals are wide and no result was statistically significant.
For patients who received CHT prior to RP, probability of disease-specific survival at 10 years was 84% (95% CI 66%, 93%) and overall survival was 78% (95% CI 60%, 89%). Overall survival favored the CHT group over the comparison group when adjusted for differences in disease characteristics; again these analyses demonstrated wide confidence intervals and no statistically significant results. Three patients in the RP only group, and one in the RP plus CHT group, experienced symptomatic local recurrences that required intervention.
Discussion
We report long term outcomes of patients enrolled in a phase II trial of neoadjuvant CHT followed by RP for the treatment of high-risk PCa. Patients had promising long term overall oncologic outcomes, with only one patient developing a symptomatic local recurrence, and few prostate cancer specific deaths. In order to better understand the impact of the CHT plus RP, we have attempted to compare the trial patients to a cohort of patients from the same time frame whose clinical variables made them potential candidates for enrollment in the trial but instead received RP alone as their primary treatment. Despite both groups being defined as high-risk by NCCN criteria, the CHT group had markedly higher baseline risk, confirming that “high-risk” prostate cancer includes patients at very different levels of risk.2 After adjustment for baseline risk, we observed a potential beneficial effect of the CHT strategy which demonstrated lower rates of metastases; confidence intervals were wide and none of these findings were statistically significant.
Taxane based chemotherapy has improved survival for patients with castrate-resistant, metastatic prostate cancer and remains the primary cytotoxic agent used for the treatment of this disease.19,20 Several investigators have attempted to evaluate the impact of neoadjuvant taxane based CHT followed by RP for high-risk PCa with the hypothesis that this approach may help extirpate tumor cells that are no longer confined to the prostate. Existing studies have primarily focused on the feasibility, toxicities and pathologic response rate with secondary outcome measures being short to intermediate BCR rates; our study is unique and important in that it is by far the longest follow of any such cohort and our outcome measures include metastasis free and overall survival which has not been examined in previous studies. Prior investigators have demonstrated that complete pathologic response occurs infrequently (0 – 11%) with encouraging but short-term BCR rates.12-18 Prayer-Galetti et al. reported a phase II trial of 21 patients who received androgen deprivation therapy (ADT) and four cycles of docetaxel plus estramustine followed by RP; pT0 was rate (5%) and with a median follow-up of 53 months, eight patients (42%) remained disease-free, nine (47%) had biochemical recurrence only and two (11%) had local recurrence.13 Sella et al. examined 22 patients who similarly received ADT and four cycles of docetaxel and estramustine however they found no patients had a complete pathologic response and with a median follow-up of 23.6 months 45% of patients had developed BCR.14 Chi et al. described the results of a phase II multicenter study of 64 patients who completed ADT and three cycles of docetaxel prior to RP; two patients (3%) had complete pathologic response and 30% experienced BCR at 42.7 months of follow-up.15 Mellado et al. reported on 51 patients who received ADT and three cycles of docetaxel followed by RP and demonstrated a complete pathologic response in three (6%) patients and BCR in 18 (31.6%) of patients with a median follow-up of 35 months.16 Garzotto et al. reported on the results of 57 patients who received docetaxel and estramustine without ADT prior to RP and demonstrated no patients had a complete pathologic response and 5-year BCR- free survival was 49.8% (95% CI, 35.5%- 64.1%).17 Variations in outcomes between studies may be attributable to differences in patients initial disease risk, type and duration of cytotoxic agents used, length of follow or use of ADT. In our original report in 2004 we demonstrated similar findings to these other cohorts; no patient achieved a pathologic complete response (0% pT0) and BCR rates were 51% with a median follow up of 20 months.18 Importantly in our study with longer follow, only 7 of 31 patients (22%; 95% CI 10%, 37%) remain free of BCR within 10 years of RP.
Prior randomized trials comparing RP alone to neoadjuvant ADT followed by RP without concomitant chemotherapy, have demonstrated decreased rates of margin positivity and tumor down staging but have repeatedly failed to establish improved oncologic outcomes.5-7 This exemplifies why pathologic and early outcomes must be followed with long term results focused on more meaningful oncologic endpoints and to our knowledge this is the first report of the long-term outcomes of neoadjuvant CHT followed by RP. Although not the primary endpoint of this phase II study we have demonstrated low rates of metastasis with CHT followed by RP; with 10 years of follow up, 63% (95% CI 44%, 77%) patients in the experimental group developed metastatic disease. Adjusted for baseline risk score, rates of metastatic disease in the CHT group were lower than those in our comparison group but the difference was not statistically significant. Additionally despite the aggressive nature of their malignancy only 16% (95% CI 7%, 34%) died from their disease and 22% (95% CI 11%, 40%) from any cause in the experimental group.
While we attempted comparisons between patients enrolled in this phase II trial and a group of patients whose clinical characteristics would have allowed them entry into the study and were treated during a similar timeframe, these comparisons were limited due to the large baseline differences in these groups. Such comparisons may provide insight into the impact of the experimental intervention but have important flaws as exemplified in this study. The experimental group had both more aggressive clinical characteristics and despite receiving neoadjuvant CHT retained more aggressive pathologic features. It is likely that in addition to these observed differences there may be unobserved differences between these groups further limiting these findings. Currently studies such as the Cancer and Leukemia Group B randomized phase III study of RP alone versus docetaxel-based CHT before RP are accruing patients in an effort to better assess the impact of neoadjuvant chemo-hormonal treatment before surgery; however long term oncologic comparisons are many years away.21
Neoadjuvant chemo-hormonal therapy followed by RP is yet to be established as superior to RP alone yet it is associated with adverse toxicities. While generally well tolerated with almost all patients completing the planned neoadjuvant therapies in this and other studies, both docetaxel and hormonal agents may have adverse side effects. Chi et al. reported thirty grade 3 or 4 adverse events related to protocol therapy in their study of 72 men14 and in the original report of our series deep venous thrombosis occurred in 8 (22%) of patients.15 Additionally despite protocol mandated recording of adverse events these studies may not fully capture common adverse effects of androgen deprivation that may negatively impact quality of life such as, decreased libido, erectile dysfunction, vasomotor symptoms, impairment of cognitive function, and risk of osteoporosis. Hormonal therapy may also induce a desmoplastic reaction and make surgery more difficult.22,23 In the future, patient reported outcomes would be essential component of these trials.24
In addition to the lack of an adequate comparison group in this study, it is limited by its small size. The central estimate for the effects of neoadjuvant therapy on metastasis was an approximate halving of risk, a large effect of clear clinical relevance, yet one that was not statistically in our analysis. Other limitations include that complete data was not available for all patients, and we attempted to address endpoints that were not the goal of the original study. Patients received a variety of treatments for BCR and metastatic disease that generally included hormonal therapy as well as additional treatments but these were not standardized and changed over the course of the study as new therapies emerged. These treatments may have impacted cancer specific and overall survival.
Conclusion
This is the first report of long term outcomes of neoadjuvant cytotoxic chemotherapy combined with hormonal therapy followed by RP. High-risk PCa patients treated with neoadjuvant CHT followed by RP experienced a trend toward improvement in metastatic-free and overall survival when compared with a group of high-risk patients undergoing RP alone; however this trend did not reach significance. Neoadjuvant chemo-hormonal therapy is associated with a number of risks and as of yet has not shown a significant clinical benefit. Patients with high-risk PCa should only pursue neoadjuvant strategies, prior to RP, in the setting of clinical trial; such trials are open and actively accruing patients.
Figure 2.
Metastatic disease-free probability for patients treated for high-risk prostate cancer treated with chemohormonal therapy and RP in a phase II multicenter trial (solid, n=34) compared to patients treated with RP alone (dashed, n=122) , adjusted for the Kattan risk score (HR= 0.55, 95% CI 0.24, 1.29; p=.2).
Key of Definitions and abbreviations
- RP
Radical Prostatectomy
- NCCN
National Comprehensive Cancer Network
- CHT
Chemohormonal therapy
- PCa
Prostate Cancer
- ADT
Androgen Depravation Therapy
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer Statistics, 2012. 2012 doi: 10.3322/caac.21153. [DOI] [PubMed] [Google Scholar]
- 2.Yossepowitch O, Eggener SE, Bianco FJ, et al. Radical prostatectomy for clinically localized, high risk prostate cancer: critical analysis of risk assessment methods. The Journal of urology. 2007;178(2):493–9. doi: 10.1016/j.juro.2007.03.105. [DOI] [PubMed] [Google Scholar]
- 3.Mohler JL, Armstrong AJ, Bahnson RR, et al. Prostate cancer, Version 3.2012: featured updates to the NCCN guidelines. Journal of the National Comprehensive Cancer Network: JNCCN. 2012;10(9):1081–7. doi: 10.6004/jnccn.2012.0114. [DOI] [PubMed] [Google Scholar]
- 4.Bolla M, Collette L, Blank L, et al. Long-term results with immediate androgen suppression and external irradiation in patients with locally advanced prostate cancer (an EORTC study): a phase III randomised trial. Lancet. 2002;360(9327):103–6. doi: 10.1016/s0140-6736(02)09408-4. [DOI] [PubMed] [Google Scholar]
- 5.Yee DS, Lowrance WT, Eastham J a, Maschino AC, Cronin AM, Rabbani F. Long-term follow-up of 3-month neoadjuvant hormone therapy before radical prostatectomy in a randomized trial. BJU international. 2010;105(2):185–90. doi: 10.1111/j.1464-410X.2009.08698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soloway MS, Pareek K, Sharifi R, et al. Neoadjuvant androgen ablation before radical prostatectomy in cT2bNxMo prostate cancer: 5-year results. The Journal of urology. 2002;167(1):112–6. [PubMed] [Google Scholar]
- 7.Klotz LH, Goldenberg SL, Jewett M a, et al. Long-term followup of a randomized trial of 0 versus 3 months of neoadjuvant androgen ablation before radical prostatectomy. The Journal of urology. 2003;170(3):791–4. doi: 10.1097/01.ju.0000081404.98273.fd. [DOI] [PubMed] [Google Scholar]
- 8.Montie JE, Clark PE, Eisenberger MA, et al. NCCN.org Bladder Cancer. 2009 [Google Scholar]
- 9.Motzer RJ, Agarwal N, Beard C, et al. NCCN clinical practice guidelines in oncology: testicular cancer. Journal of the National Comprehensive Cancer Network: JNCCN. 2009;7(6):672–93. doi: 10.6004/jnccn.2009.0047. [DOI] [PubMed] [Google Scholar]
- 10.Carlson RW, Allred DC, Anderson BO, et al. Metastatic breast cancer, version 1.2012: featured updates to the NCCN guidelines. Journal of the National Comprehensive Cancer Network: JNCCN. 2012;10(7):821–9. doi: 10.6004/jnccn.2012.0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walsh TN, Noonan N, Hollywood D, Kelly A, Keeling N, Hennessy TP. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. The New England journal of medicine. 1996;335(7):462–7. doi: 10.1056/NEJM199608153350702. [DOI] [PubMed] [Google Scholar]
- 12.Gleave M, Kelly WK. High-risk localized prostate cancer: a case for early chemotherapy. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2005;23(32):8186–91. doi: 10.1200/JCO.2005.03.3068. [DOI] [PubMed] [Google Scholar]
- 13.Prayer-Galetti T, Sacco E, Pagano F, et al. Long-term follow-up of a neoadjuvant chemohormonal taxane-based phase II trial before radical prostatectomy in patients with non-metastatic high-risk prostate cancer. BJU international. 2007;100(2):274–80. doi: 10.1111/j.1464-410X.2007.06760.x. [DOI] [PubMed] [Google Scholar]
- 14.Sella A, Zismon A, Kovel S, et al. Neoadjuvant chemohormonal therapy in poor-prognosis localized prostate cancer. Urology. 2008;71(2):323–327. doi: 10.1016/j.urology.2007.08.060. [DOI] [PubMed] [Google Scholar]
- 15.Chi KN, Chin JL, Winquist E, Klotz L, Saad F, Gleave ME. Multicenter phase II study of combined neoadjuvant docetaxel and hormone therapy before radical prostatectomy for patients with high risk localized prostate cancer. The Journal of urology. 2008;180(2):565–70. doi: 10.1016/j.juro.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 16.Mellado B, Font A, Alcaraz A, et al. Phase II trial of short-term neoadjuvant docetaxel and complete androgen blockade in high-risk prostate cancer. British Journal of Cancer. 2009;101(8):1248–1252. doi: 10.1038/sj.bjc.6605320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garzotto M, Higano C, O'Brien C, et al. Phase 1/2 study of preoperative docetaxel and mitoxantrone for high-risk prostate cancer. Cancer. 2010;116(7):1699–1708. doi: 10.1002/cncr.24960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Konety BR, Eastham J a, Reuter VE, et al. Feasibility of radical prostatectomy after neoadjuvant chemohormonal therapy for patients with high risk or locally advanced prostate cancer: results of a phase I/II study. The Journal of urology. 2004;171(2 Pt 1):709–13. doi: 10.1097/01.ju.0000108122.36893.5a. [DOI] [PubMed] [Google Scholar]
- 19.Kattan MW, Eastham JA, Stapleton AM, Wheeler TM, Scardino PT. A preoperative nomogram for disease recurrence following radical prostatectomy for prostate cancer. Journal of the National Cancer Institute. 1998;90(10):766–71. doi: 10.1093/jnci/90.10.766. [DOI] [PubMed] [Google Scholar]
- 20.Stephenson AJ, Scardino PT, Eastham JA, et al. Preoperative nomogram predicting the 10-year probability of prostate cancer recurrence after radical prostatectomy. Journal of the National Cancer Institute. 2006;98(10):715–7. doi: 10.1093/jnci/djj190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vickers AJ, Bianco FJ, Boorjian S, Scardino PT, Eastham J a. Does a delay between diagnosis and radical prostatectomy increase the risk of disease recurrence? Cancer. 2006;106(3):576–80. doi: 10.1002/cncr.21643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Petrylak DP, Tangen CM, Hussain MH a, et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. The New England journal of medicine. 2004;351(15):1513–20. doi: 10.1056/NEJMoa041318. [DOI] [PubMed] [Google Scholar]
- 23.De Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376(9747):1147–54. doi: 10.1016/S0140-6736(10)61389-X. [DOI] [PubMed] [Google Scholar]
- 24.Eastham J a, Kelly WK, Grossfeld GD, Small EJ. Cancer and Leukemia Group B (CALGB) 90203: a randomized phase 3 study of radical prostatectomy alone versus estramustine and docetaxel before radical prostatectomy for patients with high-risk localized disease. Urology. 2003;62:55–62. doi: 10.1016/j.urology.2003.09.052. [DOI] [PubMed] [Google Scholar]
- 25.Hugosson J, Abrahamsson PA, Ahlgren G, et al. The risk of malignancy in the surgical margin at radical prostatectomy reduced almost three-fold in patients given neo-adjuvant hormone treatment. European urology. 1996;29(4):413–9. doi: 10.1159/000473789. [DOI] [PubMed] [Google Scholar]
- 26.Soloway MS, Sharifi R, Wajsman Z, McLeod D, Wood DP, Puras-Baez A. Randomized prospective study comparing radical prostatectomy alone versus radical prostatectomy preceded by androgen blockade in clinical stage B2 (T2bNxM0) prostate cancer. The Lupron Depot Neoadjuvant Prostate Cancer Study Group. The Journal of urology. 1995;154(2 Pt 1):424–8. [PubMed] [Google Scholar]
- 27.Basch E. Toward patient-centered drug development in oncology. The New England journal of medicine. 2013;369(5):397–400. doi: 10.1056/NEJMp1114649. [DOI] [PubMed] [Google Scholar]